Abstract
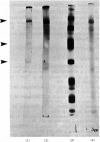
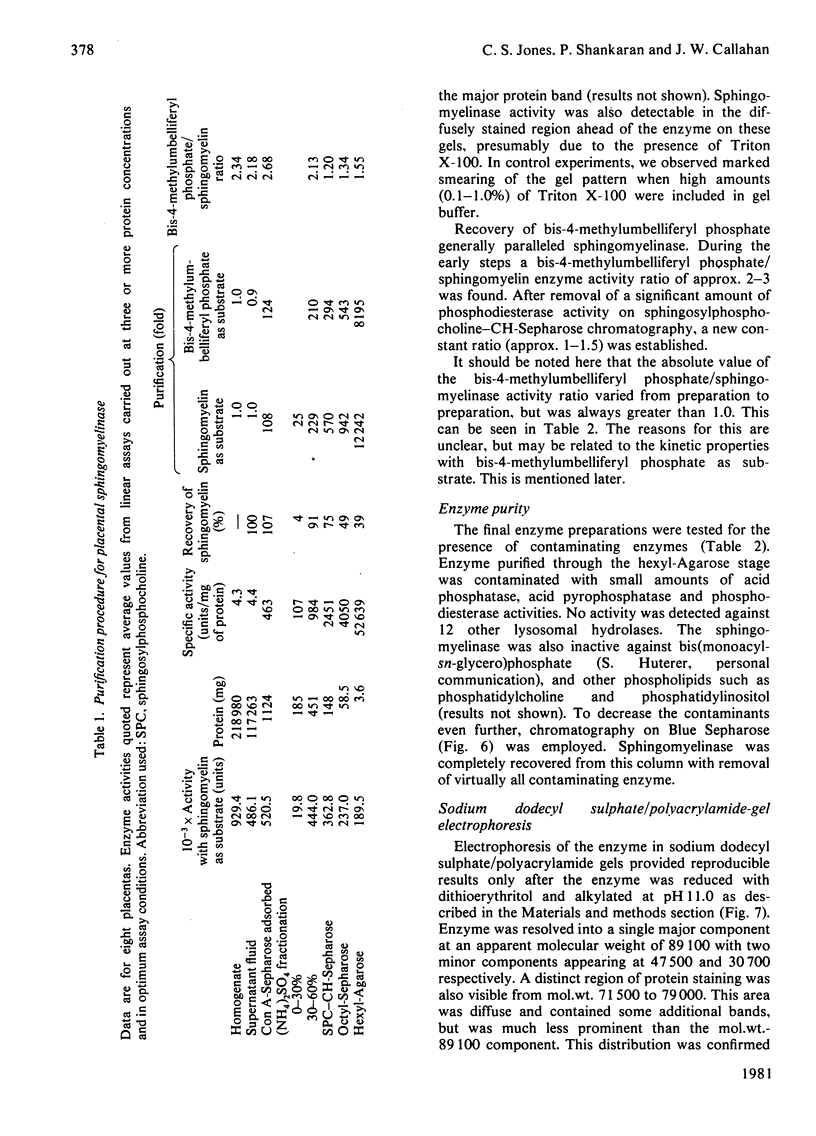
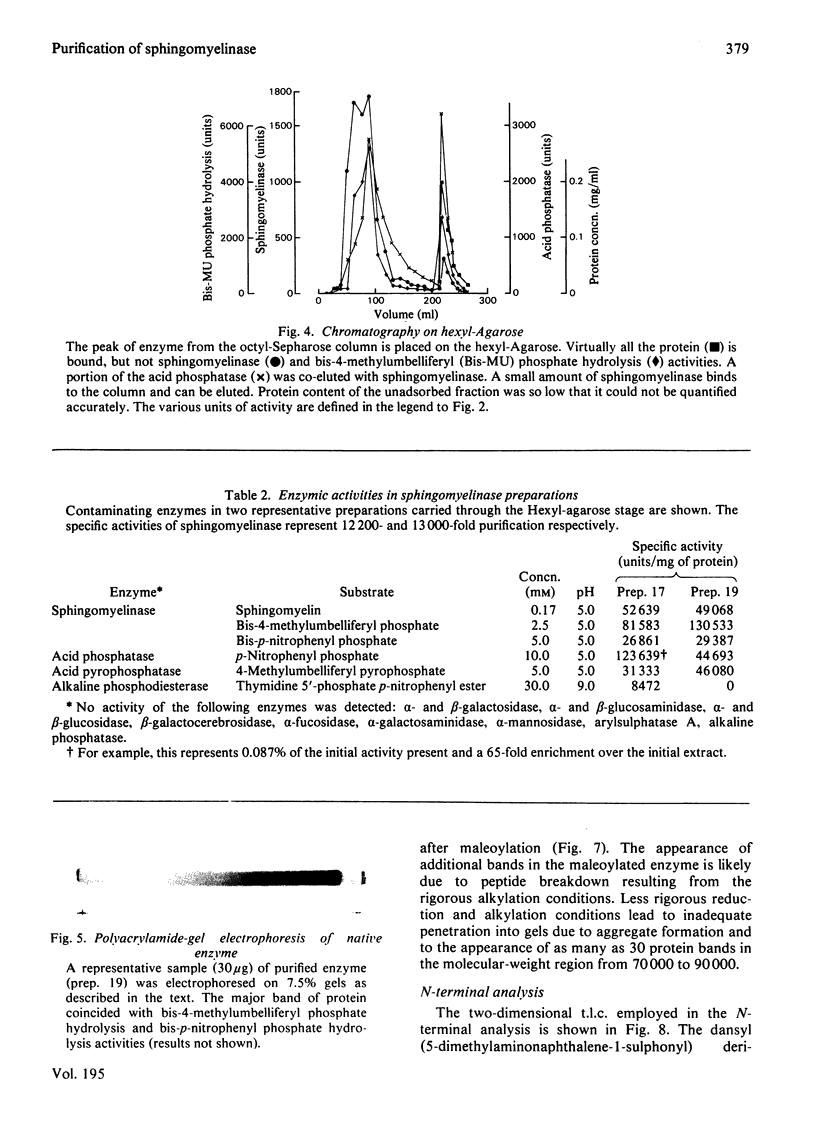
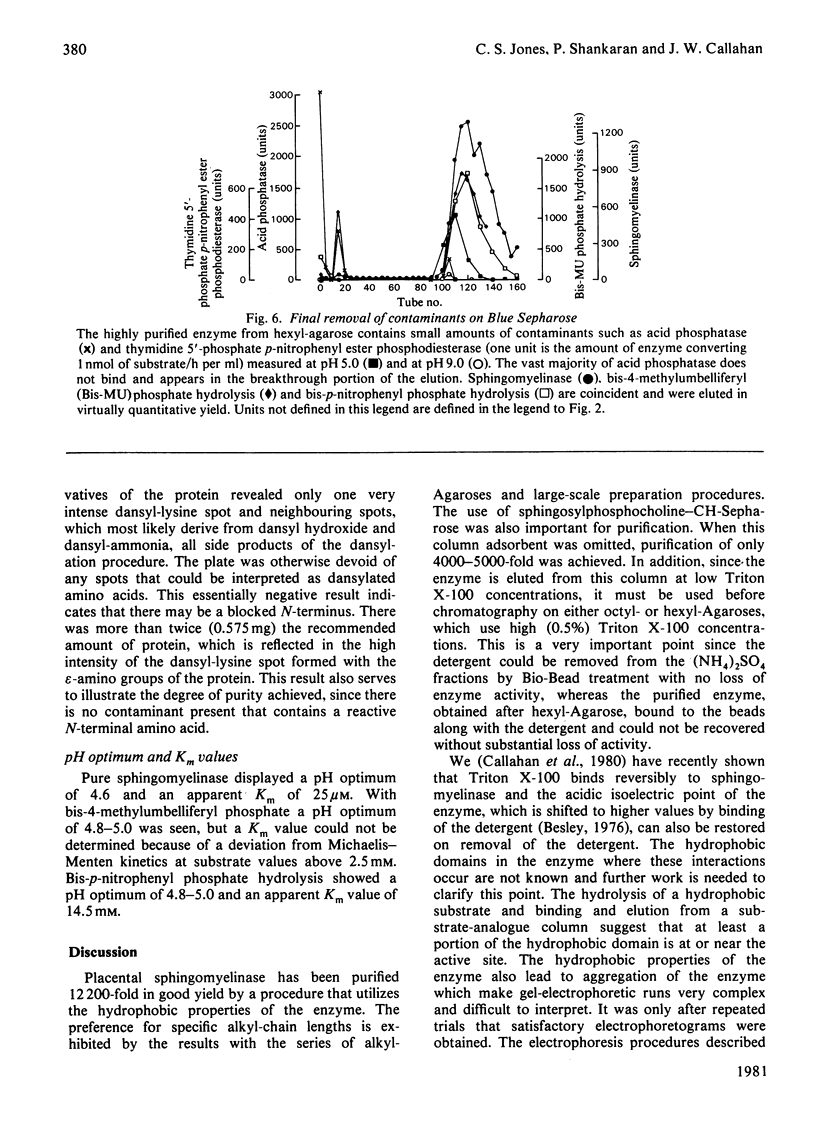
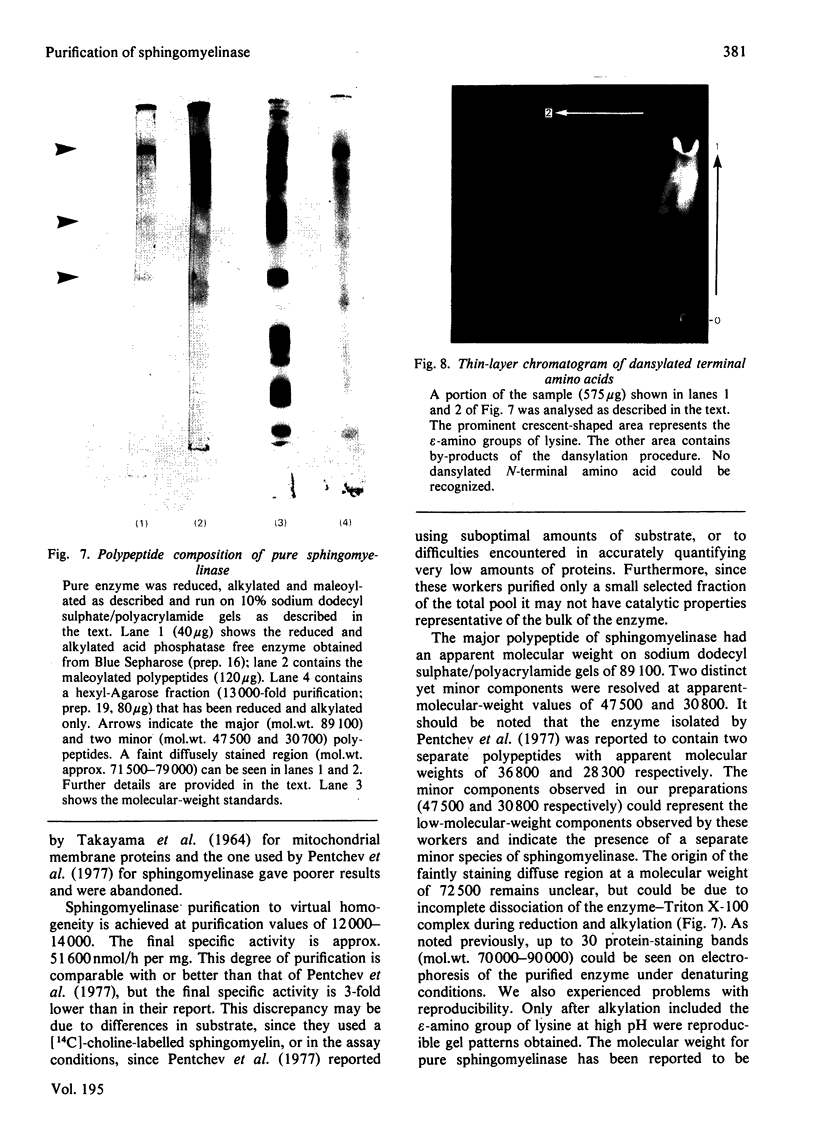
Placental sphingomyelinase has been purified to apparent homogeneity by a procedure that makes extensive use of hydrophobic interaction chromatography on sphingosylphosphocholine-CH-, octyl-, hexyl- and Blue-Sepharoses. Enzyme purification is about 10000- 14000-fold over starting extract with excellent yield (usually greater than 28%). Purification of bis-4-methylumbelliferyl phosphate phosphodiesterase activity generally paralleled that of sphingomyelinase during the final stages of the procedure. The enzyme also hydrolysed bis-p-nitrophenyl phosphate, but at a lower rate compared with bis-4-methylumbelliferyl phosphate. A single major protein was observed under non-denaturing conditions. Sphingomyelinase, denatured by reduction and alkylation, is composed of a major polypeptide chain with an apparent molecular weight of 89 100 on sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. Two minor lower-molecular-weight components were consistently obtained at 47 500 and 30 700. These results were also obtained after maleoylation of the reduced