Abstract
Background
The use of low titer group O whole blood (LTOWB) for resuscitation of patients with traumatic hemorrhage is becoming increasingly common. Practices regarding the administration of RhD-positive LTOWB to childbearing age females (CBAFs) vary between institutions due to concerns about RhD alloimmunization. This study examined practices related to LTOWB transfusion as they pertain to age and sex.
Methods
This was a secondary analysis of the Shock, Whole blood, and Assessment of TBI (traumatic brain injury) trial, a prospective, multicenter observational cohort study where outcomes following LTOWB transfusion were analyzed at seven level 1 trauma centers between 2018 and 2021, as well as a survey on transfusion practices at these centers conducted in 2023. The proportion of patients who received LTOWB or components was examined over the course of the study and grouped by age and sex, and the RhD group of injured CBAFs was documented.
Results
A total of 1046 patients were evaluated: 130 females aged <50 years (CBAFs), 77 females aged ≥50 years; 661 males aged <50 years, and 178 males aged ≥50 years. Among them, 26.2% of CBAFs received RhD-positive LTOWB, whereas 57.1%–66.3% of other sex/age groups received LTOWB. The proportion of CBAFs who received LTOWB increased significantly throughout the 4 years of this study. Except for older women in years 2 and 4, CBAFs were significantly less likely to receive LTOWB than all other groups for the study period and individual years. Among the 33 CBAFs who received LTOWB and for whom an RhD type was available, 4/33 (12.1%) were RhD-negative, while 9/95 (9.5%) CBAFs who received component therapy were RhD-negative. RhD blood product selection practices varied considerably between institutions.
Conclusions
Many institutions transfused LTOWB to CBAFs. Policies regarding RhD product selection varied. Of the total cohort, the proportion of RhD-negative CBAFs who received LTOWB increased over time but remained lower than all other groups.
Level of evidence
3.
Keywords: whole blood, resuscitation, transfusion
WHAT IS ALREADY KNOWN ON THIS TOPIC
Low titer O whole blood (LTOWB) is being used more widely in the USA.
Although there is increasing evidence for its survival benefit in severe trauma patients, there are persistent concerns regarding D-alloimmunization particularly for childbearing age females.
WHAT THIS STUDY ADDS
This study elucidates current policies and practice patterns in relation to the RhD selection of LTOWB for different age and sex groups of trauma patients from a sample of level 1 trauma centers.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Providers are encouraged to include childbearing age females in LTOWB transfusion programs.
Introduction
With increasing data supporting the safety, practicality, and benefits of transfusing low titer group O whole blood (LTOWB), its use has expanded beyond the military setting and has become more commonplace in civilian trauma centers.1,3 The civilian adoption of LTOWB required some adaptation to differences in blood supply and regulatory framework compared with the military setting. As only about 15% of North American blood donors are RhD-negative4 and about 7% are group O RhD-negative,5 most LTOWB is RhD-positive. As with red blood cells (RBCs), the relative scarcity of RhD-negative LTOWB means that not every trauma patient with significant hemorrhage whose RhD type is unknown at the time they require a transfusion can receive RhD-negative LTOWB units. Prioritization for administering the scarce RhD-negative LTOWB or RBCs at an institution or population level often considers the sex and age of the patient. This is primarily due to the concern for D-alloimmunization and the small potential risk of hemolytic disease of the fetus and newborn (HDFN) in future pregnancies in D-alloimmunized RhD-negative childbearing age females (CBAFs).1 Observational data show reduced mortality among the recipients of LTOWB compared with conventional components (COMP) transfusion, particularly for trauma patients with severe hemorrhage or increased prehospital probability of death.6,9 Given the survival improvement associated with LTOWB transfusion, policies regarding its use for injured CBAFs are an important health equity issue. A massively bleeding RhD-negative or RhD-type unknown CBAF should never be denied LTOWB or RBCs, even if only RhD-positive products are available. Part of the difficulty in making data-driven decisions regarding the risks and benefits of LTOWB in CBAFs is that they constitute a small proportion of the overall trauma population and have often been excluded from many studies on LTOWB, particularly when only RhD-positive LTOWB is available.10 11 This has resulted in limited data on practice patterns and clinical outcomes of CBAFs. The Shock, Whole blood, and Assessment of TBI (traumatic brain injury) (SWAT) study was a prospective, multicenter observational cohort study performed at seven level 1 trauma centers participating in the Linking Investigations in Trauma and Emergency Services (LITES) clinical trials network. This study evaluated outcomes in trauma patients who were resuscitated using LTOWB compared with conventional components. In this secondary analysis of the SWAT study database, we examined the evolution of trauma center practices during the enrollment period of the SWAT study as they pertain to age, sex, and the RhD selection of blood products for use in the initial resuscitation of injured patients.
Materials and methods
In conjunction with the LITES Network SWAT study group, we sought to examine the hospital policies for selecting the RhD types of RBC-containing blood products issued to hemorrhaging trauma patients during their initial resuscitation. Complete methods regarding inclusion and exclusion criteria for the SWAT study have been reported previously.6 Responses for the seven SWAT-participating sites were extracted from a national survey on the RhD-selection practices of blood units that are issued in emergencies.12 The survey was encoded in SurveyMonkey and the link to the survey was electronically sent to the transfusion and/or trauma medical directors of the seven hospitals that participated in the SWAT study in the spring and summer of 2023. When necessary, individual hospitals were contacted to clarify ambiguous responses. Notably, patients <15 years of age were excluded from the SWAT database but policies on pediatric transfusions were included in the survey.
Patient data from the prospectively collected SWAT database were examined to determine the institutional practices related to selecting the RhD type of the blood products used in trauma resuscitation, as well as the patients’ sexes and ages. Patients who received a blood product during their resuscitation were identified and characterized by receiving either LTOWB or COMP, sex (male or female), and age (<50 or ≥50 years of age). The age and sex groups compared were females aged <50 years (also referred to as CBAFs), females aged ≥50 years, males aged <50 years and males aged ≥50 years. The proportion of patients receiving LTOWB over time was compared across the sex by age groups with a repeated measures, generalized linear mixed model. Post hoc group by year comparisons were performed using a Bonferroni correction to account for multiple tests (alpha 0.05 divided by 24 tests=p<0.0021). The age threshold of 50 was chosen as it is the most frequent age used to define the upper limit of childbearing age for females both in prior studies as well as by survey of the seven participating trauma centers in this study. The RhD status of the women aged <50 years in the SWAT study and the nature of the blood products used in their resuscitation (ie, LTOWB or COMP) were collected.
