Abstract
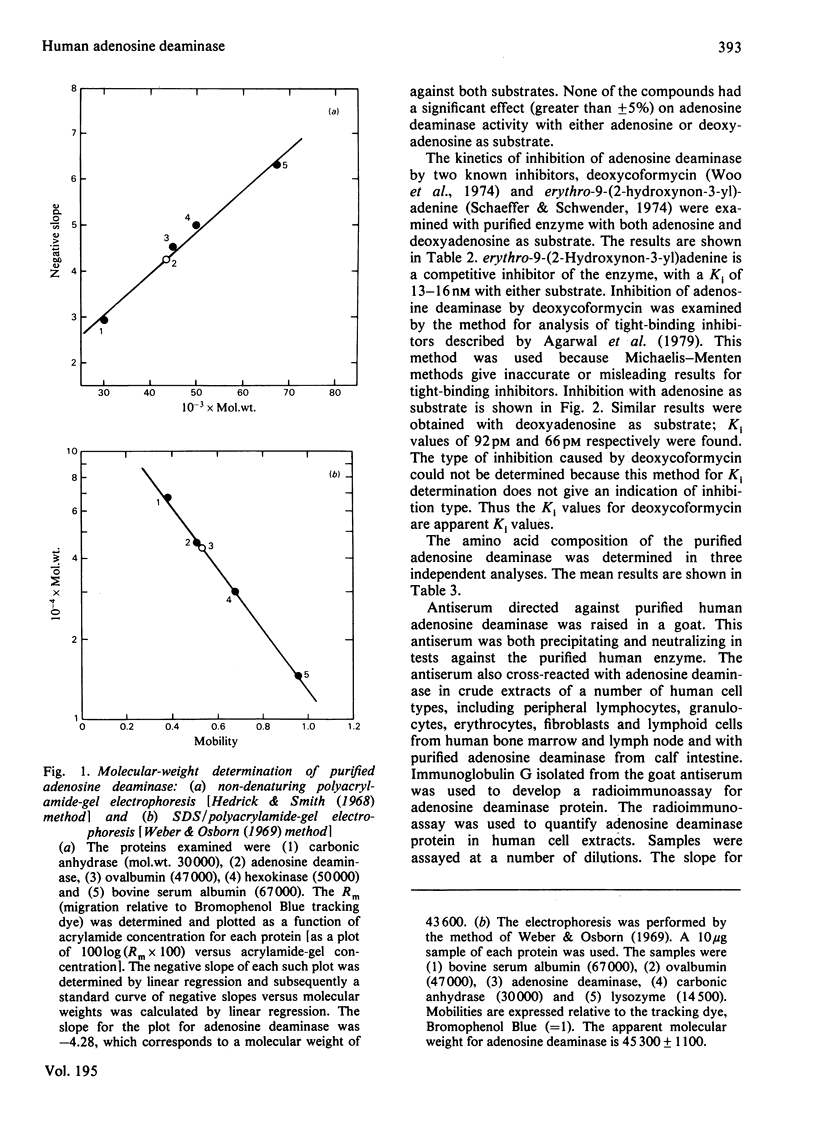
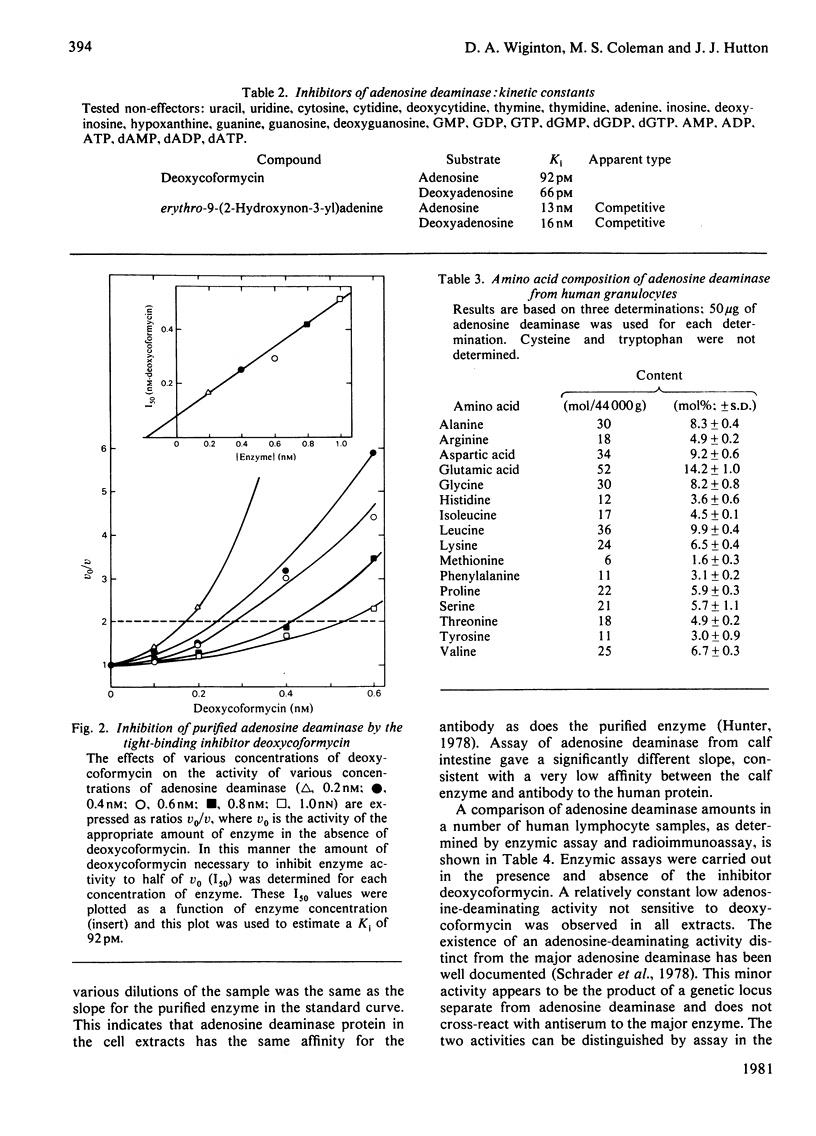
Adenosine deaminase was purified 3038-fold to apparent homogeneity from human leukaemic granulocytes by adenosine affinity chromatography. The purified enzyme has a specific activity of 486 mumol/min per mg of protein at 35 degrees C. It exhibits a single band when subjected to sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, non-denaturing polyacrylamide-gel electrophoresis and isoelectric focusing. The pI is 4.4. The enzyme is a monomeric protein of molecular weight 44000. Both electrophoretic behaviour and molecular weight differ from those of the low-molecular-weight adenosine deaminase purified from human erythrocytes. Its amino acid composition is reported. Tests with periodic acid-Schiff reagent for associated carbohydrate are negative. Of the large group of physiological compounds tested as potential effectors, none has a significant effect. The enzyme is specific for adenosine and deoxyadenosine, with Km values of 48 microM and 34 microM respectively. There are no significant differences in enzyme function on the two substrates. erythro-9-(2-Hydroxy non-3-yl) adenine is a competitive inhibitor, with Ki 15 nM. Deoxycoformycin inhibits deamination of both adenosine and deoxyadenosine, with an apparent Ki of 60-90 pM. A specific antibody was developed against the purified enzyme, and a sensitive radioimmunoassay for adenosine deaminase protein is described.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P., Sagar S. M., Parks R. E., Jr Adenosine deaminase from human erythrocytes: purification and effects of adenosine analogs. Biochem Pharmacol. 1975 Mar 15;24(6):693–701. doi: 10.1016/0006-2952(75)90245-2. [DOI] [PubMed] [Google Scholar]
- Chechik B. E., Jason J., Shore A., Baker M., Dosch H. M., Gelfand E. W. Quantitation of human thymus/leukemia-associated antigen by radioimmunoassay in different forms of leukemia. Blood. 1979 Dec;54(6):1400–1406. [PubMed] [Google Scholar]
- Chechik B. E., Percy M. E., Gelfand E. W. Human thymus-leukemia-associated antigen: isolation and partial characterization. J Natl Cancer Inst. 1978 Jan;60(1):69–75. doi: 10.1093/jnci/60.1.69. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Cohen A., Hirschhorn R., Horowitz S. D., Rubinstein A., Polmar S. H., Hong R., Martin D. W., Jr Deoxyadenosine triphosphate as a potentially toxic metabolite in adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1978 Jan;75(1):472–476. doi: 10.1073/pnas.75.