and alkylated sample. The enzyme contains a blocked-N-terminal amino acid. An extensive search for contaminating enzymes revealed the presence of minor amounts of acid phosphatase, which were removed from the final enzyme sample. The highly purified enzyme is stable for several weeks when stored with Triton X-100 at 4 degrees C. The pure enzyme aggregates under denaturing and electrophoretic conditions and special care must be taken to ensure that hydrophobic bonding of the protein is decreased as much as possible. The reproducibility and large scale of this procedure should facilitate further study on the structure and kinetic properties of the enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besley G. T. Diagnosis of Niemann-Pick disease using a simple and sensitive fluorimetric assay of sphingomyelinase activity. Clin Chim Acta. 1978 Dec 15;90(3):269–278. doi: 10.1016/0009-8981(78)90266-8. [DOI] [PubMed] [Google Scholar]
- Besley G. T. Effect of Triton X-100 on the isoelectric focusing profile of fibroblast sphingomyelinase. FEBS Lett. 1976 Dec 15;72(1):101–104. doi: 10.1016/0014-5793(76)80822-8. [DOI] [PubMed] [Google Scholar]
- Callahan J. W., Gerrie J., Jones C. S., Shankaran P. Studies on the hydrophobic properties of sphingomyelinase. Biochem J. 1981 Jan 1;193(1):275–283. doi: 10.1042/bj1930275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan J. W., Khalil M., Philippart M. Sphingomyelinases in human tissues. II. Absence of a specific enzyme from liver and brain of Niemann-Pick disease, type C. Pediatr Res. 1975 Dec;9(12):908–913. doi: 10.1203/00006450-197512000-00009. [DOI] [PubMed] [Google Scholar]
- Callahan J. W., Lassila E. L., Philippart M. Phosphodiesterases in human tissues. I. Identification and separation of enzymes active on bis(p-nitrophenyl)phosphate. Biochem Med. 1974 Nov;11(3):250–261. doi: 10.1016/0006-2944(74)90122-7. [DOI] [PubMed] [Google Scholar]
- Callahan J. W., Lassila E. L., Philippart M. Phosphodiesterases in human tissues. II. Decreased hydrolysis of synthetic substrate by tissues from patients with the Niemann-Pick syndrome. Biochem Med. 1974 Nov;11(3):262–274. doi: 10.1016/0006-2944(74)90123-9. [DOI] [PubMed] [Google Scholar]
- Callahan J. W., Shankaran P., Khalil M., Gerrie J. Sphingomyelinases in human tissues. IV. Purification of sphingomyelinase from human placenta and effect of Triton X-100. Can J Biochem. 1978 Sep;56(9):885–891. doi: 10.1139/o78-137. [DOI] [PubMed] [Google Scholar]
