Abstract
The interface between soft and hard tissues is constituted by a gradient change of cell types and matrix compositions that are optimally designed for proper load transmission and injury protection. In the musculoskeletal system, the soft-hard tissue interfaces at tendon-bone, ligament-bone, and meniscus-bone have been extensively researched as regenerative targets. Similarly, extensive research efforts have been made to guide the regeneration of multi-tissue complexes in periodontium. However, the other soft-hard tissue interfaces in the dental and craniofacial system have been somewhat neglected. This review discusses the clinical significance of developing regenerative strategies for soft-hard tissue interfaces in the dental and craniofacial system. It also discusses the research progress in the field focused on bioengineering approaches using 3D scaffolds equipped with spatially controlled bioactivities. The remaining challenges, future perspectives, and considerations for the clinical translation of bioactive scaffolds are also discussed.
Keywords: Multi-tissue interface, Regenerative engineering, Bioactive scaffold, Tissue engineering, Tissue regeneration, Periodontium, Temporomandibular joint disorders
Graphical abstract
Highlights
-
•
This review provides an overview of the research progress in regenerating soft-hard tissue interfaces.
-
•
This review also discusses the research outcomes, advantages, and limitations of various bioactive scaffolds.
-
•
It also discusses perspectives for future research directions in consideration of the remaining challenges.
1. Introduction
The soft-hard tissue interface is a highly specialized structure that connects bone to soft tissue, facilitating the transmission of mechanical loads. Such interfaces are commonly located in areas where ligaments, tendons, dental soft tissues, and joint capsules connect to bone, bridging the differences between tissues with distinct properties. These interfaces exhibit unique characteristics, including gradient cell and matrix compositions, accommodating the seamless transition from unmineralized to mineralized tissues [1]. In addition, an increasing body of research findings suggests that these soft-hard tissue interfaces are closely involved in the initiation and progression of degenerative injuries [2,3]. More importantly, the successful clinical outcome of repairing connective tissues is dependent mainly on the functional restoration of the soft-hard tissue interfaces [1,2,[4], [5], [6]]. Accordingly, there is an emerging research interest in understanding the biochemical compositions of the soft-hard tissue interfaces, the associated biomechanical features, and their developmental process for both musculoskeletal and dento-craniofacial systems [[7], [8], [9], [10]]. These research efforts are aligned with the goal of regenerating the soft-hard tissue interfaces to enhance the clinical outcome in replacing or regenerating diseased tissues [8,9,11,12].
This review provides an overview of the research progress in regenerating the dento-craniofacial soft-hard tissue interfaces, emphasizing bioengineering strategies to reconstruct integrated multi-tissue interfaces using advanced biomaterials, spatiotemporally controlled delivery systems, and biologics. Although the soft-hard tissue interfaces at different anatomical locations have certain similarities, several unique features distinguish the soft-hard tissue interfaces in the dento-craniofacial system from the musculoskeletal (MSK) tissue interfaces. Nonetheless, similar bioengineering approaches have been applied for both dento-craniofacial and MSK soft-hard tissue interfaces, from layering multiple cell types to constructing multi-layered bioactive scaffolds. Thus, this review also discusses the unique features of the dento-craniofacial soft-hard tissue interfaces to be considered for the regenerative approaches, in addition to the summary of the research outcomes from various bioactive scaffolds, their advantages and limitations, and perspectives for future research directions in consideration of the remaining challenges.
2. Unique soft-hard tissue interfaces in the dental and craniofacial system
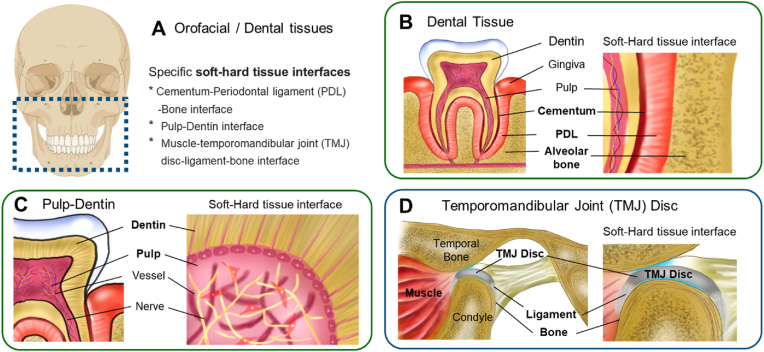
Specific soft-hard tissue interfaces within the dental and craniofacial system (Fig. 1A) include the cementum-periodontal ligament (PDL)-alveolar bone interface (Fig. 1B), the pulp-dentin interface (Fig. 1C), and the muscle-temporomandibular joint (TMJ) disc ligament-bone interface (Fig. 1D). PDL plays a crucial role in its adjacent tissues by transmitting and absorbing mechanical stresses to the alveolar bone while facilitating nutrient transfer through its vascular connections to the cementum and alveolar bone [13,14]. The interface at the pulp and dentin exhibits unique anti-inflammatory functions against the progressing carries [15], given the polarized odontoblasts at the interface, of which processes extended into dentinal tubules, serve as the first line of detection of bacterial infection and secrete antibacterial agents [16]. The TMJ disc, positioned between the condyle and temporal bone, is a vital component for stress absorption and distribution during jaw movement. The TMJ disc is connected to the mandibular bone through medial and lateral ligaments [[17], [18], [19]]. In addition, there are several ligaments connecting mandibular and temporal bones, including stylomandibular, sphenomandibular ligament, oto-mandibular, discomalleolar, and anterior malleolar ligaments, as attached to bones [20].
Fig. 1.
Illustration of the soft-hard tissue interfaces in orofacial and dental tissues.
Cementum-PDL-Bone: The periodontium comprises cementum, PDL, and alveolar bone and is critically diseased in advanced periodontitis. The PDL connects the mineralized cementum of the tooth to the alveolar bone of the tooth socket, with all three components playing a pivotal role in the oral cavity's stability, health, and development. The cementum, a mineralized structure attached to tooth root surface, provides a tight anchorage with PDL by embedding highly oriented collagen fibers, referred to as the Sharpey's fibers [21]. As densely aligned fibrous connective tissue, PDL plays roles in physiologic mobility of tooth and load distribution [21]. PDL is a source of PDL stem/progenitor cells (PDLSCs) that can give rise into PDL and mineralized tissues in the periosteum [22]. PDL-cementum makes approximately 170–580 μm in thickness with 150–380 μm PDL and 20–200 μm cementum [23].
Pulp-Dentin: Healthy pulp tissue comprises fibroblasts, odontoblasts, immune cells as the cellular component, and collagen fibers, nerves, and blood vessels [8]. A unique feature of the pulp-dentin interface is the polarized, single-layered, column-shaped odontoblasts that extend processes into highly oriented dentinal tubules [8,24,25]. Odontoblasts are not only responsible for the synthesis, secretion, organization and mineralization of dentin [8,24,25] but also believed to be involved in mechanosensory through their processes embedded into dentinal tubules [8,24,25]. Importantly, the pulp-dentin interface is suggested to have anti-inflammatory functions against the progressing carries [15]. The pulp-dentin interface is less than 100 μm given ∼50 μm-thick odontoblast layer and 14–40 μm pre-dentin structure [26].
TMJ disc ligament-bone: Consistent with other ligament-bone interfaces in the MSK system, TMJ disc ligament-bone interface is comprised of a gradient change from highly oriented collagen fibers to unmineralized and mineralized fibrocartilage zone and bone [27,28]. The ligament zone is populated by spindle-shaped fibroblasts, and fibrochondrocytes, or mixed population of fibroblasts and chondrocytes, reside in the fibrocartilage zone [[27], [28], [29]]. Such a graduate change in the matrix and cellular phenotypes provide seamless load transfer across the ligament and bone and protection against impact-derived injuries [27,28]. To date, the primary research focuses in on the replacement, engineering, or regeneration of TMJ disc with limited attention to the functional reconstruction of TMJ disc ligament-bone interface, playing a crucial role in the long-term functionality and sustainability of the joints [27,28]. Although there has been no direct measurement in the micro-resolution of TMJ disc ligament-bone interface, the width of the interface is estimated at 300–800 μm based on that of MSK ligament enthesis [30].
Distinguishable features of dento-craniofacial soft-hard tissue interfaces: Although the dental and craniofacial soft-hard tissue interfaces share characteristics with tissue interfaces in the MSK, they exhibit several unique features. For example, the interfaces at PDL-alveolar bone (AB) and PDL-cementum lack fibrocartilage interface observed in MSK ligament to bone attachments. Instead, the attachment of PDL to AB and cementum exhibits unique characteristics, featured by the Sharpey's fibers where highly aligned collagen fibers are inserted into the well-organized mineralized matrix [21]. Unfortunately, we do not have a clear understanding of the mechanical roles of such a unique structure of PDL-cementum interface, distinctive from fibrocartilaginous interfaces at MSK system. We postulate that the structural differences between PDL-cementum and MSK ligament-bone may be associated with their mechanical environments. Although MSK ligament-bone interface is mostly under tensile loadings, PDL-cementum undergoes compressive occlusal loads leading to a mixture of shear, tension, and bending [31]. In addition, PDL-cementum attachment can undergo non-pathological tissue remodeling during the orthodontic movements [32]. Although migratory enthesis is observed in the development of tendon/ligament enthesis [4], such a non-pathological tissue remodeling has been rarely reported in soft-hard tissue interfaces in adult MSK system, as tissue remodeling after injuries to tendon enthesis frequently result in pathological tissue lacked fibrocartilaginous interface [3,33].
Another unique feature in the dental and craniofacial soft-hard tissue interfaces is the polarized odontoblasts extending their processes into dentinal tubules at the pulp-dentin interface, different from any other soft-hard tissue interface in the MSK system [16]. Although MSK connective tissue cells, including ones at soft-hard tissue interfaces, sense external mechanical forces being translated into biological signals, the sensing environmental cues conducted by odontoblasts through their long processes is quite distinguishing. While MSK connective tissue cells sense mechanical forces through their binding to ECM and associated cytoskeletons, ion channels, and specialized surface receptors (e.g., CD44) [34], odontoblasts form dense network with unmyelinated nerve fibers allowing to serve them as a signal transduction, not only for mechano-sensing cells [35]. In addition, odontoblasts sense not only mechanical loading via mechano-sensitive calcium and potassium channels but also heat and cold via various types of transient receptor potential (TRP) ion channels [35,36]. As they have extension running into dentinal tubules as embedded in the dentinal fluids, odontoblasts serve as effective sensory cells that transduce physiological signals to nerve system [35,36].
Table 1 summarizes the features of dental and craniofacial soft-hard tissue interfaces with changes in tissue matrix and cellular compositions.
Table 1.
