Abstract
Obstructive sleep apnea (OSA) has been reported to influence the ocular surface and may lead to dry eye disease (DED). Continuous positive airway pressure (CPAP) is the first-line conservative treatment for OSA. However, CPAP might also have mask-related side effects that could deteriorate DED simultaneously. This study investigated the impact of OSA on DED (Aim 1), and CPAP on DED (Aim 2). Five databases were searched for articles published up to May, 2024. OSA severity, CPAP usage, and DED parameters, including tear breakup time (TBUT), Schirmer test, Ocular Surface Disease Index (OSDI), and Corneal Fluorescence Staining Score (CFS), were analyzed. For Aim 1, the random-effects model was used for meta-analysis, and the leave-one-out method was used for sensitivity analysis. For Aim 2, a narrative synthesis with critical appraisal of the literature was performed. Eleven studies with 1,526 patients for Aim 1 and three studies with 180 patients for Aim 2 were included. For Aim 1, OSA patients had poorer dry eye profiles of TBUT, Schirmer test, and OSDI when compared to non-OSA patients. For Aim 2, it seemed that those wearing CPAP for less than half a year did not have enough improvement in dry eye status. Instead, those wearing CPAP for at least a year reached greater therapeutic effects for OSA and DED. We concluded that OSA patients may suffer from poorer dry eye condition compared to non-OSA patients. Besides, wearing CPAP for long enough duration (at least 1 year) seemed to have better improvement in DED.
Keywords: Obstructive sleep apnea, continuous positive airway pressure, dry eye disease, meta-analysis
Introduction
Obstructive sleep apnea (OSA) is a worldwide disease characterized as intermittent airway collapse that contributes to repetitive airflow reduction or cessation during sleep.1 Emerging evidence has proved that OSA is not merely a single-organ disease, but a systemic disorder that could generate profound adverse impact on human’s health, including but are not limited to metabolic factors dysregulation,2 cardio- and cerebrovascular diseases,3 gut barrier dysfunction,4 cognitive impairment,5 sexual dysfunction,6 and changes in ocular microstructure.7 Treatment for OSA can not only improve sleep quality, result in alterations of metabolic and body status but also further lead to better quality of life.8–13
For these vulnerable patients, nasal continuous positive airway pressure (CPAP) is widely seen as the first-line treatment, which is non-invasive and efficient in reducing upper airway resistance, and contributes to better respiration.14 Despite its benefits, long-term use of CPAP in OSA patients may be accompanied with mask-related side-effects, in which unintentional air leakage being the most prevalent;15,16 the air-leaking effect of the mask edge could potentially lead to dry mouth, dry nose, or even dry eye.16,17 Interestingly, some previous studies have also reported that OSA may be an independent risk factor for dry eye disease (DED),18 which is also a worldwide health concern for ophthalmologists. The pathophysiology of DED includes majorly decreased production of tear and increase of evaporation; and usually, these can be due to chronic inflammation of the ocular surface resulting from dysfunction or disorders of lacrimal gland, conjunctiva, and cornea, which may be secondary to various clinical situations and systemic disease as well.19,20 Despite being multifactorial, the exact impact and mechanisms between OSA and DED have not yet been fully investigated.
Given the therapeutic effect of CPAP on OSA,3 DED may also ameliorate along with the improvement of OSA severity theoretically. However, this is paradoxical to the statement that long-term CPAP use may be related to worsening of dry eye due to inevitable air leakage.17 To study further about the complex relationship among OSA, CPAP use, and DED, the present study aimed to synthesize the currently available literature to understand more about the effect of OSA on DED, and CPAP on DED.
Materials and Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline21 was followed. Additionally, we registered the study to the International Prospective Register of Systematic Reviews (PROSPERO); the registration number was CRD42024503983.