- Fensom A. H., Benson P. F., Babarik A. W., Grant A. R., Jacobs L. Fibroblast phosphodiesterase deficiency in Niemann-Pick disease. Biochem Biophys Res Commun. 1977 Feb 7;74(3):877–883. doi: 10.1016/0006-291x(77)91600-x. [DOI] [PubMed] [Google Scholar]
- Garewal H. S. A procedure for the estimation of microgram quantities of triton X-100. Anal Biochem. 1973 Aug;54(2):319–324. doi: 10.1016/0003-2697(73)90359-x. [DOI] [PubMed] [Google Scholar]
- Gatt S., Gottesdiner T. Solubilization of sphingomyelinase by isotonic extraction of rat brain lysosomes. J Neurochem. 1976 Feb;26(2):421–422. doi: 10.1111/j.1471-4159.1976.tb04498.x. [DOI] [PubMed] [Google Scholar]
- Hostetler K. Y., Yazaki P. J. The subcellular localization of neutral sphingomyelinase in rat liver. J Lipid Res. 1979 May;20(4):456–463. [PubMed] [Google Scholar]
- Kanfer J. N., Young O. M., Shapiro D., Brady R. O. The metabolism of sphingomyelin. I. Purification and properties of a sphingomyelin-cleaving enzyme from rat liver tissue. J Biol Chem. 1966 Mar 10;241(5):1081–1084. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pentchev P. G., Brady R. O., Gal A. E., Hibbert S. R. The isolation and characterization of sphingomyelinase from human placental tissue. Biochim Biophys Acta. 1977 Aug 24;488(2):312–321. doi: 10.1016/0005-2760(77)90189-8. [DOI] [PubMed] [Google Scholar]