ECM and cellular compositions of the soft-hard tissue interface in dental and craniofacial tissues.
| Interface | ECM components | Cell phenotypes | Unique features | References |
|---|---|---|---|---|
| Cementum-PDL-bone | Cementum: Collagen, CEMP-1, and inorganic mineral PDL: Collagen I fibrils, elastin, hyaluronate, GAGs, and PG Bone: Collagen and inorganic mineral |
Cementoblasts PDL fibroblastsc and PDLSC Osteoblasts and osteoclasts |
Lack of fibrocartilage interface, distinguishable from MSK ligament to bone interfaces | [[37], [38], [39]] |
| Pulp-Dentin | Pulp: Collagen type I & III and fibronectin. Dentin: Collagen and inorganic mineral |
Fibroblastsc Odontoblastsb |
Polarized odontoblasts extending processes into dentinal tubules; not observed in the MSK interfaces | [40,41] |
| TMJ disc ligament-bone | Ligament: Collagen I fibrils, fibronectin, and elastin Fibrocartilage interface: Collagen I & II, GAGs, and PG Bone: Collagen and inorganic mineral |
Fibroblastsc Fibroblasts + Chondrocytes (or Fibrochondrocytesa) Osteoblasts and osteoclasts |
Despite the similarities with knee meniscus anchors, these tissues developed from neural crest, not mesoderm. | [33] Ligament-to-bone interface in general, not specific to TMJ |
Existence of fibrochondrocytes or a mixed population of fibroblasts and chondrocytes is still controversial [29].
Odontoblasts at pulp-dentin interface form polarized structure with long process inserted into dentinal tubules, playing essential role in transmitting sensory stimuli [42,43].
Fibroblasts at different tissues and locations exhibit distinct phenotype and genetic profiles [44].
3. Clinical needs for reconstructing dento-craniofacial soft-hard tissue interfaces
The soft-hard tissue interface is critical for various diseases, including periodontitis, deep carious lesions, and TMJ disorders (TMD). Periodontitis, characterized by the inflammation of supporting structures of teeth, occurs at the interface between the cementum and PDL [45]. Data from the National Health and Nutrition Examination Survey (NHANES) in 2009–2014 revealed that approximately 42 % of adults in the United States are affected by periodontitis, with 7.8 % experiencing the severe form of the disease [13]. In 2019, approximately 11 % of the world's population, totaling 743 million individuals, was estimated to have severe periodontitis [13]. The magnitude of this issue is underscored by the fact that the prevalent cases of severe periodontitis increased to 1.1 billion in 2019 [13]. The gravity of the situation is further accentuated by the noteworthy increase in the age-standardized prevalence rate of severe periodontitis, which surged by 8.44 % from 1990 to 2019 [46]. Moreover, the repercussions of periodontal disease extend beyond oral health, as evidenced by its association with other severe conditions. The incidence of oral cancer was notably higher among individuals with periodontal disease, with 57.1 % of cases observed in this group compared to only 28.6 % among those without periodontal issues [47]. A significant revelation from collaborative efforts between the European Federation of Periodontology and the American Academy of Periodontology demonstrated consistent and robust epidemiological evidence linking periodontitis to an increased risk of future atherosclerotic cardiovascular disease [48]. The findings collectively emphasize the imperative for public health interventions, early detection, and effective regeneration strategies of the tissue interface to mitigate the prevalence and clinical impact of periodontal disease on a global scale.
Periodontitis treatment most commonly consists of scaling and root planing to remove plaque and calculus; however, this procedure can only slow the disease's progression, not reverse it. Current regenerative methods, such as guided tissue regeneration (GTR) membranes, have been shown to induce positive outcomes; however, the results are often unpredictable. The lack of consistent, long-lasting regeneration results in end-stage periodontal disease treatment being centered arFound tooth removal with implant placement instead [49]. The most likely reason for this unpredictability revolves around the need for the three components of the periodontium to require multiple levels of coordination and spatially controlled bioactivities [50]. Furthermore, close attention must be paid to the orientations of the regenerated periodontal tissues to ensure proper alignment with the natural architecture of the surrounding tissues [51]. Thus, a reconstruction of the soft-hard tissue interface is considered critical for the functional restoration of periodontal tissues [5,9,14,[52], [53], [54], [55]].
As remotely related with periodontitis, peri-implant disease has gained attention due to increased prevalence of peri-implantitis in the recent years [7,56], with a 10–40 % of incident rate [57,58]. Current treatment of peri-implantitis is based on guided bone regeneration (GBR) technique, but a successful reconstruction of osteointegration is still challenging [[59], [60], [61], [62], [63]]. Moreover, dental implants are highly vulnerable to bacterial penetration through the gingival adhesion in peri-implant tissue [7,64,65], likely associated with the lack of periodontal fibers attached to cementum [66,67]. Due to this limitation of current dental implants, ideas of peri-implant regeneration, including PDL, cementum, and alveolar bone using scaffold technologies have been explored, but evidence and clinical implementation are limited at the preliminary stage [10,68].
The pulp-dentin interface is closely involved with deep carious lesions. Untreated dental infections mediate a demineralization of tooth enamel and dentin, leading to pulpal exposure, necrosis, periapical lesions, and, eventually, tooth loss. Clinically, compromised pulpal tissue is removed, depriving the remaining tooth of its innervation and vascularization, rendering the devitalization of the tooth. However, endodontically treated teeth can become brittle, be vulnerable to fracture, and can be re-infected [24]. Abscess can form in 46.4 % of cases of deep carious lesions [69]. Furthermore, the prevalence of dental caries, a precursor to deep carious lesions, was estimated at 90 % among adults aged 20–64 in 2011–2016. This highlights the substantial burden of deep carious lesions, even in relatively young populations, and underscores the need for early intervention and prevention efforts [69]. Notably, the pulp-dentin interface exhibits unique anti-inflammatory functions against the progressing carries [15], given the polarized odontoblasts at the interface, of which processes extended into dentinal tubules, serve as the first line of detection of bacterial infection and secrete antibacterial agents [16]. Thus, the promotion of minimally invasive treatment, rather than a destructive removal of all carious dentine, has been advocated [15]. The regenerative endodontic procedure (REP), which uses a blood clot-mediated tissue healing strategy, has been attempted. However, ∼39 % of cases fail at two-year follow-ups with regards to root fracture and uncontrolled dosage negatively affecting apical stem cells [8,24]. Recurrent infections remained a major problem after REP therapy, as they were attributed to 79 % of the failed experiments [8]. Accordingly, there has been growing interest in bioengineering approaches for regenerating functional pulp-dentin complexes [70].
The TMJ ligament to the condylar bone interface may have significant implications in the TMD, as the disc is predominantly associated with TMD, affecting 31.1 % of adults and 11.3 % of children and adolescents [19]. Disc displacement accounts for 25.9 % of TMJD [71], frequently leading to disc perforation, and represents a significant clinical problem warranting attention in proper diagnosis and advanced treatment strategies. Although there is an increasing interest in regenerative approaches for the TMJ disc, consideration has rarely been given to the reconstruction of TMJ disc ligament-bone interface. As two collateral ligaments connect the disc to the condyle and play an important role in preventing disc displacement, achieving functional restoration of the collateral ligaments-to-bone interface is imperative for the successful reconstruction of the TMJ disc.
As summarized in Table 2, the treatment landscape for mitigating diseases involved with the soft-hard tissue interfaces is diverse, presenting distinct challenges. The prevalence of periodontal disease, deep carious lesions, and TMJ disorders, and the limitations of the current treatment modalities emphasize the critical need for further research in diseases involving soft-hard tissue interfaces. Understanding the complexities and interactions at this interface is essential for advancing preventive and therapeutic strategies, ultimately improving the quality of oral healthcare.
Table 2.
Clinical significance of soft-hard tissue interface.
| Interface | Physiological functions | Associated disease | Current treatments and limitations | Potential bioengineering approaches |
|---|---|---|---|---|
| Cementum-PDL-bone | Tooth attachment to bone; Protection against oral microflora. | Periodontitis | Scaling and root planing: only slow the disease progress, not reverse it. Guided tissue regeneration (GTR): Lack of consistent, long-lasting regeneration results, likely associated with three components of the periodontium to require multiple levels of coordination and spatially controlled bioactivities. |
Multi-phase bioactive scaffolds guiding regeneration of the multi-tissue complex Injectable hydrogel carriers to mitigate inflammation and promote regeneration |
| Pulp-Dentin | Anti-inflammation against progressing carries. | Deep caries | Root canal: Devitalization of the tooth, often leading to brittle tooth vulnerable to fracture and re-infection. Regenerative endodontic procedure (REP): ∼39 % of cases fail at two-year follow-ups with regards to root fracture and uncontrolled dosage negatively affecting apical stem cells. Recurrent infections accounted for 79 % of failure. |
Spatiotemporally delivered bioactive cues to guide reconstruction of functional pulp-dentin Injectable hydrogel carriers stimulating regeneration |
| TMJ disc ligament-bone | Structural integration between TMJ disc and bone | TMJ disorders, predominantly involved with TMJ disc (e.g., displacement, perforation, and degeneration). | Conservative therapies: Only for relieving pain and symptoms with no long-term results. Surgical treatment: Lack of evidence, suffering from ineffectiveness. TMJ disc regeneration: No clinical translation; graft integration on disc-ligament interfaces has been neglected. |
Bioactive scaffold system integrated with tissue engineered TMJ disc grafts Spatially patterned interface construct to support mechanical integirity |
4. Trend analysis for soft-hard tissue interface regeneration in orofacial and dental tissues
We analyzed the research trends in the regeneration of dental/orofacial soft-hard tissue interfaces using PubMed and Google Scholar literature search. The broadest searches queried “Tissue Engineering”, “Periodontal Tissue Engineering”, “Dental Tissue Engineering”, “Periodontium regeneration”, and “Regenerative Medicine”, followed by specific keywords to narrow down to different types of tissue interfaces: “Periodontium regeneration”, “cementum-periodontal ligament”, “pulp-dentin”, “implant fixture-alveolar bone”, and “TMJ-temporomandibular ligament”. Further specification was made using “spatially controlled bioactivities”, “drug delivery”, and “in situ engineering”. To identify trends in publication rates, we used UiPath® (New York, NY), an integrated automation software for systematic literature search and analysis.
