Abstract
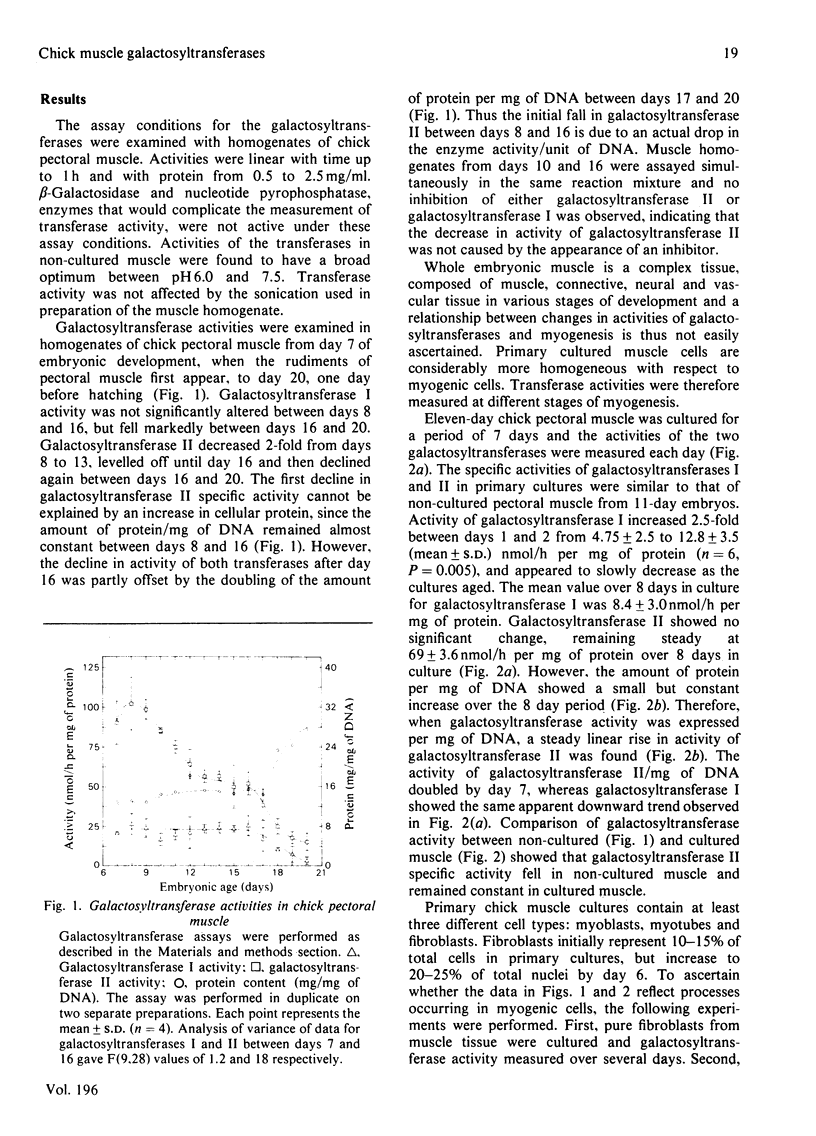
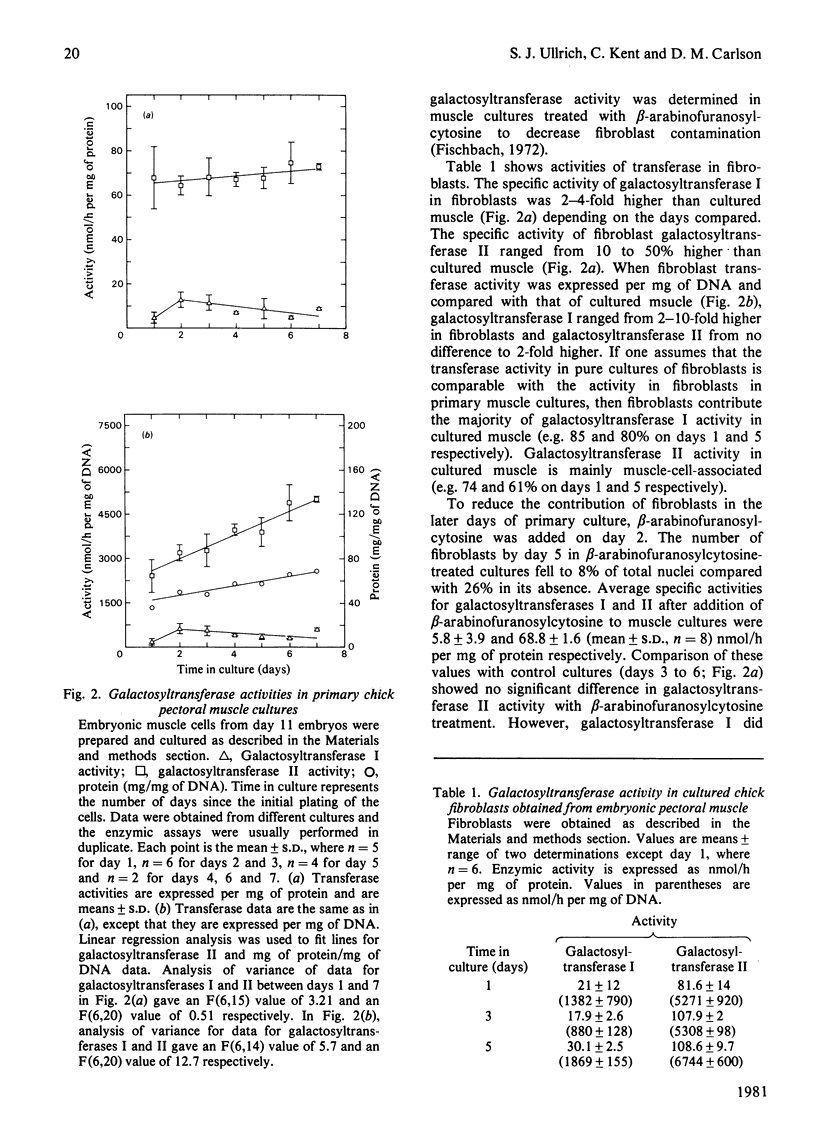
The two major vertebrate galactosyltransferases have been investigated in developing chick muscle in ovo and in vitro, and in cultured chick fibroblasts. The two enzymes were UDP-galactose-N-acetylglucosamine galactosyltransferase (galactosyltransferase I) and UDP-galactose-N-acetylgalactosamine galactosyltransferase (galactosyltransferase II). Both activities fell during muscle development in ovo. Galactosyltransferase I activity was constant from day 7 to day 16, after which it declined 5-fold, whereas galactosyltransferase II activity fell markedly from day 9 to 13 and 16 to 20, displaying an overall 8-fold decrease. In primary muscle cultures, galactosyltransferase I activity fell slightly during 7 days in culture, whereas galactosyltransferase II increased 2-fold during the same period. No significant change in activity of either galactosyltransferase was observed during intercellular recognition and fusion. Analysis of muscle cultures treated with cytosine arabinoside and of fibroblast cultures revealed that the majority of galactosyltransferase I activity in primary muscle cultures is associated with fibroblasts, whereas the majority of galactosyltransferase II activity is muscle-associated. The addition of 5-bromodeoxyuridine to primary muscle cultures resulted in a 3-fold rise in activities of both transferases.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott J., Mayne R., Holtzer H. Inhibition of cartilage development in organ cultures of chick somites by the thymidine analog, 5-bromo-2'-deoxyuridine. Dev Biol. 1972 Jun;28(2):430–442. doi: 10.1016/0012-1606(72)90024-3. [DOI] [PubMed] [Google Scholar]
- Bischoff R., Holtzer H. Inhibition of myoblast fusion after one round of DNA synthesis in 5-bromodeoxyuridine. J Cell Biol. 1970 Jan;44(1):134–150. doi: 10.1083/jcb.44.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson D. M., David J., Rutter W. J. Galactosyltransferase activities in pancreas, liver and gut of the developing rat. Arch Biochem Biophys. 1973 Aug;157(2):605–612. doi: 10.1016/0003-9861(73)90680-2. [DOI] [PubMed] [Google Scholar]