Results
Provider survey
All seven SWAT-participating sites were university affiliated teaching hospitals that were designated as adult level 1 facilities; 3/7 (43%) were designated as pediatric level 1 facilities and a fourth hospital (ie, 1/7, 14%) was designated as a pediatric level 2 hospital. Six of the seven respondents (86%) came from facilities with >500 beds, and the remaining respondent was from a facility with 100–499 beds. The minimum age at which patients were considered to be adults at these centers is shown in table 1 and the age at which women were considered to be beyond childbearing potential for the purposes of providing RhD-positive blood products to RhD-negative or RhD-type unknown women is shown in table 1.
Table 1. (A) The minimum age at which patients are considered to be adults for selecting the RhD type of blood products (the ages at the two facilities where the threshold is <16 years were 14 and 15 years); (B) the age at which women are considered to be beyond childbearing potential for the purposes of providing RhD-positive blood products to RhD-negative women (the age at the facility where the threshold is <50 years was 45 years).
| A. At what age is a patient considered to be an adult for RhD selection at your institute? | Number of respondents (% of respondents) |
| <16 years | 2 (28.6) |
| 16 years | 1 (14.3) |
| 18 years | 2 (28.6) |
| Not defined because RhD-positive products issued for all patients and all sexes | 1 (14.3) |
| Did not specify an age because RhD- negative products exclusively provided | 1 (14.3) |
| B. What is the upper age limit for a woman to be considered of childbearing potential at your institute? | Number of respondents (% of respondents) |
| <50 years | 1 (14.3) |
| 50 years | 4 (57.1) |
| 55 years | 1 (14.3) |
| Not defined because RhD-positive products issued for all patients and all sexes | 1 (14.3) |
In terms of the first blood product(s) administered to massively bleeding patients, 3/7 (43%) of the respondents issued LTOWB, while 3/7 (43%) issued both LTOWB and RBCs as part of COMP therapy. One facility (1/7, 14%) had not yet implemented a LTOWB program and issued RBCs only as part of COMP therapy as their first products in a resuscitation.
One facility that issued both LTOWB and RBCs as the first product in a resuscitation had the same RhD-selection policy for both products; this facility preferentially issued RhD-negative products for women and girls, but RhD-positive products can be issued depending on the RhD-negative product inventory and/or if the bleeding is predicted to be extensive and ongoing. This facility issued only RhD-positive products for men and boys.
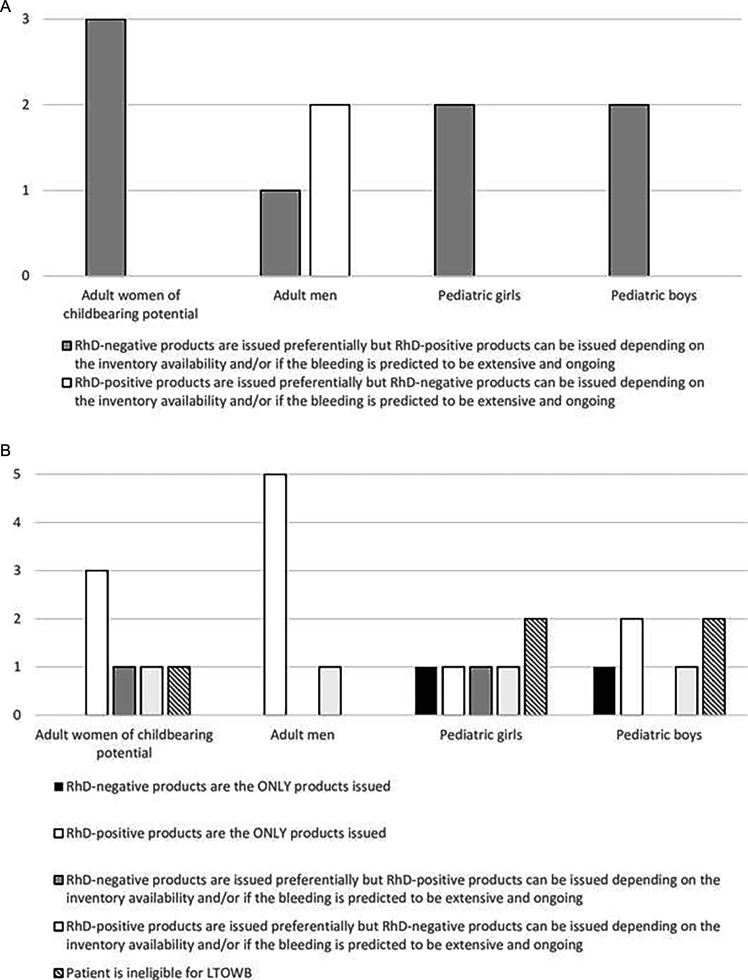
For the three facilities that either only issued RBCs in massive bleeding or if both RBCs and LTOWB were issued and the RhD-selection policy for these products was different than for LTOWB, the hospital policies for RhD selection for RBCs are shown in figure 1A. Note that one of the facilities did not treat pediatric patients so there were only two responding facilities in this category that provided their RhD-selection policy for pediatric patients. For the six facilities that either only issued LTOWB in massive bleeding or if both RBCs and LTOWB were issued and the RhD-selection policy was different, the hospital policies for RhD selection for LTOWB are shown in figure 1B.
Figure 1. (A) RhD-selection policies among the three SWAT centers that either only issue RBCs in massive bleeding or if both RBCs and LTOWB are issued and the RhD-selection policy is different for these two products. One facility does not treat pediatric patients, hence the total number of responses for pediatric patients is two. (B) RhD-selection policies among the six facilities that either only issue LTOWB in massive bleeding or if both RBCs and LTOWB are issued and the RhD-selection policy is different for these two products. LTOWB, low titer group O whole blood; RBC, red blood cell; SWAT, Shock, Whole blood, and Assessment of TBI (traumatic brain injury).