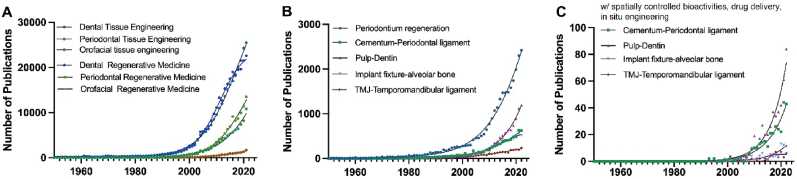
Since 1950, research related to dental and periodontal tissue engineering has been rising. After the early 2000s, there was a significant increase in the publication rate of all terms except orofacial tissue engineering (Fig. 2A). Literatures with a specific keyword, tissue interface, showed a clear upward trend, with notably accelerated growth rate around 2010 (Fig. 2B). Apparently, periodontium has been the most prevalent target for regeneration among the dental and craniofacial tissue interfaces (Fig. 2B). Interestingly, when additional keywords (e.g., spatially controlled bioactivities and drug delivery) were incorporated in the trend analysis, the pulp-dentin showed a sharp increase in the recent years, and cementum-periodontal tissue showed a gradual increase in research output (Fig. 2C). These data indicate a sustained and progressive rise in research endeavors within dental and periodontal tissue engineering. We also noted that investigations related to multi-tissue interface regeneration employing in-situ strategies, such as drug delivery and spatial control delivery, are actively and prominently featured in the research pursuits in the last decade (Fig. 2C).
Fig. 2.
Large-scale review trends in publication across different types of dental tissues and dental engineering. The number of publications per year between 1950 and 2022 for (A) Dental Tissue Engineering, Periodontal Tissue Engineering, and Orofacial Tissue Engineering shown with a sigmoidal fit, (B) specific-scale review trends in publication for each dental tissue interface: Periodontium, Cementum-Periodontal ligament, Pulp-Dentin, Implant Fixture-Alveolar Bone, and TMJ-Temporomandibular ligament, (C) with spatially controlled bioactivities, drug delivery, in situ engineering.
5. Bioengineering approaches employed for spatially controlled bioactivities targeting soft-hard tissue interfaces
5.1. Cementum-PDL-bone complex
Multiple bioactive factors have been identified to specifically target and promote regeneration in each component of the periodontium complex. Cementum protein 1 (CEMP1), ε-aminocaproic acid (ACA), and amelogenin have demonstrated potential to induce differentiation of PDLSCs into cementoblasts when delivered via various scaffolds [5,50,52,[72], [73], [74]]. Bioactive factors explored for PDL regeneration include connective tissue growth factor (CTGF), fibroblast growth factor-2 (FGF-2), platelet-derived growth factor (PDGF), insulin-like growth factor-I and -II (IGF-I & II), bone morphogenetic protein-3 (BMP-3), and growth differentiation factor-5 (GDF-5) [45,75]. The anticipated functions of CTGF, FGF-2, and GDF-5 are to promote fibrogenesis, whereas those of IGF-1 and BMP-3 are to promote osteogenic differentiation [45,75]. These factors could be co-delivered with other cues having distinct biological functions. For example, spatiotemporal delivery of CTGF with other osteogenic and cementogenic cues in 3D-printed PCL scaffolds led to the formation of PDL-like tissues integrating cementum and bone both in vitro and in vivo [5]. Similarly, FGF-2 and carbonated apatite (CO3Ap) were delivered to a one-wall periodontal defect model in beagle dogs to guide regeneration of PDL and cementum [76]. In another study, poly(lactic-co-glycolic acids) (PLGA) scaffolds loaded with plasmid DNA encoding FGF-2 demonstrated the ability to produce more regular PDL-like tissues and less root surface resorption in a beagle dog model [77]. Besides growth factors, bioactive materials have been tested to promote osteogenic differentiation of PDLSCs targeting alveolar bone regeneration. For instance, titanium carbide MXene (Ti3C2Tx) nanoflakes were used to promote new bone formation by PDL cells and inhibit osteoclast activity [78]. Cerium oxide (CeO2) nanoparticles have shown to similar osteogenic effects on PDLSCs [79]. The above-listed bioactive materials and cues have various biological functions useful for tissue regeneration, but a direct comparison between those factors has been limited, making it challenging to estimate their relative potential for periodontal regeneration [[80], [81], [82], [83]].
The abovementioned bioactive factors can be delivered through scaffolds in various configurations with or without stem/progenitor cells or tissue-specific cells to promote regeneration of target tissue(s) in the periodontium complex. Scaffolds can provide physical substrates for cell and tissue ingrowth, mechanical integrity or augmentation, and precise control of spatiotemporal delivery of cells and bioactive cues [80]. For example, a monophasic PCL membrane releasing simvastatin, seeded with PDL stem/progenitor cells (PDLSCs), induced an ectopic formation of cementum-like structure on the dentin surface [84]. Double-layered PCL scaffolds loaded with osteoblasts and PDLSCs showed a formation of cementum-like tissue [85]. Triphasic scaffolds have also been tested for integrated healing of cementum, PDL, and alveolar bone simultaneously. In our previous study, tri-layer 3D-printed PCL/HA scaffolds were fabricated with region-specific microstructures and spatiotemporally delivered bioactive cues specific to the respective tissue types [5]. The triphase PCL/HA scaffolds with spatiotemporal delivery of multiple cues led to de novo formation of integrated multi-tissues, including cementum-like, PDL-like, and alveolar bone-like constructs. In another study, a composite construct consisting of three layers with graded nanoapatite (NAp) and lauric acid (LA) in a PLGA matrix was developed, aiming at providing quick bone mineral formation and continuous antimicrobial concentration [86]. Another type of tri-layered scaffold, comprising of chitin-PLGA/nanobioactive glass ceramic(nBGC)/CEMP1 as the cementum layer, chitin–PLGA/FGF-2 as the PDL layer, and chitin–PLGA/nBGC/PDGF for the bone layer, showed promising in vivo outcome in healing periodontal tissues in rabbits [9]. These previous studies suggest the potential of multi-phase bioactive scaffolds in leading to endogenous healing of integrated periodontal tissues, even without cell delivery.
More advanced fabrication technologies have been employed to better mimic native-like multi-phase structures in periodontal tissues. For example, 3D printing with layered deposition of polymeric fibers has proved its unique advantages in fabricating scaffolds with integrated, multi-phase microstructures, mimicking multi-tissue interfaces [5,45,75]. In addition, 3D printing has been incorporated with bioprinting, enabling precisely controlled cell seeding or spatial delivery of bioactive cues, helpful in constructing multi-tissue interfaces [5,45,75]. These properties are also highly beneficial for designing spatiotemporal delivery system integrated in scaffolds [83]. Despite the positive features, 3D printing with fused deposition has a limited size of printable fibers, restricted by the dispensing nozzle. Although fine printing nozzles as small as 50 μm are available, the heat-based extrusion of widely used polyester-based biodegradable polymers is limited to 100–200 μm in the fiber diameter to maintain reliable printability. Moreover, precise and versatile controlling of fiber orientation is limited in extrusion-based 3D-printing [87]. A more recently emerged technique, such as Melt ElectroWriting (MEW) combining 3D printing and electrospinning, may overcome those limitations by enabling the printing of micron-to nano-sized filaments, over 10 times thinner than fibers from conventional 3D-printing [50]. Graded MEW scaffolds with osteoconductive coating enhanced the healing of calvaria defects [88]. MEW PCL scaffolds with fluorinated calcium phosphate (F/CaP) facilitated osteogenic differentiation of PDLSCs in vitro and healing of fenestration periodontal defects (Daghreryetal.,2021). Although the MEW has unique advantages in creating organized, micro-thin fibers, it may not be suitable for fabricating a thick 3D structure with a consistent fabrication resolution, as limited by the strength and depth of electromagnetic field [89].
Although signaling mediators regulating the regeneration process of periodontal tissues are not well described, signaling pathways involved in periodontal tissues development and homeostasis have been reported. For example, Wnt signaling play critical roles in periodontal complex, as knocking down Wnt signaling network leads to a pathological widening associated with reduced osteogenic signals and disorganized fibrous matrix [90]. Another study reported that Wnt5a maintains a non-mineralized stats of PDL and regulates bone homeostasis [91]. Sonic hedgehog (Shh) is also closely involved in periodontal complex development and homeostasis [92]. Shh signaling regulates formation of dentin, cementum, and PDL, and Gli1-positive cells responsive to Shh signaling are identified in PDL, as progenitor cells developing into soft-hard tissue interfaces [92]. These signaling pathways involved in periodontal tissue development and homeostasis can have potential to be incorporated into bioactive scaffolds guiding periodontium regeneration.
5.2. Peri-implant interface
Due to the scarcity of PDL and its attachment to cementum, the peri-implant region is highly vulnerable to bacterial infections and consequent bone loss [64,67]. To prevent the recurrence of peri-implantitis, peri-implant regeneration of PDL, cementum, and alveolar bone has been explored despite being at a preliminary stage [12,93]. A recent study developed PCL microfibers loaded with cholecalciferol for the treatment of peri-implantitis, showing promising in vitro osteogenic potential [12]. In another study, delivery of microRNAs, miR-27a, targeting Dickkopf2 (DKK2) and secreted frizzled-related protein 1 (SFRP1) has improved healing of bone defects around implants in a canine peri-implantitis model [94]. Engineering periodontium-like structure surrounding implants has also been attempted as a preventive measure against peri-implantitis [93]. Titanium implants wrapped by PDLSC sheets formed cementum- and PDL-like structures on the titanium surface after implantation in tibial and mandibular bone defects [93]. To date, studies have been predominantly focused on delivering of single bioactive cue with a goal to regenerate peri-implant bone, with limited attempts to multiple periodontal tissues for the treatment of peri-implantitis. Nonetheless, the above-discussed state-of-the-art scaffolds with spatiotemporal delivery designed for periodontal regeneration can be applied as regenerative treatments for peri-implantitis with necessary modifications. We speculate that the approaches for spatially controlled bioactivities can be applied on the surface of dental implants prior to implantation to prevent peri-implantitis or along with the reconstruction treatment for severe peri-implantitis resulting in loss of bony integration and soft tissue junction.
5.3. Pulp-dentin interface
Regenerative endodontics (RE) has attempted to regenerate pulp, predominantly using injectable hydrogel scaffolds (e.g., collagen, gelatin, alginate, and hyaluronic acids) with bioactive molecules and/or cells [70,95]. Synthetic materials have also been evaluated for pulp-dentin regeneration as a carrier of bioactive molecules and cells or a substrate supporting tissue healing [70,95]. Bioactive molecules tested for pulp regeneration include TGF-β1, FGF-2, BMP-2, PDGF, placenta growth factor, epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF) [70]. TGF-β1 and FGF-2 were used given their function to promote formation of soft tissues [96,97], whereas PDGF was used to enhance the proliferation of dental pulp cells and osteogenesis [98]. BMP-2 and EGF were applied for dental pulp given their well-established functions for osteogenesis [99], and VEGF was used to promote angiogenesis in pulp tissue and pulp-dentin interfaces [100]. Atlhough Wnt/β-cathenin, Shh, BMP-associated Smad have been reported as signaling mediators in the development and homeostasis of pulp-dentin interface [101,102], these signaling pathways have not extensively investigated as incorporated bioactive scaffold systems.