Literature Search and Selection
Two of the authors (J.D.L. and Y.C.S.) systematically searched five databases, including PubMed, Embase, ScienceDirect, the Cochrane Library, and Web of Science, for relevant articles on May 1st, 2024. Two aims were set, including the effect of OSA on DED (Aim 1), and the effect of CPAP use in OSA patients on DED (Aim 2). The major keywords for searching in Aim 1 were (“OSA” OR “obstructive sleep apnea” OR “sleep breathing disorder”) AND (“dry eye” OR “ocular surface”), and the major keywords for searching in Aim 2 were (“CPAP” OR “positive airway pressure”) AND (“dry eye” OR “ocular surface”); a detailed searching strategy is presented in Supplementary Table 1. There was no restriction on language or publish date of the literature. The references of the enrolled articles were also viewed for including extra studies if necessary. After removing duplicates, two authors (J.D.L. and Y.C.S.) independently screened the articles for eligibility, and enrolled the final eligible literature. In both of the aims, all of the final enrolled studies underwent qualitative analysis. However, to guarantee better statistical power, we only performed quantitative analysis in each of the aim if the number of study (which could provide extractable data) exceeded five, and every single study should contain a control group for comparison (ie Aim 1: non-OSA patients; Aim 2: non-CPAP patients) to ensure promising statistical evidence.
The inclusion criteria for both aims were (i) Prospective and retrospective cohort studies, case–control studies, cross-sectional studies, and clinical trials (however, in Aim 1, clinical trials were not enrolled); (ii) Articles with abstract and full-text for critical appraisals; (iii) Studies reporting OSA diagnosed by apnea–hypopnea index (AHI/hr).; and (iv) Studies providing dry eye data. The exclusion criteria for both aims were (i) Studies not clearly stating whether OSA was measured or diagnosed by polysomnography (PSG) (eg by ICD codes, questionnaire); (ii) Patients receiving dry eye medication or ocular surgery within 6 months before dry eye evaluation; and (iii) Patients with other concurrent underlying conjunctival, corneal, lacrimal gland diseases, or systemic disease that may directly influence dry eye evaluation (eg Sjögren disease) within 6 months before dry eye assessment; besides, for Aim 2, (iv) Studies reporting patients receiving OSA treatment other than CPAP (such as oral appliances, and any type of sleep surgery) were also excluded. Any inconsistency during the process was discussed with a third author (P.W.L.).
Data Extraction
One author (C.W.L.) extracted the required data, which was cross-examined by the other two authors (Y.C.S. and S.C.S.). For unextractable data, we also contacted the authors of each study for original data if possible. The data for both aims included: (i) Name of the first author and the publication year; (ii) Country or region of the study; (iii) Sample size; (iv) Number of OSA patients (including the distribution based on OSA severity); (v) Number of non-OSA patients; (vi) Data of 4 common dry eye assessment tool, including tear breakup time (TBUT), Schirmer test, Ocular Surface Disease Index (OSDI), and Corneal Fluorescence Staining Score by Oxford grading system (CFS); and (vii) Treatment for OSA. For Aim 2, we also extracted data of (viii) Length of CPAP use for OSA patients.
Following are the details of the dry eye profiles:22 TBUT indicates the time duration from tear secretion to the first tear film break (within normal: < 5 sec). Schirmer test is conducted with a filter paper put inside the lower eyelid without anesthesia; the paper is removed 5 min later and is tested for the distance of wetting (within normal: < 15 mm). OSDI is a validated questionnaire based on subjective feeling of dry eye symptoms, which is composed of twelve questions and a total of one-hundred points (within normal: < 13 points). CFS uses punctate fluorescein staining to detect the degree of corneal surface damage, and is rated by Oxford grading system (from 0 (absent) to 5 (severe)). For all of the studies, we only extracted the data of the above dry eye parameters but not the exact percentage of DED of each single study (if available) for further data synthesis. This is due to the inconsistency of the diagnostic criteria for DED in different medical settings; therefore, direct statistical data pooling may generate imprecise results.
Data Processing
For studying the effect of OSA on DED, data of each dry eye profile was compared between OSA patients and non-OSA patients; for studying the effect of CPAP on DED, data of each dry eye profile was compared between CPAP patients and non-CPAP patients. The effect size was weighted mean difference with a 95% confidence interval (CI). The severity of OSA was derived from the American Academy of Sleep Medicine (AASM) guidelines,23 in which the definitions based on AHI (events/hr) were: “<5”: non-OSA; “5 to <15”: mild OSA; “15 to <30”: moderate OSA; and “≥30”: severe OSA.