- Rao B. G., Spence M. W. Sphingomyelinase activity at pH 7.4 in human brain and a comparison to activity at pH 5.0. J Lipid Res. 1976 Sep;17(5):506–515. [PubMed] [Google Scholar]
- Sakiyama T., Robinson J. C., Chou J. Y. Characterization of alkaline phosphatases from human first trimester placentas. J Biol Chem. 1979 Feb 10;254(3):935–938. [PubMed] [Google Scholar]
- Takayama K., MacLennan D. H., Tzagoloff A., Stoner C. D. Studies on the electron transfer system. LXVII. Polyacrylamide gel electrophoresis of the mitochondrial electron transfer complexes. Arch Biochem Biophys. 1966 Apr;114(1):223–230. doi: 10.1016/0003-9861(66)90324-9. [DOI] [PubMed] [Google Scholar]
- Vanha-Perttula T. Chromatographic fractionation and characterization of rat testicular acid phosphatases. Biochim Biophys Acta. 1971 Feb 10;227(2):390–401. doi: 10.1016/0005-2744(71)90070-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Suzuki K. A novel magnesium-independent neutral sphingomyelinase associated with rat central nervous system meylin. J Biol Chem. 1978 Jun 25;253(12):4090–4092. [PubMed] [Google Scholar]
- Yamaguchi S., Suzuki K. Purification and characterization of sphingomyelinase from human brain. J Biol Chem. 1977 Jun 10;252(11):3805–3813. [PubMed] [Google Scholar]