Recently, multi-layered scaffolds have been explored for pulp-dentin regeneration given the unique, heterogenous interface between the soft and hard tissues [70,103]. Multi-phased scaffolds offer a system of specific compositional properties for co-culturing heterotypic cell populations that were lacking in homogenous hydrogel scaffolds [103]. Bilayer PLGA scaffolds with two different pore sizes on each side resulted in spatial distribution and odontoblastic differentiation of DPSCs [104]. Although the study was not focused on the pulp-dentin interface, the unique spatial distribution of cells within a multi-layered scaffold can be further developed by targeting the interface between dentin and pulp tissues. Another recent study showed a 3D-printed bi-layered scaffold with PCL/45S5 and PCL/HA, designed for dentin and pulp, respectively, promoted mechanical properties, surface roughness, and bioactivity toward dentin formation [105]. Although pulp-related marker expressions were lacked in PCL/HA scaffold, the presented bi-layered scaffolds hold the potential to be developed into an anatomically shaped, patient-specific structure to support pulp-dentin regeneration. Despite the few promising previous reports, there have been rare research efforts for regenerating pulp-dentin interface.
5.4. TMJ disc ligament-bone interface
The central focus of ongoing research involves the reconstruction of TMJ discs through a combination of scaffolds, biomolecules, and cells. Various synthetic materials have also been investigated for TMJ disc regeneration [6,18,19]. These advanced scaffolds with synthetic biomaterials are capable of readily modifying microstructures, anatomical shape, and structural stability to achieve optimal structures to replace TMJ discs [6,18]. However, the unique structure and compositions suitable for the attachment of TMJ disc to ligament/bone have not been implemented in the previous TMJ disc scaffolds. Instead, the previous TMJ disc scaffolds were implanted by suture-fixation through bone tunnels or adjacent soft tissue attachments [6,106]. Unfortunately, previous studies paid little or no attention to the functional restoration of the attachment sites, which play critical roles in the functionality of regenerated TMJ discs in the joint system.
Recently, an application of MEW in combination with a hydrogel has been proposed for the regeneration of osteochondral interfaces of the TMJ by emulating viscoelasticity and stress relaxation of cartilage and ligaments in response to mechanical load [87]. Despite the paucity of experimental outcomes, MEW may have notable potential to build a specific microstructure that mimics the gradient changes at the TMJ disc ligament-bone interface. The approaches used for reconstructing the knee meniscus to bone insertion interface can also be applicable to the TMJ disc ligament-bone interface. For example, collagen gel-based scaffolds were infused with bone plugs to engineer a stable meniscus-bone interface [2]. Multi-phase 3D printed micro-architecture, in combination with spatiotemporal delivery of bioactive cues, widely used for periodontium regeneration, can also be readily implemented in the existing 3D printed TMJ disc scaffolds. It is postulated that the specific geometries and microarchitectures of scaffolds for the interface would significantly facilitate the functional recovery and long-term success of tissue engineered TMJ discs.
Like MSK ligament enthesis, the development and homeostasis of TMJ disc ligament-bone interface is estimated to be regulated by various signaling pathways, including but not limited to TGF-β, FGF, and Shh, as associated with Gli1+ progenitor cells [4]. Thus, transcription factors involved in the pathways, such as Scleraxis, Sox9, Pbx1, and Krüppel-like (KLF) transcription factors [4], may have potential to serve as bioactive cues as incorporated into the bioactive scaffold system to facilitate augmentation of TMJ disc ligament-bone interface.
5.5. Potential technologies enabling micro-spatially controlled bioactivities for interface regeneration
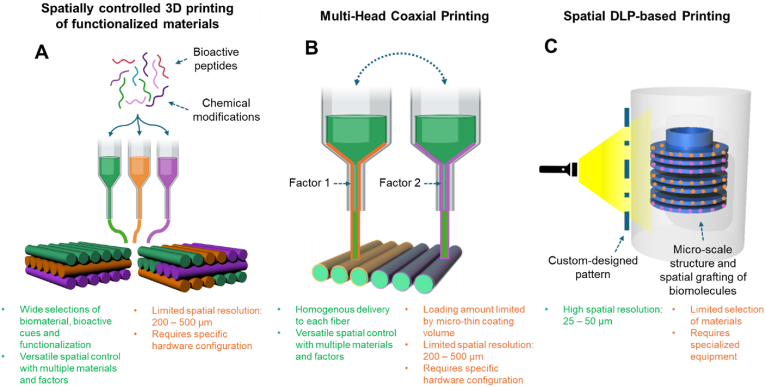
This section introduces several potential technologies applied for reconstructing MSK soft-hard tissue interfaces, which have rarely been adopted to build spatially controlled bioactivities for the dento-craniofacial tissue interfaces (Fig. 3).
Fig. 3.
Micro-scale scaffold fabrication techniques with potential in creating micro-spatial delivery of bioactive cues in 3D scaffolds for dento-craniofacial soft-hard tissue interfaces: (A) 3D printing of multiple biomaterials, pre-functionalized with bioactive peptides or specific chemical modifications, (B) Multi-head coaxial 3D printing, and (C) spatially controlled DLP-based printing.
As one of the potential technologies, spatially functionalized scaffolds were fabricated by 3D-printing bioactive peptide-conjugated polymers [107,108]. Chondrogenic, hyaluronic acid (HA)-binding (HAbind–PCL) and mineralizing peptide (E3)-conjugated PCL were prepared, followed by printing two materials into integrated multiphase 3D scaffolds for osteochondral tissue engineering [107,108]. These peptides-conjugated PCL showed potential to guide spatially controlled chondrogenic and osteogenic differentiation of mesenchymal stem/progenitor cells (MSCs) [107,108]. This approach allows for establishing micro-precise, spatial control of desired bioactivities as integrated in the layer-by-layer 3D printing process. Given the wide availability of bioactive peptides applicable for dental and craniofacial tissues, including cementum, dentin, bone, and PDL [[108], [109], [110], [111], [112], [113]], 3D-printed scaffolds with peptide-conjugated polymer may provide useful tool to create spatially controlled microenvironment for engineering multiphase dental and craniofacial tissue interfaces.
Coaxial 3D printing/bioprinting may also have potential to enable micro-precise, spatial control of delivery of multiple bioactive cues in a 3D scaffold [[114], [115], [116]]. Coaxial extrusion, simultaneously dispensing two or more bioinks or other biomaterials arranged concentrically in a single filament, has been utilized to deliver bioactive cues as incorporated in 3D-printed scaffolds [[114], [115], [116]]. Previous coaxial printing works have mostly used the solid, structural polymer as the core filament and shell layer material as delivery vehicle of selected growth factors [[114], [115], [116]]. Although this method has been widely used to build 3D scaffolds equipped with temporally controlled delivery, it has yet been applied for spatial delivery of multiple factors [114]. A few recent studies have used coaxial electrospinning to build nano-sized segments containing different bioactive cues that were prepared as bioinks to build stratified scaffolds for interface tissue engineering [117,118]. As coaxial printing enables a delivery of bioactive cues via micro-thin shell layer of each 3D printed microfilaments, we believe that a combination of coaxial printing and multi-head printing technique can provide with a micro-precisely controlled spatial delivery of multiple bioactive cues, thus showing a notable promise in the regeneration of soft-hard tissue interfaces.
The recently reported spatial-selective volumetric 4D printing with single-photon grafting of biomolecules [119] may also have significant potential to be applicable for dento-craniofacial tissue interfaces. A novel light-based volumetric printing using thiol–ene click chemistry enabled on-demand photo-grafting of thiolated compounds post-printing, resulting in high spatiotemporal control in forming 3D geometries with a high spatiotemporal control of growth factor delivery [119]. This technology can be readily applicable to fabricate 3D bioactive scaffolds suitable for cementum-PDL-bone and pulp-dentin.
Digital light processing (DLP)-based 3D bioprinting has also exhibited notable potential in developing scaffolds with high-resolution and micro-scale structural complexity [120,121]. Extrusion-based 3D printing, widely used tissue engineering applications, mainly builds 3D structure by directing extruding bioinks with predesigned CAD structure. Thus, the resolution of spatial control of extrusion printing is limited by the physical nozzle of dispending unit, typically around 200–500 μm [120,121]. Distinguishably, DLP-based 3D bioprinting enables the 3D complex structures by using digitalized light triggering in situ photopolymerization, resulting in 25–50 μm spatial resolution determined by parameters such as the pixel size of the light projection patterns and the magnification of the projecting optics [120,121]. DLP-based 3D bioprinting has recently reported its superior capacity to build scaffolds with high-resolution composite gradient [120]. Another study utilized DLP-based 3D bioprinting to build a bone scaffold with micro-spatially controlled multi-cellular delivery [122]. To date, DLP-based bioprinting has not been tested for dento-craniofacial tissue engineering. We speculate that the high-precision bioprinting with superior capacity to build complex structures of DLP bioprinting will be highly beneficial for developing bioactive scaffolds for dento-craniofacial soft-hard tissue interfaces with unique micro-scale complexity.
6. Challenges and future perspectives
Despite the research progress, there has been only few clinical applications of biomaterial scaffolds with biologics for the soft-hard tissue interface [82,83,[123], [124], [125], [126], [127], [128]]. To date, only monophasic scaffolds with or without growth factor or autologous cell sheet have been tested in clinical trials for periodontal tissue regeneration, without any clinical application of spatially controlled bioactivities [82,83,[123], [124], [125], [126], [127], [128]]. The remaining hurdles toward clinical translation of the bioactive scaffolds for interface regeneration include our limited understanding of in vivo degradation of biomaterials. Although biomaterials explored for tissue regeneration are biodegradable, undergoing degradation upon implantation in the body, as replaced by newly forming tissue. However, balancing in vivo degradation with new tissue formation is technically challenging. Despite the well-characterized in vitro degradation rate, the in vivo degradation can be quite different due to the biochemical environment associated with blood supply, inflammation, and metabolism [129,130]. Unfortunately, in vivo degradation of implanted scaffolds has been rarely examined to balance the degradation with regeneration due to technological challenges [6,131]. Thus, we may need to consider active adaptation of advanced imaging modalities for non-invasive tracking of in vivo scaffold degradation [131], thus achieving patient-specific balancing of tissue regeneration with scaffold degradation.