When asked about the policies for providing additional blood products to RhD-negative CBAFs who received RhD-positive blood products during resuscitation, the majority of centers (3/7, 43%) would keep issuing RhD-positive products during the acute resuscitation and then switch to RhD-negative products once the patient was more hemodynamically stable and not requiring a massive transfusion, while 2/7 (29%) would switch to RhD-negative products for the duration of the acute resuscitation and hospital stay. One facility (1/7, 14%) would continue issuing RhD-positive blood products for the rest of the admission, and another facility (1/7, 14%) indicated that this situation would not likely happen as their policy was to issue only RhD-negative products; at this center, if RhD-positive blood products were issued the transfusion physician would be consulted for D-alloimmunization prevention advice.
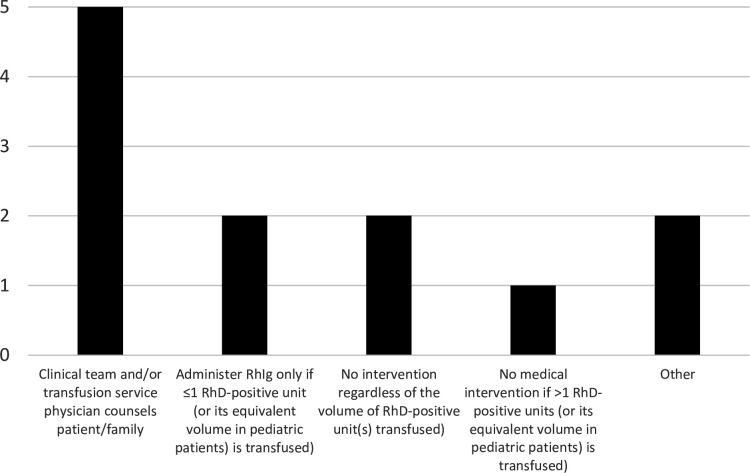
The follow-up policies at these seven facilities for RhD-negative CBAFs who received RhD-positive blood products during their resuscitation are shown in figure 2. At most centers (5/7, 71%), a member of the clinical team or the transfusion service counseled the patient or their family and at 2/7 (29%) centers no further treatment was administered, while at 2/7 (29%) other centers, Rh immunoglobulin (RhIg) was administered if only one RhD-positive unit was transfused. One of the two ‘other’ responses indicated that it was the clinician’s responsibility to determine if RhIg was needed and the second ‘other’ response indicated that this situation was unlikely to happen at the facility as their policy is to use RhD-negative products.
Figure 2. The follow-up policies at the seven SWAT facilities for RhD-negative women of childbearing age who receive RhD-positive blood products during their resuscitation. The total number of replies exceeds seven because multiple answers were permitted. See text for description of the ‘other’ responses. RhIg, Rh immunoglobulin; SWAT, Shock, Whole blood, and Assessment of TBI (traumatic brain injury).
Patient data
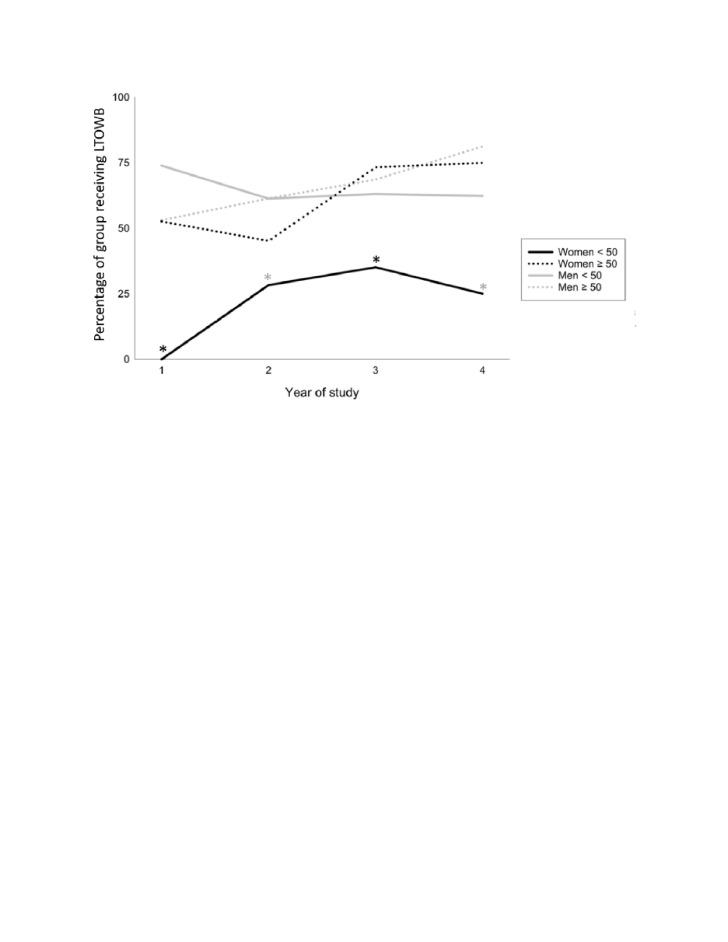
There were a total of 1046 patients enrolled in the SWAT study, of which 621/1046 (59.4%) received LTOWB (figure 3). There were 130 CBAFs enrolled in the study, of which 34/130 (26.2%) received LTOWB. The RhD group was available on 128/130 (98.0%) CBAF patients; overall, 13/128 (10.2%) were RhD-negative (figure 3). From the beginning to the end of the study, all groups were significantly more likely to receive LTOWB except for men aged <50 years who were significantly less likely to receive LTOWB over time (figure 4, table 2). Notably, the absolute number of patients receiving LTOWB over the 3 complete years of the study increased, indicating increased availability and/or comfort with the use of LTOWB overall. Proportions of age/sex groups receiving LTOWB over the study period were evaluated to better elucidate relative differences in transfusion practices for these groups. With the exception of females aged ≥50 years at years 2 and 4, where the proportional difference did not reach statistical significance, females aged <50 years were significantly less likely (p< 0.0021) to receive LTOWB compared with all other groups (table 2).