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. S., Donofrio J., Hutton J. J., Hahn L., Daoud A., Lampkin B., Dyminski J. Identification and quantitation of adenine deoxynucleotides in erythrocytes of a patient with adenosine deaminase deficiency and severe combined immunodeficiency. J Biol Chem. 1978 Mar 10;253(5):1619–1626. [PubMed] [Google Scholar]
- Coleman M. S., Hutton J. J. Micromethod for quantitation of adenosine deaminase activity in cells from human peripheral blood. Biochem Med. 1975 May;13(1):46–55. doi: 10.1016/0006-2944(75)90139-8. [DOI] [PubMed] [Google Scholar]
- Daddona P. E., Frohman M. A., Kelley W. N. Human adenosine deaminase and its binding protein in normal and adenosine deaminase-deficient fibroblast cell strains. J Biol Chem. 1980 Jun 25;255(12):5681–5687. [PubMed] [Google Scholar]
- Daddona P. E., Kelley W. N. Human adenosine deaminase. Purification and subunit structure. J Biol Chem. 1977 Jan 10;252(1):110–115. [PubMed] [Google Scholar]
- Deibel M. R., Jr, Coleman M. S. Purification of a high molecular weight human terminal deoxynucleotidyl transferase. J Biol Chem. 1979 Sep 10;254(17):8634–8640. [PubMed] [Google Scholar]
- Donofrio J., Coleman M. S., Hutton J. J., Daoud A., Lampkin B., Dyminski J. Overproduction of adenine deoxynucleosides and deoxynucletides in adenosine deaminase deficiency with severe combined immunodeficiency disease. J Clin Invest. 1978 Oct;62(4):884–887. doi: 10.1172/JCI109201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards Y. H., Hopkinson D. A., Harris H. Adenosine deaminase isozymes in human tissues. Ann Hum Genet. 1971 Oct;35(2):207–219. doi: 10.1111/j.1469-1809.1956.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R. Conversion of human erythrocyte-adenosine deaminase activity to different tissue-specific isozymes. Evidence for a common catalytic unit. J Clin Invest. 1975 Mar;55(3):661–667. doi: 10.1172/JCI107974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Levytaka V., Pollara B., Meuwissen H. J. Evidence for control of several different tissue-specific isozymes of adenosine deaminase by a single genetic locus. Nat New Biol. 1973 Dec 19;246(155):200–202. doi: 10.1038/newbio246200a0. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R., Spencer N. Partial purification and properties of the common inherited forms of adenosine deaminase from human erythrocytes. Biochem J. 1973 May;133(1):117–123. doi: 10.1042/bj1330117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polmar S. H. Metabolic aspects of immunodeficiency disease. Semin Hematol. 1980 Jan;17(1):30–43. [PubMed] [Google Scholar]