- Cates G. A., Holland P. C. Biosynthesis of plasma-membrane proteins during myogenesis of skeletal muscle in vitro. Biochem J. 1978 Sep 15;174(3):873–881. doi: 10.1042/bj1740873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B. Alteration in cell surface LETS protein during myogenesis. Cell. 1977 Mar;10(3):393–400. doi: 10.1016/0092-8674(77)90026-5. [DOI] [PubMed] [Google Scholar]
- Den H., Malinzak D. A., Rosenberg A. Lack of evidence for the involvement of a beta-D-galactosyl-specific lectin in the fusion of chick myoblasts. Biochem Biophys Res Commun. 1976 Apr 5;69(3):621–627. doi: 10.1016/0006-291x(76)90921-9. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D. Synapse formation between dissociated nerve and muscle cells in low density cell cultures. Dev Biol. 1972 Jun;28(2):407–429. doi: 10.1016/0012-1606(72)90023-1. [DOI] [PubMed] [Google Scholar]
- Garfield S., Ilan J. Galactosyltransferase activities during embryonic development of chick neural tissue. Biochim Biophys Acta. 1976 Aug 24;444(1):154–163. doi: 10.1016/0304-4165(76)90232-4. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Podleski T. R. Evidence that a membrane bound lectin mediates fusion of L6 myoblasts. Biochem Biophys Res Commun. 1975 Dec 1;67(3):972–978. doi: 10.1016/0006-291x(75)90770-6. [DOI] [PubMed] [Google Scholar]
- Jeffrey P. L., Appel S. H. Denervation alterations in surface membrane glycoprotein glycosyltransferases of mammalian skeletal muscle. Exp Neurol. 1978 Sep 1;61(2):432–441. doi: 10.1016/0014-4886(78)90258-3. [DOI] [PubMed] [Google Scholar]
- Kent C. Stimulation of phospholipid metabolism in embryonic muscle cells treated with phospholipase C. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4474–4478. doi: 10.1073/pnas.76.9.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent C., Truesdale N. J., Constantine L. A. 5'-nucleotidase activity in developing chick pectoral muscle. Exp Cell Res. 1979 Jun;121(1):63–70. doi: 10.1016/0014-4827(79)90444-0. [DOI] [PubMed] [Google Scholar]
- Kobiler D., Barondes S. H. Lectin activity from embryonic chick brain, heart, and liver: changes with development. Dev Biol. 1977 Oct 1;60(1):326–330. doi: 10.1016/0012-1606(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Leyva A., Jr, Kelley W. N. Measurement of DNA in cultured human cells. Anal Biochem. 1974 Nov;62(1):173–179. doi: 10.1016/0003-2697(74)90378-9. [DOI] [PubMed] [Google Scholar]
- McEvoy F. A., Ellis D. E. Glycolipids and myoblast differentiation [proceedings]. Biochem Soc Trans. 1977;5(6):1719–1721. doi: 10.1042/bst0051719. [DOI] [PubMed] [Google Scholar]