Figure 3. Age and sex of the LTWOB recipients in the SWAT study. Details of the nature of the transfused blood products and the RhD groups of the CBAFs in this study are also provided. Note that the RhD group was available on 128/130 (98.0%) of the CBAF patients. Patients aged <15 years of age were excluded from inclusion in the SWAT database. CBAF, childbearing age female; COMP, conventional components; LTOWB, low titer group O whole blood; SWAT, Shock, Whole blood, and Assessment of TBI (traumatic brain injury).
Figure 4. Percentage of patients aged >15 years of age who received LTOWB over the study period. Year 1: April 2018 to May 2019, year 2: April 2019 to May 2020, year 3: April 2020 to May 2021, year 4: May 2021 to September 2021. All groups were significantly more likely to receive LTOWB over time except for men aged <50 years who were significantly less likely to receive LTOWB over time. With the exception of females aged ≥50 years at years 2 and 4, where the proportional difference did not reach statistical significance, females aged <50 years were significantly less likely to receive LTOWB than all other groups in every year of the study. Enrollment began in May 2018 and ended in September 2021. Year 4 includes 5 months of enrollment from May 2021 to September 2021. Given the seasonal nature of trauma, transfusion practices over 5 months of summer may not be directly comparable to a full year. Black asterisk denotes females aged <50 years significantly lower proportion compared with all groups, grey asterisk denotes females aged <50 years significantly lower proportion compared with all males. LTOWB, low titer group O whole blood.
Table 2. Blood product groups by age and sex.
| All | Female aged <50 years | Female aged ≥50 years | Male aged <50 years | Male aged ≥50 years | ||||||
| Blood product transfused | N | N (%n) | N | N (%n) | N | N (%n) | N | N (%n) | N | N (%n) |
| All | 1046 | 130 | 77 | 661 | 178 | |||||
| Whole and components | 621 (59.4) | 34 (26.2) | 44 (57.1) | 425 (64.3) | 118 (66.3) | |||||
| Components only | 425 (40.6) | 96 (73.8) | 33 (42.9) | 236 (35.7) | 60 (33.7) | |||||
| Year 1 | 182 | 16 | 19 | 115 | 32 | |||||
| Whole and components | 112 (61.5) | 0 (0.0) | 10 (52.6) | 85 (73.9) | 17 (53.1) | |||||
| Components only | 70 (38.5) | 16 (100) | 9 (47.4) | 30 (26.1) | 15 (46.9) | |||||
| P (vs female aged <50 years) | 0.0006* | <0.0001 | 0.0003 | |||||||
| Year 2 | 327 | 53 | 31 | 199 | 44 | |||||
| Whole and components | 178 (54.4) | 15 (28.3) | 14 (45.2) | 122 (61.3) | 27 (61.4) | |||||
| Components only | 149 (45.6) | 38 (71.7) | 17 (54.8) | 77 (38.7) | 17 (38.6) | |||||
| P (vs female aged <50 years) | 0.1168 | <0.0001 | 0.0011 | |||||||
| Year 3 | 355 | 37 | 15 | 233 | 70 | |||||
| Whole and components | 219 (61.7) | 13 (35.1) | 11 (73.3) | 147 (63.1) | 48 (68.6) | |||||
| Components only | 136 (38.3) | 24 (64.9) | 4 (26.7) | 86 (36.9) | 22 (31.4) | |||||
| P (vs female aged <50 years) | 0.0002 | <0.0001 | <0.0001 | |||||||
| Year 4† | 182 | 24 | 12 | 114 | 32 | |||||
| Whole and components | 112 (61.5) | 6 (25.0) | 9 (75.0) | 71 (62.3) | 26 (81.3) | |||||
| Components only | 70 (38.5) | 18 (75.0) | 3 (25.0) | 43 (37.7) | 6 (18.8) | |||||
| P (vs female aged <50 years) | 0.0041 | 0.0008 | <0.0001 | |||||||
Year 1: April 2018 to May 2019, year 2: April 2019 to May 2020, year 3: April 2020 to May 2021, year 4: May 2021 to September 2021. With the exception of females aged ≥50 years at years 2 and 4, where the proportional difference did not reach statistical significance, females aged <50 years were significantly less likely to receive LTOWB than all other groups in every year of the study. Given the seasonal nature of trauma, transfusion practices over 5 months of summer may not be directly comparable to a full year.
Chi-square probability values in bold are significant after a Bonferroni correction (alpha 0.05 divided by 24 tests =p < 0.0021; group comparisons excluding feamles < 50 are not listed)
May 2021 to September 2021, inclusive
Discussion
In the survey component of this study, we found that CBAFs were included in many but not all programs where LTOWB is used in the resuscitation of trauma patients, with varying policies regarding the RhD type of the provided blood products. We also found that rate of transfusing LTOWB to CBAFs has increased over time, but the percentage of CBAFs who received LTOWB remained lower than all other age/sex categories for every year of the study. While we do not know the RhD type of the COMP or LTOWB units that were transfused, we found that a minority of CBAFs who received both LTOWB and COMP were RhD-negative and that the proportion of transfused RhD-negative CBAFs was lower than the average for North America (10.2% vs 15% respectively).