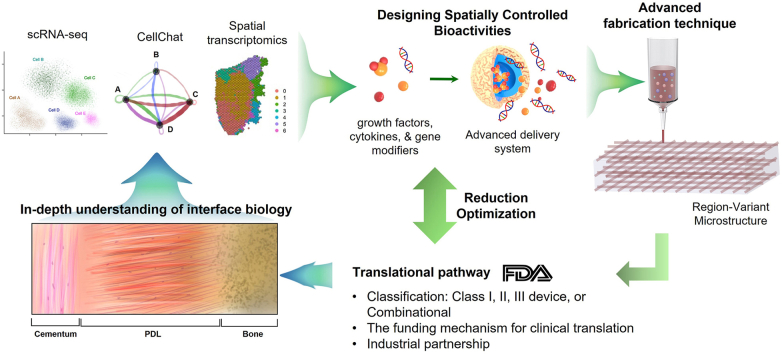
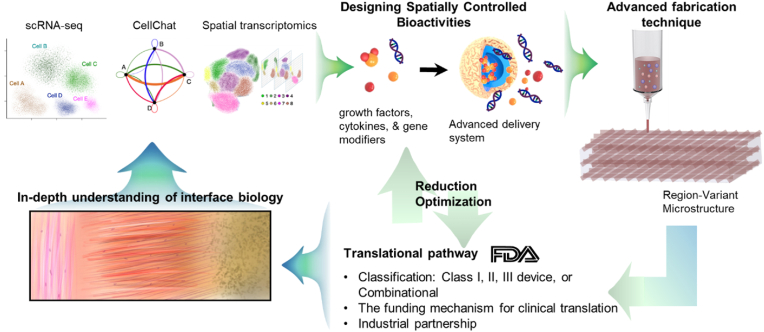
Our limited understanding of interface biology may serve as another translational hurdle. In the musculoskeletal system, the developmental biology of soft-hard tissue interfaces has significantly been studied to learn timely regulated molecular signaling mediating the formation and maturation of the tissue interfaces [4]. Knowledge from developmental biology may hold promise to provide signaling targets with robust effectiveness in facilitating post-natal regeneration of soft-hard tissue interfaces [4]. However, we have a relatively limited understanding of the development of dento-craniofacial tissue interfaces. As the biological processes regulating the development and regeneration of soft-hard tissue interfaces are complex, we may need to apply emerging research tools such as single-cell RNA sequencing (scRNA-seq) with CellChat analysis [132] and spatial transcriptomics [133] to understand interactions between multiple signaling molecules and cross-talks between different types of cells [134], beyond investigating roles of selected signaling mediators. Deepening our knowledge in the complex signaling regulations in conjunction with multi-tissue cross-talks, potentially associated with unique features in the dento-craniofacial tissue interfaces, may lead to designing of more effective bioactive scaffold system targeting the soft-hard tissue interfaces in the dental and craniofacial system.
A more precisely controlled delivery of bioactive cues may be necessary to orchestrate the regeneration process for multi-tissue interfaces better. Given the unavoidable involvement of inflammation during the disease initiation, progress, and repair/healing process, a precisely controlled timing of the bioactivities of anti-inflammatory cues. Similarly, bioactive cues to simulate differentiation and tissue formation need to be controlled in a timely manner. As the previous delivery modes used in scaffolds, such as simple adsorption and loaded in a polymer, are not suitable for precise release control, advanced control-delivery modalities such as nano-layer-by-layer fabrication and core-shell nanoparticles can be considered for precisely controlled sequential release of multiple factors [135]. In addition, timely and/or stimulation-sensitive release of multiple factors can be achieved through the emerging, “smart” hydrogel-based delivery systems incorporated into various scaffold structures [136].
It is imperative to consider the translational pathway at the stage of designing the scaffolds containing bioactive cues to guide in situ regeneration of the soft-hard tissue interface. Depending on the type and functional mode, the biomaterial-based scaffold can go through different regulatory pathways. Biomaterial scaffolds without biologics, proteins, or chemicals can be classified as Class I, II, or III devices, coming with distinct FDA regulatory pathways [137]. Most likely, tissue engineering products containing two or more components of material and biologics are categorized as combinational products that would generally require additional safety and efficacy data [137]. Completion of all the FDA requirements for a market launch takes a long journey and a tremendous cost. On average, the development of new regenerative therapeutics from target identification through approval for marketing takes over 12 years with an estimated cover over $2.6 billion [137]. The cost would be significantly increased for the bioactive scaffolds containing multiple bioactive factors. The significant financial barrier is mainly responsible for the “Valley of Death” across pre-clinical develop to clinical translation [137]. Thus, finding an optimal balance between the recapitulation of the complex bioactivities for interface regeneration and the design simplicity to minimize the financial hurdle is critically essential. The cost of clinical translation can be dramatically reduced if the desired bioactivities can be achieved by drug repurposing [138]. Moreover, investigators may consider a ‘reduction optimization’ process [139] to achieve the most straightforward possible design of bioactive scaffold to result in the desired clinical outcome to facilitate the clinical translation.
Although the complex multi-tissue structure at the soft-hard tissue interface is a challenging feature to reconstruct, a need for interface regeneration is inevitably linked with various diseases in the dental and craniofacial connective tissues. Despite the challenges, our long-lasting dedication has made significant progress in developing effective bioactive scaffolds with notable potential to regenerate complex soft-hard tissue interfaces. Robust collaboration between biomaterial scientists, biologists, chemists, clinicians, business partners, and regulatory experts will facilitate the process of developing regenerative products for interface regeneration. An in-depth understanding of interface biology and development, adoption of rapidly advancing technologies in scaffold fabrication and delivery system, and early-stage consideration of translational pathway and funding requirements are also expected to play critical roles in our journey toward functional regeneration of the dental and craniofacial soft-hard tissue interfaces (Fig. 4).
Fig. 4.
Potential strategies to facilitate development and clinical translation of bioactive scaffold for regeneration of soft-hard tissue interface. In-depth understanding of the interface biology using advanced technologies (e.g., scRNA-seq, CellChat, & spatial transcriptomics) will guide the designing of effective bioactive cues and delivery systems. The spatiotemporally controlled bioactivities can be implemented in a 3D scaffold system produced by advanced fabrication techniques (e.g., 3D printing & MEW). The designing of spatially controlled bioactivities and the biomaterial selection necessitate the understanding of expected translational pathways, dependent on the FDA classification and associated requirements for premarket approval. Consideration of the funding mechanism for translational pathway and potential industrial partnerships is also required to be visited at the early stage of project development. If necessary, the components in the bioactive scaffolds may need to be simplified through reduction optimization process, seeking the simplest form to achieve desired efficacy, which may lead to a cost-reduction in the translational pathway.
CRediT authorship contribution statement
Hun Jin Jeong: Writing – review & editing, Writing – original draft, Visualization, Software, Investigation, Data curation. Lan Anh P. Hoang: Writing – review & editing, Writing – original draft, Visualization, Software, Formal analysis, Data curation. Neeve Chen: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Elen Zhu: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Albert Wang: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Bozhi Chen: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Emma Y. Wang: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Christopher L. Ricupero: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation. Chang H. Lee: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Ethics approval and consent to participate
No applicable, as this manuscript is a review paper without any human subject or animal study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Funding: This manuscript was partially supported by NIH Grants 1R01DE029321 to CHL.
References
- 1.Li Y., Zhou M., Zheng W., Yang J., Jiang N. Scaffold-based tissue engineering strategies for soft-hard interface regeneration. Regen Biomater. 2023;10 doi: 10.1093/rb/rbac091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCorry M.C., Mansfield M.M., Sha X., Coppola D.J., Lee J.W., Bonassar L.J. A model system for developing a tissue engineered meniscal enthesis. Acta Biomater. 2017;56:110–117. doi: 10.1016/j.actbio.2016.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apostolakos J., Durant T.J., Dwyer C.R., Russell R.P., Weinreb J.H., Alaee F., et al. The enthesis: a review of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 2014;4(3):333–342. [PMC free article] [PubMed] [Google Scholar]
- 4.Killian M.L. Growth and mechanobiology of the tendon-bone enthesis. Semin. Cell Dev. Biol. 2022;123:64–73. doi: 10.1016/j.semcdb.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee C.H., Hajibandeh J., Suzuki T., Fan A., Shang P., Mao J.J. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng Part A. 2014;20(7–8):1342–1351. doi: 10.1089/ten.tea.2013.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarafder S., Koch A., Jun Y., Chou C., Awadallah M.R., Lee C.H. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication. 2016;8(2) doi: 10.1088/1758-5090/8/2/025003. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz F., Derks J., Monje A., Wang H.L. Peri-implantitis. J. Clin. Periodontol. 2018;45(Suppl 20):S246–S266. doi: 10.1111/jcpe.12954. [DOI] [PubMed] [Google Scholar]
- 8.Shah D., Lynd T., Ho D., Chen J., Vines J., Jung H.D., et al. Pulp-dentin tissue healing response: a discussion of current biomedical approaches. J. Clin. Med. 2020;9(2) doi: 10.3390/jcm9020434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sowmya S., Mony U., Jayachandran P., Reshma S., Kumar R.A., Arzate H., et al. Tri-layered nanocomposite hydrogel scaffold for the concurrent regeneration of cementum, periodontal ligament, and alveolar bone. Adv. Healthcare Mater. 2017;6(7) doi: 10.1002/adhm.201601251. [DOI] [PubMed] [Google Scholar]