We performed qualitative analysis to critically appraise the literature for both aims. If a further quantitative analysis was performed, the random-effects model was more statistically appropriate and was adopted for pooling data considering the expected population variation in the real-world settings. The proportion of heterogeneity was calculated with the I2 statistic. Sensitivity analysis by “leave-one-out” method and subgroup analysis stratified by OSA severity and study design would be performed for determining the heterogeneity. To better determine the heterogeneity changes, we performed subgroup analysis only when more than three studies were included in each of the subgroup. Publication bias would be analyzed with Egger’s test and demonstrated as funnel plot if the available studies exceeded ten for achieving better power.24 All of the statistical data were processed with the Cochrane RevMan 5.4 software (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark).
Risk of Bias Assessment & Quality of Evidence
Two authors (Y.C.S. and S.C.S.) independently assessed the risk of bias of the studies and quality of evidence. The Newcastle-Ottawa Scale (NOS) was used for assessing the cohort studies and case–control studies;25 the modified NOS was used for assessing the cross-sectional studies.26 For clinical trials, the Cochrane risk of bias 2 (RoB 2) tool was used.27 The Grading of the Recommendations, Assessment, Development, and Evaluations (GRADE) system was used for evaluating the quality of evidence.28 Any discrepancy was discussed with a third author (H.C.L.).
Results
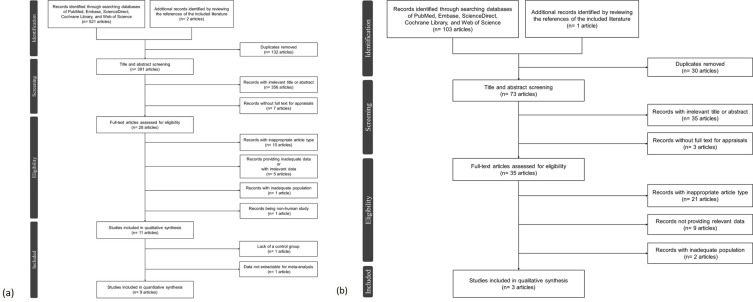
A total of 627 articles (523 for Aim 1 and 104 for Aim 2) were collected after the initial search of the databases and the reference (Figure 1). Eventually, thirteen studies29–41 with 1,617 patients were included (eleven studies29–39 with 1,526 patients for studying the effect of OSA on DED, and three studies33,40,41 with 180 patients for studying the effect of CPAP on DED; the study by Kadyan et al33 analyzed both aims, and was therefore enrolled in both groups) after excluding the ineligible articles. The above-mentioned thirteen studies were all evaluated and processed with qualitative analysis; however, two of the studies investigating the effect of OSA on DED were excluded for further quantitative analysis despite meeting the entire inclusion criteria (one of the studies39 was due to lack of a control group for further comparisons, and one of these studies38 was due to unextractable statistical data for synthesis).
Figure 1.
(a) The PRISMA flowchart of the literature selection regarding the impact of OSA on DED. (b) The PRISMA flowchart of the literature selection regarding the impact of CPAP on DED.
The Impact of OSA on DED
General results
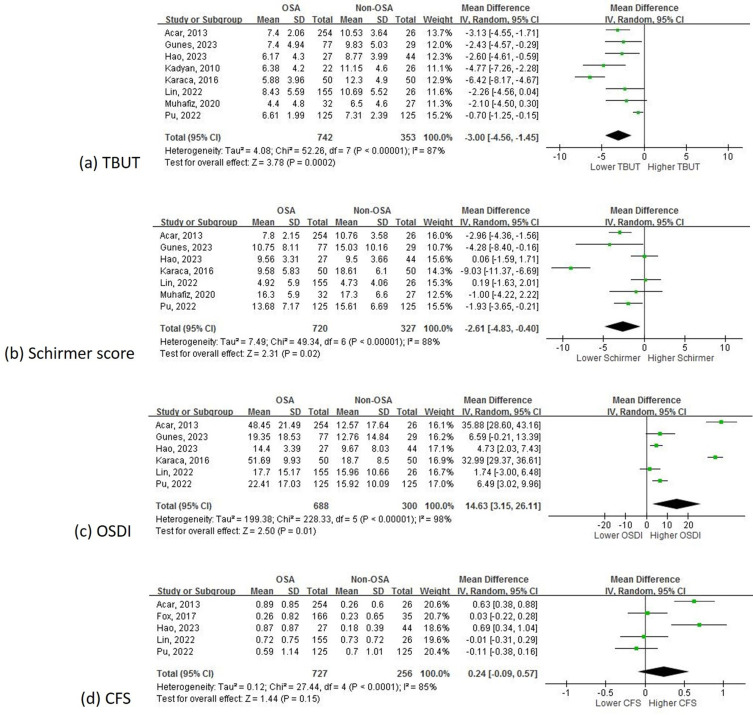
Among the 1,526 patients in the eleven included studies (Table 1), which consisted of six cohort studies,29,33–36,39 four cross-sectional studies,30–32,38 and one case-control study,37 qualitative analysis generally revealed poorer dry eye profiles along with OSA severity; 1,363 of these patients (in nine out of the eleven included studies29–37) were further studied with quantitative analysis. The pooled statistical results (Table 2 and Figure 2a-d) showed that except for CFS (OSA versus non-OSA: mean difference (95% CI): 0.24 (−0.09, 0.57); p = 0.15), the rest of the dry eye parameters, including TBUT (OSA versus non-OSA: mean difference (sec; 95% CI): −3.00 (−4.56, −1.45); p < 0.01), Schirmer test (OSA versus non-OSA: mean difference (mm; 95% CI): −2.61 (−4.83, −0.40); p = 0.02), and OSDI (OSA versus non-OSA: mean difference (95% CI): 14.63 (3.15, 26.11); p = 0.01), were significantly poorer in OSA patients when compared with non-OSA patients.