- Prentice H. G., Smyth J. F., Ganeshaguru K., Wonke B., Bradstock K. F., Janossy G., Goldstone A. H., Hoffbrand A. V. Remission induction with adenosine-deaminase inhibitor 2'-deoxycoformycin in Thy-lymphoblastic leukaemia. Lancet. 1980 Jul 26;2(8187):170–172. doi: 10.1016/s0140-6736(80)90060-4. [DOI] [PubMed] [Google Scholar]
- Rodbard D., Bridson W., Rayford P. L. Rapid calculation of radioimmunoassay results. J Lab Clin Med. 1969 Nov;74(5):770–781. [PubMed] [Google Scholar]
- Schaeffer H. J., Schwender C. F. Enzyme inhibitors. 26. Bridging hydrophobic and hydrophilic regions on adenosine deaminase with some 9-(2-hydroxy-3-alkyl)adenines. J Med Chem. 1974 Jan;17(1):6–8. doi: 10.1021/jm00247a002. [DOI] [PubMed] [Google Scholar]
- Schrader W. P., Pollara B., Meuwissen H. J. Characterization of the residual adenosine deaminating activity in the spleen of a patient with combined immunodeficiency disease and adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1978 Jan;75(1):446–450. doi: 10.1073/pnas.75.1.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader W. P., Stacy A. R., Pollara B. Purification of human erythrocyte adenosine deaminase by affinity column chromatography. J Biol Chem. 1976 Jul 10;251(13):4026–4032. [PubMed] [Google Scholar]
- Schrader W. P., Stacy A. R. Purification and subunit structure of adenosine deaminase from human kidney. J Biol Chem. 1977 Sep 25;252(18):6409–6415. [PubMed] [Google Scholar]
- Siaw M. F., Mitchell B. S., Koller C. A., Coleman M. S., Hutton J. J. ATP depletion as a consequence of adenosine deaminase inhibition in man. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6157–6161. doi: 10.1073/pnas.77.10.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow D. M., Evans L., Hopkinson D. A. Several of the adenosine deaminase isozymes are glycoproteins. Nature. 1977 Sep 15;269(5625):261–262. doi: 10.1038/269261a0. [DOI] [PubMed] [Google Scholar]
- Van der Weyden M. B., Kelley W. N. Human adenosine deaminase. Distribution and properties. J Biol Chem. 1976 Sep 25;251(18):5448–5456. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]