CBAFs represent a small proportion of the trauma population, and they were not included in the intervention arm of early studies of LTOWB in trauma due to concerns about D-alloimmunization with possible subsequent HDFN.10 13 As we have shown, they remain excluded from some LTOWB programs leading to low representation in retrospective studies.14 15 A recent retrospective review of TQIP data showed similar proportions (22% vs 23%) of females received either LTOWB or COMP, but the ages of the females in the study were not reported.8 In this study, we found that the percentage of females aged ≥50 years who received LTOWB was higher than CBAFs at all time points examined (although this difference only reached significance in years 1 and 3), so it is unclear if CBAFs are represented proportionally in studies examining LTOWB use that group all females together. This makes studying clinical outcomes in CBAFs difficult as meaningful statistical analysis is limited with small numbers. For example, part of the original plan for this secondary analysis was to study outcomes for CBAFs specifically but despite the size of this dataset from seven large trauma centers, our analyses were underpowered for interpretation. In the trauma population at large, LTOWB is safe10 16 17 with no significant increase in hemolysis or transfusion reactions.11 18 19 It also has been associated with decreased mortality,7 13 15 20 21 especially in patients with increased prehospital risk of mortality.6 Although there is strong evidence for sex dimorphisms in coagulation and general trauma outcomes,22,27 there is no direct evidence that LTOWB is associated with less benefit for females, nor is there evidence that balanced resuscitation with COMP benefits females less than males.28 29
Much of the concern about LTOWB transfusions in CBAFs and the reason for their exclusion from LTOWB programs is the risk of D-alloimmunization and the risk of HDFN in future pregnancies with a blood source that is predominantly RhD-positive. As demonstrated here and previously,30 there is practice variation across institutions regarding transfusion strategies. Many centers differentiate between sexes in pediatric as well as adult patients. In injured RhD-negative females, the risk of having a future pregnancy affected by HDFN after receiving a RhD-positive product is higher at younger ages, with a risk of approximately 6.5% at 18 years and decreasing to nearly 0% by 43 years.31 There are myriad steps that need to occur for an RhD-negative CBAF who receives an RhD-positive LTOWB or RBC unit during their trauma resuscitation to have a future pregnancy that is affected by HDFN.532,35 While it is generally assumed that 15% of the general North American population will be RhD-negative,4 this study found a lower rate (10.2%) of RhD-negative transfused CBAFs, thereby indicating that fewer CBAFs than expected were at risk of potential D-alloimmunization and future HDFN in this study. However, if only RhD-positive blood products are available, they should be used; several recent surveys have shown that a majority of females would accept an emergent transfusion if it increased their probability survival even by a very small amount, despite the potential for some degree of risk to a potential future fetus.36,39 There is increasing evidence showing a mortality benefit from LTOWB transfusion in trauma1 6 7 as well as increasing data for safety of RhD-positive transfusion in CBAFs.418,20 30 A recent model of injured CBAFs found that a >0.1% relative reduction in mortality from receipt of RhD-positive LTOWB compared with RhD-negative COMP during their resuscitation offset the life years of potential future fetuses lost due to anti-D-mediated HDFN mortality and severe neurological injury.40If one is persuaded that LTOWB is the superior blood product for resuscitating trauma patients and recognizes that CBAFs have consistently expressed that they prioritize survival over potential future adverse events, it is no longer logical or equitable to deny CBAFs a mortality reducing therapy due to the small risk to a potential future fetus that is a non-issue in 85%–90% of CBAFs.
There are several limitations of this study. Surveys were completed after the study period so it is possible that practices had changed after the study period, and questions about changes in practice over time were not included. The RhD types of the transfused LTOWB and component units were not collected, which precluded a direct comparison of RhD-positive exposure between the CBAFs who received RBCs and those who received LTOWB. However, 5/6 centers with LTOWB use only RhD-positive units for massively bleeding CBAFs regardless of their RhD type and since LTOWB is intended to be the first product transfused to bleeding patients, it is reasonable to conclude that the resuscitation of the CBAFs at 5/6 centers that use LTOWB began with RhD-positive LTOWB. Additionally, RhIg use was not recorded and as such we were not able to examine practice patterns for this therapy. Notably, it has been recently demonstrated that the use of RhIg to prevent D-alloimmunization across the USA is highly variable.43 Despite containing a large amount of clinical data, the number of CBAFs in the SWAT database was too small to examine outcomes following LTOWB transfusion relative to COMP in CBAFs compared with other age and sex groups. We also did not have access to follow-up data on the RhD-negative CBAFs who received RhD-positive blood products to determine their D-alloimmunization rate or their fertility rates. The fourth ‘year’ of the SWAT study only included 5 months which, given the seasonal nature of trauma presentations in most centers, makes it difficult to compare with the preceding three full years. Although the participating sites in the SWAT study spanned the USA, all had relatively advanced trauma systems and may not be representative of other centers nationwide particularly in regard to application of emerging data for which these sites are generally early adopters, further limiting generalizability. As data on safety of RhD-positive transfusion in CBAFs have increased over the past 5 years and full implementation generally takes longer than the timeline of this study,44 it is possible these practices would change over time in all centers without further intervention. However, when it comes to equitable care for our patients, education and advocacy are vital to speed the process of matching data with practice.
Conclusion
While the RhD-type selection of blood products for use in trauma policies varied considerably, we found that the use of LTOWB for injured CBAFs has significantly increased but this population of injured patients remains less likely to receive LTOWB than other age/sex groups despite better understanding of low risk and increasing evidence for benefit. In addition to provider education on the low risk of HDFN, continuous prospective and retrospective evaluation of outcomes in all age and sex demographics is vital to elucidate small differences in risk/benefits balance and provide high-quality and equitable patient care.
Acknowledgements
University of Pittsburgh, Presbyterian Hospital; Clinical & Data Coordinating Center and enrolling site: Barbara J Early-Young, Meghan L Buck, Peter W Adams, Rachel L Molinaro, Alexandra Merti, Ashely M Harner, Elizabeth A Gimbel, Logan Owens, Hannah Hayes, Alan Jackson, Laurie Silfies, Lisa Over, Steve Knopf, Melody Macey-Kalcevic, Angela Pattison, Megan E Buhay, Brianna J Higginbottom, Marissa L Marcin, MACRO Research Specialists, MACRO Clinical Trials Research Associates, and Cara Battistella. McGovern Medical School at UTHealth: Yu Bai, Kandice L Motley, Yao-Wei Wang, Victoria Herrick; Garrett Woodruff, Veda Pa, Rhonda Hobbs, Jeanette Podbielski, Laura Vincent, Christy Allen, Subin Alexander, Natolie Hamilton, Symantha Lopez, Selina Hernandez Gonzalez, Jason Rashall, James Seymour, and Nicole Zarate. University of Pennsylvania: Alea Zone, Sarah Joergensen, Liam Forsythe, Daria Zaitseva, Paul Callahan, Komal Khan, Olivia Doran, Sarah Gamblin, Lydia Fisher, Daniela Schmulevich, Steve Balian, Carrie Diamond, Jonathan Kolansky, and Dena Torrente. Oregon Health & Science University: Sean Van Walchren, Diane Lape. Denver Health Medical Center: Angela Sauaia, Jason Haukoos, Lee Anne Ammons, James Chandler, Marcela Fitzpatrick, Emmalee Vittatoe, Nick Brant, Stephanie Kennedy, and Megan Swope. University of Miami: Ronald Manning, Cristina Botero Fonnegra, Sebastian Brito, Vivian Calderon, Majid Chammas, Anthony Dure, Chelsea Ferreira, Allison Ferreira, Richard Guerra, Ivonne Guzman, Aaliyah Jolly, and Rajan Ramdev. University of Texas Southwestern: Shreedhar Reddy, Nadia Nassaj.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the US Army Medical Research Acquisition Activity.