Table 1.
Characteristics of the Included Studies for Studying the Effect of OSA on DED
| Study | Country & region | Study type | Mean age (year) | Gender (male;%) | BMI (kg/m2) | Sample size | OSA patients | Non-OSA patients (control) | Dry eye assessment tool | Treatment for OSA patients | General results (OSA patients) | Enrolled for quantitative analysisa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acar, 2013 29 | Turkey | Prospective cohort | 48.1 ± 10.5 | 70.4 | Non-OSA: 28.3 ± 4.7; OSA: 31.5 ± 5.9 | 280 | 254 (mild: 60; moderate: 72; severe: 122) | 26 | TBUT, Schirmer test, OSDI, CFS | - | Poorer dry eye data among OSA subgroups (all parameters) | v |
| Fox, 2017 30 | US | Cross- sectional |
53.2 ± 13.5 | 56.7 | Non-OSA: 31.0 ± 12.5; OSA: 33.0 ± 6.8 | 201 | 166 (mild: 33; moderate: 70; severe: 63) | 35 | CFS | - | No significant impact on CFS among OSA subgroups | v |
| Gunes, 2023 31 | Turkey | Cross- sectional |
47.1 ± 12.0 | 68.9 | - | 106 | 77 (mild: 23; moderate: 27; severe: 27) | 29 | TBUT, Schirmer test, OSDI | Nil | Poorer dry eye data among OSA subgroups (all parameters) | v |
| Hao, 2023 32 | China | Cross- sectional |
39.7 ± 17.8 | 66.2 | Non-OSA: 26.6 ± 2.0; OSA: 30.5 ± 4.9 | 71 | 27 (mild and moderate: 14; severe: 13) | 44 | TBUT, Schirmer test, OSDI, CFS | - | Poorer dry eye data among OSA subgroups (most parameters); no significant impact on Schirmer test | v |
| Kadyan, 2010 33 | UK | Prospective cohort | 55.6 ± 10.9 | 80.0 | Non-OSA: 32.9 ± 5.2; OSA: 35.0 ± 7.2 | 115 | 89 (all were moderate to severe) | 26 | TBUT | CPAP (67/89) | Poorer dry eye data (all parameters) | v |
| Karaca, 2016 34 | Turkey | Cohort | 47.5 ± 11.5 | 67.0 | Non-OSA: 25.9 ± 3.6; OSA: 30.4 ± 5.0 | 100 | 50 (mild: 15; moderate: 15; severe: 20) | 50 | TBUT, Schirmer test, OSDI | Nil | Poorer dry eye data (all parameters) | v |
| Lin, 2022b 35 | Taiwan | Prospective cohort | 40.9 ± 10.0 | 69.6 | Non-OSA: 23.7 ± 3.6; OSA: 26.5 ± 3.9 | 181 | 155 (mild: 53; moderate: 42; severe: 60) | 26 | TBUT, Schirmer test, OSDI, CFS | Nil | Poorer dry eye data among OSA subgroups (TBUT); no significant impact on Schirmer test, OSDI, and CFS | v |
| Muhafiz, 2020 36 | Turkey | Prospective cohort | 46.3 ± 10.8 | - | - | 59 | 32 (mild: 9; moderate: 6; severe: 17) | 27 | TBUT, Schirmer test | Nil | Poorer TBUT in OSA patients; no significant impact on Schirmer test | v |
| Pu, 2022 37 | China | Case- control |
53.9 ± 13.0 | 61.6 | Non-OSA: 26.6 ± 4.9; OSA: 30.4 ± 5.5 | 250 | 125 (mild: 32; moderate: 54; severe: 39) | 125 | TBUT, Schirmer test, OSDI, CFS | - | Poorer dry eye data among OSA subgroups (all parameters) | v |