- McLaughlin J., Bosmann H. B. Glycolipid and glycoprotein sialytransferase enzyme activity in denervated skeletal muscle. Exp Neurol. 1975 Jun;47(3):381–391. doi: 10.1016/0014-4886(75)90071-0. [DOI] [PubMed] [Google Scholar]
- Moss M., Norris J. S., Peck E. J., Jr, Schwartz R. J. Alterations in iodinated cell surface proteins during myogenesis. Exp Cell Res. 1978 May;113(2):445–450. doi: 10.1016/0014-4827(78)90388-9. [DOI] [PubMed] [Google Scholar]
- O'Neill M. C., Stockdale F. E. 5-Bromodeoxyuridine inhibition of differentiation. Kinetics of inhibition and reversal in myoblasts. Dev Biol. 1974 Mar;37(1):117–132. doi: 10.1016/0012-1606(74)90173-0. [DOI] [PubMed] [Google Scholar]
- Puri E. C., Turner D. C. Serum-free medium allows chicken myogenic cells to be cultivated in suspension and separated from attached fibroblasts. Exp Cell Res. 1978 Aug;115(1):159–173. doi: 10.1016/0014-4827(78)90413-5. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Shur B. D., Bennett D. A specific defect in galactosyltransferase regulation on sperm bearing mutant alleles of the T/t locus. Dev Biol. 1979 Aug;71(2):243–259. doi: 10.1016/0012-1606(79)90167-2. [DOI] [PubMed] [Google Scholar]
- Turkington R. W., Brew K., Vanaman T. C., Hill R. L. The hormonal control of lactose synthetase in the developing mouse mammary gland. J Biol Chem. 1968 Jun 25;243(12):3382–3387. [PubMed] [Google Scholar]
- Turkington R. W., Majumder G. C., Riddle M. Inhibition of mammary gland differentiation in vitro by 5-bromo-2'-deoxyuridine. J Biol Chem. 1971 Mar 25;246(6):1814–1819. [PubMed] [Google Scholar]
- Weeds A. G., Trentham D. R., Kean C. J., Buller A. J. Myosin from cross-reinnervated cat muscles. Nature. 1974 Jan 18;247(5437):135–139. doi: 10.1038/247135a0. [DOI] [PubMed] [Google Scholar]
- Whatley R., Ng S. K., Rogers J., McMurray W. C., Sanwal B. D. Developmental changes in gangliosides during myogenesis of a rat myoblast cell line and its drug resistant variants. Biochem Biophys Res Commun. 1976 May 3;70(1):180–185. doi: 10.1016/0006-291x(76)91125-6. [DOI] [PubMed] [Google Scholar]
- Winand R., Luzzati D. Cell surface changes during myoblast differentiation: preparation and carbohydrate composition of plasma membranes. Biochimie. 1975;57(6-7):764–771. [PubMed] [Google Scholar]