Footnotes
Funding: This material is based on work supported by the US Army Medical Research Acquisition Activity under contract no. W81XWH-16-D-0024-0001.
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics approval: The protocol for this study was approved by the University of Pittsburgh’s Institutional Review Board (IRB), serving as the single IRB for the LITES Network (Study ID: STUDY20110385), and by the Human Research Protection Office of the Department of Defense. The survey was determined not to meet the definition of human subjects research by the University of Pittsburgh IRB and, therefore, did not require IRB review.
Contributor Information
Alexandra MP Brito, Email: a.brito.26@gmail.com.
Mark H Yazer, Email: yazermh@upmc.edu.
Jason L Sperry, Email: sperryjl@upmc.edu.
James F Luther, Email: jim.luther@pitt.edu.
Stephen R Wisniewski, Email: stevewis@pitt.edu.
Frances Guyette, Email: guyefx@UPMC.EDU.
Ernest E Moore, Email: ernest.moore@dhha.org.
Bryan A Cotton, Email: Bryan.A.Cotton@uth.tmc.edu.
Laura Vincent, Email: vincentl3@upmc.edu.
Erin Fox, Email: erin.e.fox@uth.tmc.edu.
Jeremy W Cannon, Email: jeremy.cannon@pennmedicine.upenn.edu.
Nicholas Namias, Email: nnamias@miami.edu.
Joseph P Minei, Email: joseph.minei@utsouthwestern.edu.
Lee Anne Ammons, Email: LeeAnne.Ammons@dhha.org.
Skye Clayton, Email: SSC38@pitt.edu.
Martin Schreiber, Email: schreibm@ohsu.edu.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.McCoy CC, Brenner M, Duchesne J, Roberts D, Ferrada P, Horer T, Kauvar D, Khan M, Kirkpatrick A, Ordonez C, et al. Back to the Future: Whole Blood Resuscitation of the Severely Injured Trauma Patient. Shock. 2021;56:9–15. doi: 10.1097/SHK.0000000000001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yazer MH, Spinella PC, Anto V, Dunbar NM. Survey of group A plasma and low-titer group O whole blood use in trauma resuscitation at adult civilian level 1 trauma centers in the US. Transfusion. 2021;61:1757–63. doi: 10.1111/trf.16394. [DOI] [PubMed] [Google Scholar]
- 3.Schauer SG, April MD, Fisher AD, Wright FL, Winkle JM, Wright AR, Rizzo JA, Getz TM, Nicholson SE, Yazer MH, et al. A survey of low titer O whole blood use within the trauma quality improvement program registry. Transfusion. 2024;64:S85–92. doi: 10.1111/trf.17746. [DOI] [PubMed] [Google Scholar]
- 4.Yazer MH, Spinella PC, Bank EA, Cannon JW, Dunbar NM, Holcomb JB, Jackson BP, Jenkins D, Levy M, Pepe PE, et al. THOR-AABB Working Party Recommendations for a Prehospital Blood Product Transfusion Program. Prehosp Emerg Care. 2022;26:863–75. doi: 10.1080/10903127.2021.1995089. [DOI] [PubMed] [Google Scholar]
- 5.Hanna M, Knittel J, Gillihan J. The Use of Whole Blood Transfusion in Trauma. Curr Anesthesiol Rep. 2022;12:234–9. doi: 10.1007/s40140-021-00514-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperry JL, Cotton BA, Luther JF, Cannon JW, Schreiber MA, Moore EE, Namias N, Minei JP, Wisniewski SR, Guyette FX, et al. Whole Blood Resuscitation and Association with Survival in Injured Patients with an Elevated Probability of Mortality. J Am Coll Surg. 2023;237:206–19. doi: 10.1097/XCS.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brill JB, Tang B, Hatton G, Mueck KM, McCoy CC, Kao LS, Cotton BA. Impact of Incorporating Whole Blood into Hemorrhagic Shock Resuscitation: Analysis of 1,377 Consecutive Trauma Patients Receiving Emergency-Release Uncrossmatched Blood Products. J Am Coll Surg. 2022;234:408–18. doi: 10.1097/XCS.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 8.Torres CM, Kent A, Scantling D, Joseph B, Haut ER, Sakran JV. Association of Whole Blood With Survival Among Patients Presenting With Severe Hemorrhage in US and Canadian Adult Civilian Trauma Centers. JAMA Surg. 2023;158:532–40. doi: 10.1001/jamasurg.2022.6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hazelton JP, Ssentongo AE, Oh JS, Ssentongo P, Seamon MJ, Byrne JP, Armento IG, Jenkins DH, Braverman MA, Mentzer C, et al. Use of Cold-Stored Whole Blood is Associated With Improved Mortality in Hemostatic Resuscitation of Major Bleeding: A Multicenter Study. Ann Surg. 2022;276:579–88. doi: 10.1097/SLA.0000000000005603. [DOI] [PubMed] [Google Scholar]
- 10.Cotton BA, Podbielski J, Camp E, Welch T, del Junco D, Bai Y, Hobbs R, Scroggins J, Hartwell B, Kozar RA, et al. A randomized controlled pilot trial of modified whole blood versus component therapy in severely injured patients requiring large volume transfusions. Ann Surg. 2013;258:527–32. doi: 10.1097/SLA.0b013e3182a4ffa0. [DOI] [PubMed] [Google Scholar]
- 11.McCoy CC, Montgomery K, Cotton ME, Meyer DE, Wade CE, Cotton BA. Can RH+ whole blood be safely used as an alternative to RH- product? An analysis of efforts to improve the sustainability of a hospital’s low titer group O whole blood program. J Trauma Acute Care Surg. 2021;91:627–33. doi: 10.1097/TA.0000000000003342. [DOI] [PubMed] [Google Scholar]