- 10.Zhu B., Liu W., Liu Y., Zhao X., Zhang H., Luo Z., et al. Jawbone microenvironment promotes periodontium regeneration by regulating the function of periodontal ligament stem cells. Sci. Rep. 2017;7 doi: 10.1038/srep40088. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Onizuka S., Iwata T. Application of periodontal ligament-derived multipotent mesenchymal stromal cell sheets for periodontal regeneration. Int. J. Mol. Sci. 2019;20(11) doi: 10.3390/ijms20112796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanhueza C., Hermosilla J., Klein C., Chaparro A., Valdivia-Gandur I., Beltrán V., et al. Osteoinductive electrospun scaffold based on PCL-Col as a regenerative therapy for peri-implantitis. Pharmaceutics. 2023;15(7):1939. doi: 10.3390/pharmaceutics15071939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon T., Lamster I.B., Levin L. Current concepts in the management of periodontitis. Int. Dent. J. 2021;71(6):462–476. doi: 10.1111/idj.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsson L., Decker A.M., Nibali L., Pilipchuk S.P., Berglundh T., Giannobile W.V. Regenerative medicine for periodontal and peri-implant diseases. J. Dent. Res. 2016;95(3):255–266. doi: 10.1177/0022034515618887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galler K.M., Weber M., Korkmaz Y., Widbiller M., Feuerer M. Inflammatory response mechanisms of the dentine-pulp complex and the periapical tissues. Int. J. Mol. Sci. 2021;22(3) doi: 10.3390/ijms22031480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veerayutthwilai O., Byers M.R., Pham T.T., Darveau R.P., Dale B.A. Differential regulation of immune responses by odontoblasts. Oral Microbiol. Immunol. 2007;22(1):5–13. doi: 10.1111/j.1399-302X.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad M., Schiffman E.L. Temporomandibular joint disorders and orofacial pain. Dent Clin North Am. 2016;60(1):105–124. doi: 10.1016/j.cden.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legemate K., Tarafder S., Jun Y., Lee C.H. Engineering human TMJ discs with protein-releasing 3D-printed scaffolds. J. Dent. Res. 2016;95(7):800–807. doi: 10.1177/0022034516642404. [DOI] [PubMed] [Google Scholar]
- 19.Van Bellinghen X., Idoux-Gillet Y., Pugliano M., Strub M., Bornert F., Clauss F., et al. Temporomandibular joint regenerative medicine. Int. J. Mol. Sci. 2018;19(2) doi: 10.3390/ijms19020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Runci Anastasi M., Centofanti A., Arco A., Vermiglio G., Nicita F., Santoro G., et al. Histological and immunofluorescence study of discal ligaments in human temporomandibular joint. J. Funct. Morphol. Kinesiol. 2020;5(4):90. doi: 10.3390/jfmk5040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang Y., Hu Z., Chang B., Liu X. Quantitative characterizations of the Sharpey's fibers of rat molars. J. Periodontal. Res. 2020;55(2):307–314. doi: 10.1111/jre.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu W., Liang M. Periodontal ligament stem cells: current status, concerns, and future prospects. Stem Cell. Int. 2015;2015 doi: 10.1155/2015/972313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J.D., Jang A.T., Kurylo M.P., Hurng J., Yang F., Yang L., et al. Periodontal ligament entheses and their adaptive role in the context of dentoalveolar joint function. Dent. Mater. 2017;33(6):650–666. doi: 10.1016/j.dental.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moussa D.G., Aparicio C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen Med. 2019;13(1):58–75. doi: 10.1002/term.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjorndal L., Simon S., Tomson P.L., Duncan H.F. Management of deep caries and the exposed pulp. Int. Endod. J. 2019;52(7):949–973. doi: 10.1111/iej.13128. [DOI] [PubMed] [Google Scholar]
- 26.Hand A.R., Frank M.E. Wiley; 2014. Fundamentals of Oral Histology and Physiology. [Google Scholar]
- 27.Lu H.H., Thomopoulos S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu. Rev. Biomed. Eng. 2013;15(1):201–226. doi: 10.1146/annurev-bioeng-071910-124656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarafder S., Brito J.A., Minhas S., Effiong L., Thomopoulos S., Lee C.H. In situ tissue engineering of the tendon-to-bone interface by endogenous stem/progenitor cells. Biofabrication. 2019;12(1) doi: 10.1088/1758-5090/ab48ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamin M., Ralphs J.R. Biology of fibrocartilage cells. Int. Rev. Cytol. 2004;233:1–45. doi: 10.1016/S0074-7696(04)33001-9. [DOI] [PubMed] [Google Scholar]
- 30.Lei T., Zhang T., Ju W., Chen X., Heng B.C., Shen W., et al. Biomimetic strategies for tendon/ligament-to-bone interface regeneration. Bioact. Mater. 2021;6(8):2491–2510. doi: 10.1016/j.bioactmat.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho S.P., Kurylo M.P., Fong T.K., Lee S.S., Wagner H.D., Ryder M.I., et al. The biomechanical characteristics of the bone-periodontal ligament-cementum complex. Biomaterials. 2010;31(25):6635–6646. doi: 10.1016/j.biomaterials.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meeran N.A. Biological response at the cellular level within the periodontal ligament on application of orthodontic force - an update. J. Orthod. Sci. 2012;1(1):2–10. doi: 10.4103/2278-0203.94769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamin M., Toumi H., Ralphs J.R., Bydder G., Best T.M., Milz S. Where tendons and ligaments meet bone: attachment sites ('entheses') in relation to exercise and/or mechanical load. J. Anat. 2006;208(4):471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphrey J.D., Dufresne E.R., Schwartz M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014;15(12):802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magloire H., Couble M.L., Thivichon-Prince B., Maurin J.C., Bleicher F. Odontoblast: a mechano-sensory cell. J. Exp. Zool. B Mol. Dev. Evol. 2009;312b(5):416–424. doi: 10.1002/jez.b.21264. [DOI] [PubMed] [Google Scholar]
- 36.Bernal L., Sotelo-Hitschfeld P., König C., Sinica V., Wyatt A., Winter Z., et al. Odontoblast TRPC5 channels signal cold pain in teeth. Sci. Adv. 2021;7(13) doi: 10.1126/sciadv.abf5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto T., Hasegawa T., Yamamoto T., Hongo H., Amizuka N. Histology of human cementum: its structure, function, and development. Jpn Dent. Sci. Rev. 2016;52(3):63–74. doi: 10.1016/j.jdsr.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grzesik W.J., Cheng H., Oh J.S., Kuznetsov S.A., Mankani M.H., Uzawa K., et al. Cementum-forming cells are phenotypically distinct from bone-forming cells. J. Bone Miner. Res. 2000;15(1):52–59. doi: 10.1359/jbmr.2000.15.1.52. [DOI] [PubMed] [Google Scholar]
- 39.Jiang N., Guo W., Chen M., Zheng Y., Zhou J., Kim S.G., et al. Periodontal ligament and alveolar bone in health and adaptation: tooth movement. Front. Oral Biol. 2016;18:1–8. doi: 10.1159/000351894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallorini M., Krifka S., Widbiller M., Schröder A., Brochhausen C., Cataldi A., et al. Distinguished properties of cells isolated from the dentin-pulp interface. Ann. Anat. 2021;234 doi: 10.1016/j.aanat.2020.151628. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg M., Smith A.J. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit. Rev. Oral Biol. Med. 2004;15(1):13–27. doi: 10.1177/154411130401500103. [DOI] [PubMed] [Google Scholar]
- 42.Lee Y.S., Park Y.-H., Lee D.-S., Seo Y.-M., Lee J.-H., Park J.-H., et al. Tubular dentin regeneration using a CPNE7-derived functional peptide. Materials. 2020;13(20):4618. doi: 10.3390/ma13204618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carda C., Peydró A. Ultrastructural patterns of human dentinal tubules, odontoblasts processes and nerve fibres. Tissue Cell. 2006;38(2):141–150. doi: 10.1016/j.tice.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Parker J.B., Valencia C., Akras D., DiIorio S.E., Griffin M.F., Longaker M.T., et al. Understanding fibroblast heterogeneity in form and function. Biomedicines. 2023;11(8) doi: 10.3390/biomedicines11082264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daghrery A., Bottino M.C. Advanced biomaterials for periodontal tissue regeneration. Genesis. 2022;60(8–9) doi: 10.1002/dvg.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen M.X., Zhong Y.J., Dong Q.Q., Wong H.M., Wen Y.F. Global, regional, and national burden of severe periodontitis, 1990-2019: an analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021;48(9):1165–1188. doi: 10.1111/jcpe.13506. [DOI] [PubMed] [Google Scholar]
- 47.György Komlós K.C., Horváth Ferenc, Pelyhe Liza, Németh Zsolt. Periodontitis as a risk for oral cancer: a case-control study. BMC Oral Health. 2021 doi: 10.1186/s12903-021-01998-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanz M., Del Castillo A.M., Jepsen S., Gonzalez-Juanatey J.R., D'Aiuto F., Bouchard P., et al. Periodontitis and cardiovascular diseases. Consensus Rep. Glob. Heart. 2020;15(1):1. doi: 10.5334/gh.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyu H., Zhou X., Qian Y., Liu X., Gopinathan G., Pandya M., et al. Long-acting PFI-2 small molecule release and multilayer scaffold design achieve extensive new formation of complex periodontal tissues with unprecedented fidelity. Biomaterials. 2022;290 doi: 10.1016/j.biomaterials.2022.121819. [DOI] [PubMed] [Google Scholar]
- 50.Bourdon L., Attik N., Belkessam L., Chevalier C., Bousige C., Brioude A., et al. Direct-writing electrospun functionalized scaffolds for periodontal regeneration: in vitro studies. J. Funct. Biomater. 2023;14(5) doi: 10.3390/jfb14050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abedi N., Rajabi N., Kharaziha M., Nejatidanesh F., Tayebi L. Layered scaffolds in periodontal regeneration. J. Oral Biol. Craniofac. Res. 2022;12(6):782–797. doi: 10.1016/j.jobcr.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin H.H., Chao P.G., Tai W.C., Chang P.C. 3D-Printed collagen-based waveform microfibrous scaffold for periodontal ligament reconstruction. Int. J. Mol. Sci. 2021;22(14) doi: 10.3390/ijms22147725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mordini L., Sun N., Chang N., De Guzman J.P., Generali L., Consolo U. Peri-implantitis regenerative therapy: a review. Biology (Basel). 2021;10(8) doi: 10.3390/biology10080773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nyman S., Gottlow J., Karring T., Lindhe J. The regenerative potential of the periodontal ligament. An experimental study in the monkey. J. Clin. Periodontol. 1982;9(3):257–265. doi: 10.1111/j.1600-051x.1982.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 55.Nyman S., Karring T., Lindhe J., Planten S. Healing following implantation of periodontitis-affected roots into gingival connective tissue. J. Clin. Periodontol. 1980;7(5):394–401. doi: 10.1111/j.1600-051x.1980.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 56.Berglundh T., Armitage G., Araujo M.G., Avila-Ortiz G., Blanco J., Camargo P.M., et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018;45(Suppl 20):S286–S291. doi: 10.1111/jcpe.12957. [DOI] [PubMed] [Google Scholar]
- 57.Kordbacheh Changi K., Finkelstein J., Papapanou P.N. Peri‐implantitis prevalence, incidence rate, and risk factors: a study of electronic health records at a U.S. dental school. Clin. Oral Implants Res. 2019;30(4):306–314. doi: 10.1111/clr.13416. [DOI] [PubMed] [Google Scholar]
- 58.Zitzmann N.U., Berglundh T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008;35(8 Suppl):286–291. doi: 10.1111/j.1600-051X.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- 59.Froum S.J., Froum S.H., Rosen P.S. Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3-to 7.5-year follow-up. Int. J. Periodontics Restor. Dent. 2012;32(1):11. [PubMed] [Google Scholar]