- 12.Clayton S, Leeper CM, Yazer MH, Spinella PC. Survey of policies at US hospitals on the selection of RhD type of low-titer O whole blood for use in trauma resuscitation. Transfusion. 2024;64:S111–8. doi: 10.1111/trf.17789. [DOI] [PubMed] [Google Scholar]
- 13.Siletz AE, Blair KJ, Cooper RJ, Nguyen NC, Lewis SJ, Fang A, Ward DC, Jackson NJ, Rodriguez T, Grotts J, et al. A pilot study of stored low titer group O whole blood + component therapy versus component therapy only for civilian trauma patients. J Trauma Acute Care Surg. 2021;91:655–62. doi: 10.1097/TA.0000000000003334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones AR, Frazier SK. Increased mortality in adult patients with trauma transfused with blood components compared with whole blood. J Trauma Nurs. 2014;21:22–9. doi: 10.1097/JTN.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanna K, Bible L, Chehab M, Asmar S, Douglas M, Ditillo M, Castanon L, Tang A, Joseph B. Nationwide analysis of whole blood hemostatic resuscitation in civilian trauma. J Trauma Acute Care Surg. 2020;89:329–35. doi: 10.1097/TA.0000000000002753. [DOI] [PubMed] [Google Scholar]
- 16.Kemp Bohan PM, McCarthy PM, Wall ME, Adams AM, Chick RC, Forcum JE, Radowsky JS, How RA, Sams VG. Safety and efficacy of low-titer O whole blood resuscitation in a civilian level I trauma center. J Trauma Acute Care Surg. 2021;91:S162–8. doi: 10.1097/TA.0000000000003289. [DOI] [PubMed] [Google Scholar]
- 17.Crowe E, DeSantis SM, Bonnette A, Jansen JO, Yamal J-M, Holcomb JB, Pedroza C, Harvin JA, Marques MB, Avritscher EBC, et al. Whole blood transfusion versus component therapy in trauma resuscitation: a systematic review and meta-analysis. J Am Coll Emerg Physicians Open . 2020;1:633–41. doi: 10.1002/emp2.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yazer MH, Corcos A, L Sperry J, Triulzi DJ, Leeper C. Receipt of at least 4 units of low titer group O whole blood with titer <100 does not lead to hemolysis in adult trauma patients. Transfusion. 2022;62:S72–9. doi: 10.1111/trf.16980. [DOI] [PubMed] [Google Scholar]
- 19.Seheult JN, Triulzi DJ, Alarcon LH, Sperry JL, Murdock A, Yazer MH. Measurement of haemolysis markers following transfusion of uncrossmatched, low-titre, group O+ whole blood in civilian trauma patients: initial experience at a level 1 trauma centre. Transfus Med. 2017;27:30–5. doi: 10.1111/tme.12372. [DOI] [PubMed] [Google Scholar]
- 20.Williams J, Merutka N, Meyer D, Bai Y, Prater S, Cabrera R, Holcomb JB, Wade CE, Love JD, Cotton BA. Safety profile and impact of low-titer group O whole blood for emergency use in trauma. J Trauma Acute Care Surg. 2020;88:87–93. doi: 10.1097/TA.0000000000002498. [DOI] [PubMed] [Google Scholar]
- 21.Morgan KM, Abou Khalil E, Feeney EV, Spinella PC, Lucisano AC, Gaines BA, Leeper CM. The Efficacy of Low-Titer Group O Whole Blood Compared With Component Therapy in Civilian Trauma Patients: A Meta-Analysis. Crit Care Med. 2024;52:e390–404. doi: 10.1097/CCM.0000000000006244. [DOI] [PubMed] [Google Scholar]
- 22.Patel RM, Lukemire J, Shenvi N, Arthur C, Stowell SR, Sola-Visner M, Easley K, Roback JD, Guo Y, Josephson CD. Association of Blood Donor Sex and Age With Outcomes in Very Low-Birth-Weight Infants Receiving Blood Transfusion. JAMA Netw Open . 2021;4:e2123942. doi: 10.1001/jamanetworkopen.2021.23942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edgren G, Ullum H, Rostgaard K, Erikstrup C, Sartipy U, Holzmann MJ, Nyrén O, Hjalgrim H. Association of Donor Age and Sex With Survival of Patients Receiving Transfusions. JAMA Intern Med. 2017;177:854–60. doi: 10.1001/jamainternmed.2017.0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgren G, Murphy EL, Brambilla DJ, Westlake M, Rostgaard K, Lee C, Cable RG, Triulzi D, Bruhn R, St Lezin EM, et al. Association of Blood Donor Sex and Prior Pregnancy With Mortality Among Red Blood Cell Transfusion Recipients. JAMA. 2019;321:2183–92. doi: 10.1001/jama.2019.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chassé M, McIntyre L, English SW, Tinmouth A, Knoll G, Wolfe D, Wilson K, Shehata N, Forster A, van Walraven C, et al. Effect of Blood Donor Characteristics on Transfusion Outcomes: A Systematic Review and Meta-Analysis. Transfus Med Rev. 2016;30:69–80. doi: 10.1016/j.tmrv.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Bösch F, Angele MK, Chaudry IH. Gender differences in trauma, shock and sepsis. Mil Med Res. 2018;5:35. doi: 10.1186/s40779-018-0182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharpe JP, Magnotti LJ, Weinberg JA, Brocker JA, Schroeppel TJ, Zarzaur BL, Fabian TC, Croce MA. Gender disparity in ventilator-associated pneumonia following trauma: identifying risk factors for mortality. J Trauma Acute Care Surg. 2014;77:161–5. doi: 10.1097/TA.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 28.Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, Adams PW, Daley BJ, Miller RS, Harbrecht BG, et al. Prehospital Plasma during Air Medical Transport in Trauma Patients at Risk for Hemorrhagic Shock. N Engl J Med. 2018;379:315–26. doi: 10.1056/NEJMoa1802345. [DOI] [PubMed] [Google Scholar]
- 29.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meshkin D, Yazer MH, Dunbar NM, Spinella PC, Leeper CM. Low titer Group O whole blood utilization in pediatric trauma resuscitation: A National Survey. Transfusion. 2022;62:S63–71. doi: 10.1111/trf.16979. [DOI] [PubMed] [Google Scholar]
- 31.Yazer MH, Spinella PC, Seheult JN. Risk of future haemolytic disease of the fetus and newborn following the transfusion of Rh(D)-positive blood products to Rh(D)-negative children. Vox Sang. 2022;117:291–2. doi: 10.1111/vox.13169. [DOI] [PubMed] [Google Scholar]
- 32.Yazer MH, Panko G, Holcomb JB, Kaplan A, Leeper C, Seheult JN, Triulzi DJ, Spinella PC. Not as 'D'eadly as once thought - the risk of D-alloimmunization and hemolytic disease of the fetus and newborn following RhD-positive transfusion in trauma. Hematology. 2023;28:2161215. doi: 10.1080/16078454.2022.2161215. [DOI] [PubMed] [Google Scholar]
- 33.Yazer MH, Emery SP, Triulzi DJ, Spinella P, Leeper C. Another piece of the hemolytic disease of the fetus and newborn puzzle after RhD-positive transfusion in trauma resuscitation: the proportion of pregnant women who produce high titer anti-D. Trauma Surg Acute Care Open . 2024;9:e001252. doi: 10.1136/tsaco-2023-001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorken-Gallastegi A, Spinella PC, Neal MD, Leeper C, Sperry J, Peitzman AB, Brown JB. Whole Blood and Blood Component Resuscitation in Trauma: Interaction and Association With Mortality. Ann Surg. 2024;280:1014–20. doi: 10.1097/SLA.0000000000006316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Horst RA, Rijnhout TWH, Noorman F, Borger van der Burg BLS, van Waes OJF, Verhofstad MHJ, Hoencamp R. Whole blood transfusion in the treatment of acute hemorrhage, a systematic review and meta-analysis. J Trauma Acute Care Surg. 2023;95:256–66. doi: 10.1097/TA.0000000000004000. [DOI] [PubMed] [Google Scholar]
- 36.Uhlich R, Hu P, Yazer M, Jansen JO, Patrician P, Reynolds L, Marques MB, Stephens SW, Gelbard RB, Kerby J, et al. Perception of risk in massive transfusion as it relates to fetal outcomes: A survey of surgeons and nurses at one American trauma center. Transfusion. 2021;61 Suppl 1:S159–66. doi: 10.1111/trf.16492. [DOI] [PubMed] [Google Scholar]
- 37.Yu G, Siegler J, Hayes J, Yazer MH, Spinella PC. Attitudes of American adult women toward accepting RhD-mismatched transfusions in bleeding emergencies. Transfusion. 2022;62:S211–7. doi: 10.1111/trf.16981. [DOI] [PubMed] [Google Scholar]
- 38.Sherwood MR, Clayton S, Leeper CM, Yazer M, Moise KJ Jr, Granger ME, Spinella PC. Receipt of RhD-positive whole blood for life-threatening bleeding in female children: A survey in alloimmunized mothers regarding minimum acceptable survival benefit relative to risk of maternal alloimmunization to anti-D. Transfusion. 2024;64:S100–10. doi: 10.1111/trf.17807. [DOI] [PubMed] [Google Scholar]
- 39.Uhlich R, Hu P, Yazer M, Jansen JO, Patrician P, Marques MB, Reynolds L, Fifolt M, Stephens SW, Gelbard RB, et al. The females have spoken: A patient-centered national survey on the administration of emergent transfusions with the potential for future fetal harm. J Trauma Acute Care Surg. 2023;94:791–7. doi: 10.1097/TA.0000000000003914. [DOI] [PubMed] [Google Scholar]
- 40.Yazer MH, Leeper C, Spinella PC, Emery SP, Horvath S, Seheult JN. Maternal and child life years gained by transfusing low titer group O whole blood in trauma: A computer simulation. Transfusion. 2024;64 Suppl 2:S93–9. doi: 10.1111/trf.17767. [DOI] [PubMed] [Google Scholar]
- 41.Susila S, Ilmakunnas M, Lauronen J, Vuorinen P, Ångerman S, Sainio S. Low titer group O whole blood and risk of RhD alloimmunization: Rationale for use in Finland. Transfusion. 2024;64 doi: 10.1111/trf.17700. [DOI] [PubMed] [Google Scholar]
- 42.Yazer MH, Delaney M, Doughty H, Dunbar NM, Al‐Riyami AZ, Triulzi DJ, Watchko JF, Wood EM, Yahalom V, Emery SP. It is time to reconsider the risks of transfusing RhD negative females of childbearing potential with RhD positive red blood cells in bleeding emergencies. Transfusion. 2019;59:3794–9. doi: 10.1111/trf.15569. [DOI] [PubMed] [Google Scholar]
- 43.Lu W, Stephens L, Shmookler A, O’Brien K, Karp JK, Hermelin D, Bakhtary S, Almozain N, George M, Fung M. Rh immune globulin immunoprophylaxis after RhD-positive red cell exposure in RhD-negative patients via transfusion: A survey of practices. Transfusion. 2024;64:839–45. doi: 10.1111/trf.17812. [DOI] [PubMed] [Google Scholar]
- 44.Ho VP, Dicker AR, Haut ER. Coalition for National Trauma Research Scientific Advisory Council. Dissemination, implementation, and de-implementation: the trauma perspective. Trauma Surg Acute Care Open. 2020;5:e000423. doi: 10.1136/tsaco-2019-000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.