- 60.Chan H.-L., Lin G.-H., Suarez F., Maceachern M., Wang H.-L. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. 2014;85(8):1027–1041. doi: 10.1902/jop.2013.130563. [DOI] [PubMed] [Google Scholar]
- 61.Renvert S., Roos-Jansåker A.-M., Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J. Clin. Periodontol. 2008;35:305–315. doi: 10.1111/j.1600-051X.2008.01276.x. [DOI] [PubMed] [Google Scholar]
- 62.Kotsakis G.A., Konstantinidis I., Karoussis I.K., Ma X., Chu H. Systematic review and meta-analysis of the effect of various laser wavelengths in the treatment of peri-implantitis. J. Periodontol. 2014;85(9):1203–1213. doi: 10.1902/jop.2014.130610. [DOI] [PubMed] [Google Scholar]
- 63.Esposito M., Grusovin M.G., Worthington H.V. Treatment of peri-implantitis: what interventions are effective? A Cochrane systematic review. Eur. J. Oral Implant. 2012;5(Suppl):S21–S41. [PubMed] [Google Scholar]
- 64.Heitz-Mayfield L.J., Lang N.P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol. 2000. 2010;53:167–181. doi: 10.1111/j.1600-0757.2010.00348.x. [DOI] [PubMed] [Google Scholar]
- 65.Berglundh T., Zitzmann N.U., Donati M. Are peri-implantitis lesions different from periodontitis lesions? J. Clin. Periodontol. 2011;38:188–202. doi: 10.1111/j.1600-051X.2010.01672.x. [DOI] [PubMed] [Google Scholar]
- 66.Ivanovski S., Lee R. Comparison of peri-implant and periodontal marginal soft tissues in health and disease. Periodontology. 2000. 2018;76(1):116–130. doi: 10.1111/prd.12150. [DOI] [PubMed] [Google Scholar]
- 67.Berglundh T., Jepsen S., Stadlinger B., Terheyden H. Peri-implantitis and its prevention. Clin. Oral Implants Res. 2019;30(2):150–155. doi: 10.1111/clr.13401. [DOI] [PubMed] [Google Scholar]
- 68.Kammerer P.W., Scholz M., Baudisch M., Liese J., Wegner K., Frerich B., et al. Guided bone regeneration using collagen scaffolds, growth factors, and periodontal ligament stem cells for treatment of peri-implant bone defects in vivo. Stem Cell. Int. 2017;2017 doi: 10.1155/2017/3548435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schnabl D., Fleischer F., Riedmann M., Laimer J., Gassner R. Prevalence and distribution of deep caries and abscess formation in children who required emergency dental general anaesthesia. A retrospective analysis. Eur. J. Paediatr. Dent. 2019;20(2):119–122. doi: 10.23804/ejpd.2019.20.02.07. [DOI] [PubMed] [Google Scholar]
- 70.Shrestha S., Kishen A. Bioactive molecule delivery systems for dentin-pulp tissue engineering. J. Endod. 2017;43(5):733–744. doi: 10.1016/j.joen.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 71.Valesan L.F., Da-Cas C.D., Reus J.C., Denardin A.C.S., Garanhani R.R., Bonotto D., et al. Prevalence of temporomandibular joint disorders: a systematic review and meta-analysis. Clin. Oral Invest. 2021;25(2):441–453. doi: 10.1007/s00784-020-03710-w. [DOI] [PubMed] [Google Scholar]
- 72.Hoz L., Lopez S., Zeichner-David M., Arzate H. Regeneration of rat periodontium by cementum protein 1-derived peptide. J. Periodontal. Res. 2021;56(6):1223–1232. doi: 10.1111/jre.12921. [DOI] [PubMed] [Google Scholar]
- 73.Bousnaki M., Beketova A., Kontonasaki E. A review of in vivo and clinical studies applying scaffolds and cell sheet technology for periodontal ligament regeneration. Biomolecules. 2022;12(3) doi: 10.3390/biom12030435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Viswanathan H.L., Berry J.E., Foster B.L., Gibson C.W., Li Y., Kulkarni A.B., et al. Amelogenin: a potential regulator of cementum-associated genes. J. Periodontol. 2003;74(10):1423–1431. doi: 10.1902/jop.2003.74.10.1423. [DOI] [PubMed] [Google Scholar]
- 75.Woo H.N., Cho Y.J., Tarafder S., Lee C.H. The recent advances in scaffolds for integrated periodontal regeneration. Bioact. Mater. 2021;6(10):3328–3342. doi: 10.1016/j.bioactmat.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagayasu-Tanaka T., Anzai J., Takedachi M., Kitamura M., Harada T., Murakami S. Effects of combined application of fibroblast growth factor (FGF)-2 and carbonate apatite for tissue regeneration in a beagle dog model of one-wall periodontal defect. Regen Ther. 2023;23:84–93. doi: 10.1016/j.reth.2023.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang L., Ding Z., Xia S., Liu Y., Lei S., Zhong M., et al. Poly lactic-co-glycolic acid scaffold loaded with plasmid DNA encoding fibroblast growth factor-2 promotes periodontal ligament regeneration of replanted teeth. J. Periodontal. Res. 2020;55(4):488–495. doi: 10.1111/jre.12734. [DOI] [PubMed] [Google Scholar]
- 78.Cui D., Kong N., Ding L., Guo Y., Yang W., Yan F. Ultrathin 2D titanium carbide MXene (Ti(3) C(2) T(x)) nanoflakes activate WNT/HIF-1alpha-Mediated metabolism reprogramming for periodontal regeneration. Adv. Healthcare Mater. 2021;10(22) doi: 10.1002/adhm.202101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ren S., Zhou Y., Zheng K., Xu X., Yang J., Wang X., et al. Cerium oxide nanoparticles loaded nanofibrous membranes promote bone regeneration for periodontal tissue engineering. Bioact. Mater. 2022;7:242–253. doi: 10.1016/j.bioactmat.2021.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamada S., Shanbhag S., Mustafa K. Scaffolds in periodontal regenerative treatment. Dent Clin North Am. 2022;66(1):111–130. doi: 10.1016/j.cden.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 81.Lemaitre M., Monsarrat P., Blasco-Baque V., Loubières P., Burcelin R., Casteilla L., et al. Periodontal tissue regeneration using syngeneic adipose-derived stromal cells in a mouse model. Stem. Cells Transl. Med. 2017;6(2):656–665. doi: 10.5966/sctm.2016-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iwata T., Yamato M., Washio K., Yoshida T., Tsumanuma Y., Yamada A., et al. Periodontal regeneration with autologous periodontal ligament-derived cell sheets – a safety and efficacy study in ten patients. Regenerat. Ther. 2018;9:38–44. doi: 10.1016/j.reth.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rasperini G., Pilipchuk S.P., Flanagan C.L., Park C.H., Pagni G., Hollister S.J., et al. 3D-printed bioresorbable scaffold for periodontal repair. J. Dent. Res. 2015;94(9_suppl) doi: 10.1177/0022034515588303. 153S-7S. [DOI] [PubMed] [Google Scholar]
- 84.Zhao B., Chen J., Zhao L., Deng J., Li Q. A simvastatin-releasing scaffold with periodontal ligament stem cell sheets for periodontal regeneration. J. Appl. Biomater. Funct. Mater. 2020;18 doi: 10.1177/2280800019900094. [DOI] [PubMed] [Google Scholar]
- 85.Vaquette C., Fan W., Xiao Y., Hamlet S., Hutmacher D.W., Ivanovski S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials. 2012;33(22):5560–5573. doi: 10.1016/j.biomaterials.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 86.Jamuna-Thevi K., Saarani N.N., Abdul Kadir M.R., Hermawan H. Triple-layered PLGA/nanoapatite/lauric acid graded composite membrane for periodontal guided bone regeneration. Mater. Sci. Eng. C Mater Biol. Appl. 2014;43:253–263. doi: 10.1016/j.msec.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 87.Daghrery A., de Souza Araujo I.J., Castilho M., Malda J., Bottino M.C. Unveiling the potential of melt electrowriting in regenerative dental medicine. Acta Biomater. 2023;156:88–109. doi: 10.1016/j.actbio.2022.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abbasi N., Ivanovski S., Gulati K., Love R.M., Hamlet S. Role of offset and gradient architectures of 3-D melt electrowritten scaffold on differentiation and mineralization of osteoblasts. Biomater. Res. 2020;24(1):2. doi: 10.1186/s40824-019-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eichholz K.F., Pitacco P., Burdis R., Chariyev-Prinz F., Barceló X., Tornifoglio B., et al. Integrating melt electrowriting and fused deposition modeling to fabricate hybrid scaffolds supportive of accelerated bone regeneration. Adv. Healthcare Mater. 2023 doi: 10.1002/adhm.202302057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lim W.H., Liu B., Cheng D., Williams B.O., Mah S.J., Helms J.A. Wnt signaling regulates homeostasis of the periodontal ligament. J. Periodontal. Res. 2014;49(6):751–759. doi: 10.1111/jre.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wei X., Liu Q., Guo S., Wu Y. Role of Wnt5a in periodontal tissue development, maintenance, and periodontitis: implications for periodontal regeneration. Mol. Med. Rep. 2021;23(3) doi: 10.3892/mmr.2020.11806. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hosoya A., Shalehin N., Takebe H., Shimo T., Irie K. Sonic hedgehog signaling and tooth development. Int. J. Mol. Sci. 2020;21(5) doi: 10.3390/ijms21051587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Washio K., Tsutsumi Y., Tsumanuma Y., Yano K., Srithanyarat S.S., Takagi R., et al. In vivo periodontium formation around titanium implants using periodontal ligament cell sheet. Tissue Eng. 2018;24(15–16):1273–1282. doi: 10.1089/ten.TEA.2017.0405. [DOI] [PubMed] [Google Scholar]
- 94.Wu X., Gu Q., Chen X., Mi W., Wu T., Huang H. MiR-27a targets DKK2 and SFRP1 to promote reosseointegration in the regenerative treatment of peri-implantitis. J. Bone Miner. Res. 2019;34(1):123–134. doi: 10.1002/jbmr.3575. [DOI] [PubMed] [Google Scholar]
- 95.Gathani K.M., Raghavendra S.S. Scaffolds in regenerative endodontics: a review. Dent. Res. J. 2016;13(5):379–386. doi: 10.4103/1735-3327.192266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rakkiettiwong N., Hengtrakool C., Thammasitboon K., Kedjarune-Leggat U. Effect of novel chitosan-fluoroaluminosilicate glass ionomer cement with added transforming growth factor beta-1 on pulp cells. J. Endod. 2011;37(3):367–371. doi: 10.1016/j.joen.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 97.Kikuchi N., Kitamura C., Morotomi T., Inuyama Y., Ishimatsu H., Tabata Y., et al. Formation of dentin-like particles in dentin defects above exposed pulp by controlled release of fibroblast growth factor 2 from gelatin hydrogels. J. Endod. 2007;33(10):1198–1202. doi: 10.1016/j.joen.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 98.Richardson T.P., Peters M.C., Ennett A.B., Mooney D.J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 99.Del Angel-Mosqueda C., Gutiérrez-Puente Y., López-Lozano A.P., Romero-Zavaleta R.E., Mendiola-Jiménez A., Medina-De la Garza C.E., et al. Epidermal growth factor enhances osteogenic differentiation of dental pulp stem cells in vitro. Head Face Med. 2015;11:29. doi: 10.1186/s13005-015-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim J.Y., Xin X., Moioli E.K., Chung J., Lee C.H., Chen M., et al. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng Part A. 2010;16(10):3023–3031. doi: 10.1089/ten.tea.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu M., Goldman G., MacDougall M., Chen S. BMP signaling pathway in dentin development and diseases. Cells. 2022;11(14):2216. doi: 10.3390/cells11142216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nemoto E., Koshikawa Y., Kanaya S., Tsuchiya M., Tamura M., Somerman M.J., et al. Wnt signaling inhibits cementoblast differentiation and promotes proliferation. Bone. 2009;44(5):805–812. doi: 10.1016/j.bone.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 103.Seidi A., Ramalingam M., Elloumi-Hannachi I., Ostrovidov S., Khademhosseini A. Gradient biomaterials for soft-to-hard interface tissue engineering. Acta Biomater. 2011;7(4):1441–1451. doi: 10.1016/j.actbio.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 104.Gangolli R.A., Devlin S.M., Gerstenhaber J.A., Lelkes P.I., Yang M. A bilayered poly (lactic-Co-glycolic acid) scaffold provides differential cues for the differentiation of dental pulp stem cells. Tissue Eng Part A. 2019;25(3–4):224–233. doi: 10.1089/ten.TEA.2018.0041. [DOI] [PubMed] [Google Scholar]
- 105.Mousavi Nejad Z., Zamanian A., Saeidifar M., Vanaei H.R., Salar Amoli M. 3D bioprinting of polycaprolactone-based scaffolds for pulp-dentin regeneration: investigation of physicochemical and biological behavior. Polymers (Basel) 2021;13(24) doi: 10.3390/polym13244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown B.N., Chung W.L., Pavlick M., Reppas S., Ochs M.W., Russell A.J., et al. Extracellular matrix as an inductive template for temporomandibular joint meniscus reconstruction: a pilot study. J. Oral Maxillofac. Surg. 2011;69(12):e488–e505. doi: 10.1016/j.joms.2011.02.130. [DOI] [PubMed] [Google Scholar]
- 107.Camacho P., Fainor M., Seims K.B., Tolbert J.W., Chow L.W. Fabricating spatially functionalized 3D-printed scaffolds for osteochondral tissue engineering. J. Biol. Methods. 2021;8(1) doi: 10.14440/jbm.2021.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Camacho P., Behre A., Fainor M., Seims K.B., Chow L.W. Spatial organization of biochemical cues in 3D-printed scaffolds to guide osteochondral tissue engineering. Biomater. Sci. 2021;9(20):6813–6829. doi: 10.1039/d1bm00859e. [DOI] [PubMed] [Google Scholar]
- 109.Balshaw T.G., Funnell M.P., McDermott E.J., Maden-Wilkinson T.M., Massey G.J., Abela S., et al. The effect of specific bioactive collagen peptides on tendon remodeling during 15 wk of lower body resistance training. Med. Sci. Sports Exerc. 2023;55(11):2083–2095. doi: 10.1249/MSS.0000000000003242. [DOI] [PubMed] [Google Scholar]
- 110.Correa R., Arenas J., Montoya G., Hoz L., López S., Salgado F., et al. Synthetic cementum protein 1-derived peptide regulates mineralization in vitro and promotes bone regeneration in vivo. Faseb. J. 2019;33(1):1167–1178. doi: 10.1096/fj.201800434RR. [DOI] [PubMed] [Google Scholar]
- 111.Bermúdez M., Hoz L., Montoya G., Nidome M., Pérez-Soria A., Romo E., et al. Bioactive synthetic peptides for oral tissues regeneration. Front. Mater. 2021;8 [Google Scholar]
- 112.Culpepper B.K., Bonvallet P.P., Reddy M.S., Ponnazhagan S., Bellis S.L. Polyglutamate directed coupling of bioactive peptides for the delivery of osteoinductive signals on allograft bone. Biomaterials. 2013;34(5):1506–1513. doi: 10.1016/j.biomaterials.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yucesoy D.T., Fong H., Hamann J., Hall E., Dogan S., Sarikaya M. Biomimetic dentin repair: amelogenin-derived peptide guides occlusion and peritubular mineralization of human teeth. ACS Biomater. Sci. Eng. 2023;9(3):1486–1495. doi: 10.1021/acsbiomaterials.2c01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Poerio A., Mano J.F., Cleymand F. Advanced 3D printing strategies for the controlled delivery of growth factors. ACS Biomater. Sci. Eng. 2023;9(12):6531–6547. doi: 10.1021/acsbiomaterials.3c00873. [DOI] [PubMed] [Google Scholar]
- 115.Zhang X., Zhou J., Xu Y. Experimental study on preparation of coaxial drug-loaded tissue-engineered bone scaffold by 3D printing technology. Proc. IME H J. Eng. Med. 2020;234(4):309–322. doi: 10.1177/0954411919890422. [DOI] [PubMed] [Google Scholar]
- 116.Gao Q., He Y., Fu J-z, Liu A., Ma L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials. 2015;61:203–215. doi: 10.1016/j.biomaterials.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 117.dos Santos D.M., de Annunzio S.R., Carmello J.C., Pavarina A.C., Fontana C.R., Correa D.S. Combining coaxial electrospinning and 3D printing: design of biodegradable bilayered membranes with dual drug delivery capability for periodontitis treatment. ACS Appl. Bio Mater. 2022;5(1):146–159. doi: 10.1021/acsabm.1c01019. [DOI] [PubMed] [Google Scholar]
- 118.He W., Li C., Zhao S., Li Z., Wu J., Li J., et al. Integrating coaxial electrospinning and 3D printing technologies for the development of biphasic porous scaffolds enabling spatiotemporal control in tumor ablation and osteochondral regeneration. Bioact. Mater. 2024;34:338–353. doi: 10.1016/j.bioactmat.2023.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Falandt M., Bernal P.N., Dudaryeva O., Florczak S., Gröfibacher G., Schweiger M., et al. Spatial-selective volumetric 4D printing and single-photon grafting of biomolecules within centimeter-scale hydrogels via tomographic manufacturing. Adv. Mater Technol. 2023;8(15) doi: 10.1002/admt.202300026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang M., Li W., Mille L.S., Ching T., Luo Z., Tang G., et al. Digital light processing based bioprinting with composable gradients. Adv. Mater. 2022;34(1) doi: 10.1002/adma.202107038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li H., Dai J., Wang Z., Zheng H., Li W., Wang M., et al. Digital light processing (DLP)-based (bio)printing strategies for tissue modeling and regeneration. Aggregate. 2023;4(2) [Google Scholar]
- 122.Zhang M., Lin R., Wang X., Xue J., Deng C., Feng C., et al. 3D printing of Haversian bone–mimicking scaffolds for multicellular delivery in bone regeneration. Sci. Adv. 2020;6(12) doi: 10.1126/sciadv.aaz6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baba S., Yamada Y., Komuro A., Yotsui Y., Umeda M., Shimuzutani K., et al. Phase I/II trial of autologous bone marrow stem cell transplantation with a three-dimensional woven-fabric scaffold for periodontitis. Stem Cell. Int. 2016;2016 doi: 10.1155/2016/6205910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cochran D.L., Oh T.J., Mills M.P., Clem D.S., McClain P.K., Schallhorn R.A., et al. A randomized clinical trial evaluating rh-FGF-2/beta-TCP in periodontal defects. J. Dent. Res. 2016;95(5):523–530. doi: 10.1177/0022034516632497. [DOI] [PubMed] [Google Scholar]
- 125.Deshoju A.K., Chandra R.V., Reddy A.A., Reddy B.H., Nagarajan S., Naveen A. Efficacy of a novel Zn-substituted monetite-based scaffold in the treatment of periodontal osseous defects. J. Int. Acad. Periodontol. 2017;19(1):2–9. [PubMed] [Google Scholar]
- 126.Kadam S., Gautam S., Dwivedi A., Jain V. Treatment of gingival recession defect using human umbilical cord mesenchymal stem cells cultured on PCL based bone regenerating scaffold: a randomized controlled clinical study. Cytotherapy. 2019;21(5) [Google Scholar]
- 127.Abdal‐Wahab M., Abdel Ghaffar KA., Ezzatt O.M., Hassan A.A.A., El Ansary M.M.S., Gamal A.Y. Regenerative potential of cultured gingival fibroblasts in treatment of periodontal intrabony defects (randomized clinical and biochemical trial) J. Periodontal. Res. 2020;55(3):441–452. doi: 10.1111/jre.12728. [DOI] [PubMed] [Google Scholar]
- 128.Lee J.H., Kim D.H., Jeong S.N. Adjunctive use of enamel matrix derivatives to porcine-derived xenograft for the treatment of one-wall intrabony defects: two-year longitudinal results of a randomized controlled clinical trial. J. Periodontol. 2020;91(7):880–889. doi: 10.1002/JPER.19-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee C.H., Rodeo S.A., Fortier L.A., Lu C., Erisken C., Mao J.J. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci. Transl. Med. 2014;6(266) doi: 10.1126/scitranslmed.3009696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakagawa Y., Fortier L.A., Mao J.J., Lee C.H., Goodale M.B., Koff M.F., et al. Long-term evaluation of meniscal tissue formation in 3-dimensional-Printed scaffolds with sequential release of connective tissue growth factor and TGF-beta3 in an ovine model. Am. J. Sports Med. 2019;47(11):2596–2607. doi: 10.1177/0363546519865513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim S.H., Park J.H., Kwon J.S., Cho J.G., Park K.G., Park C.H., et al. NIR fluorescence for monitoring in vivo scaffold degradation along with stem cell tracking in bone tissue engineering. Biomaterials. 2020;258 doi: 10.1016/j.biomaterials.2020.120267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jin S., Guerrero-Juarez C.F., Zhang L., Chang I., Ramos R., Kuan C.-H., et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 2021;12(1):1088. doi: 10.1038/s41467-021-21246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jerby-Arnon L., Regev A. DIALOGUE maps multicellular programs in tissue from single-cell or spatial transcriptomics data. Nat. Biotechnol. 2022;40(10):1467–1477. doi: 10.1038/s41587-022-01288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang X., Wang D., Mak K.-L.K., Tuan R.S., Ker D.F.E. Engineering musculoskeletal grafts for multi-tissue unit repair: lessons from developmental biology and wound healing. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.691954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pavlukhina S., Sukhishvili S. Polymer assemblies for controlled delivery of bioactive molecules from surfaces. Adv. Drug Deliv. Rev. 2011;63(9):822–836. doi: 10.1016/j.addr.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 136.Mantha S., Pillai S., Khayambashi P., Upadhyay A., Zhang Y., Tao O., et al. Smart hydrogels in tissue engineering and regenerative medicine. Materials (Basel) 2019;12(20) doi: 10.3390/ma12203323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cousin M.A., Greenberg A.J., Koep T.H., Angius D., Yaszemski M.J., Spinner R.J., et al. The value of systematic reviews in estimating the cost and barriers to translation in tissue engineering. Tissue Eng. Part B Rev. 2016;22(6):430–437. doi: 10.1089/ten.teb.2016.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schcolnik-Cabrera A., Juárez-López D., Duenas-Gonzalez A. Perspectives on drug repurposing. Curr. Med. Chem. 2021;28(11):2085–2099. doi: 10.2174/0929867327666200831141337. [DOI] [PubMed] [Google Scholar]
- 139.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]