| Karaca, 2019 38 | Turkey | Cross- sectional |
49.6 ± 9.3 | 82.0 | Mixeda | 60 | Mixeda (simple snoring and mild: 24; severe: 36) | TBUT, Schirmer test, OSDI, CFS | Nil | Severe-OSA patients had significantly poorer TBUT only when compared to mild- and non-OSA patients; no significant difference in the rest of the parameters | - | |
| Liu, 2022 39 | China | Cohort | 38.9 ± 8.8 | 93.2 | 27.2 ± 3.1 | 103 | 103 (mild: 21; moderate: 20; severe: 62) | - | TBUT, Schirmer test, OSDI, CFS | Nil | Severe-OSA patients had the poorest TBUT, Schirmer test, and CFS; no significant impact on OSDI | - |
Notes: aFor studies not suitable for quantitative analysis despite meeting the whole selection criteria, one study (Liu et al, 2022) is due to lack of a control group for further comparison; one study (Karaca et al, 2019) is due to unextractable statistical data for synthesis. bOriginal data obtained from author.
Abbreviations: OSA, obstructive sleep apnea; DED, dry eye disease; TBUT, tear breakup time; OSDI, ocular surface disease index; CFS, corneal fluorescence-staining score (Oxford grading system); CPAP, continuous positive airway pressure.
Table 2.
Subgroup Analysis of Dry Eye Profiles Based on OSA Severity When Comparing with Non-OSA Patients
| Dry Eye Profiles | OSA Subgroup | Study Number | Sample Size | Mean Difference (95% CI)a | P value |
|---|---|---|---|---|---|
| TBUT (sec) | Overall | 8 | 1,095 | −3.00 (−4.56, −1.45) | <0.01 |
| Mild OSA | 5 | 439 | −0.66 (−1.98, 0.67) | 0.33b | |
| Moderate OSA | 5 | 466 | −3.40 (−5.82, −0.99) | <0.01 | |
| Severe OSA | 6 | 581 | −4.13 (−6.11, −2.14) | <0.01 | |
| Schirmer (mm) | Overall | 7 | 1,047 | −2.61 (−4.83, −0.40) | 0.02 |
| Mild OSA | 5 | 439 | −0.92 (−2.48, 0.65) | 0.25b | |
| Moderate OSA | 5 | 466 | −3.37 (−6.70, −0.04) | 0.05 | |
| Severe OSA | 6 | 581 | −4.18 (−7.18, −1.19) | <0.01 | |
| OSDI | Overall | 6 | 988 | 14.63 (3.15, 26.11) | 0.01 |
| Mild OSA | 5 | 439 | 6.99 (−4.08, 18.06) | 0.22b | |
| Moderate OSA | 5 | 466 | 15.77 (0.94, 30.61) | 0.04 | |
| Severe OSA | 6 | 581 | 19.68 (0.96, 38.40) | 0.04 | |
| CFS | Overall | 5 | 983 | 0.24 (−0.09, 0.57) | 0.15 |
| Mild OSA | 4 | 390 | −0.10 (−0.38, 0.18) | 0.49b | |
| Moderate OSA | 4 | 450 | 0.11 (−0.34, 0.55) | 0.63 | |
| Severe OSA | 5 | 553 | 0.45 (0.03, 0.87) | 0.04 |
Notes: a“OSA subgroup” minus “non-OSA patients”. bDecreased heterogeneity after subgroup analysis.
Abbreviations: OSA, obstructive sleep apnea; TBUT, tear breakup time; OSDI, ocular surface disease index; CFS, corneal fluorescence-staining score (Oxford grading system).
Figure 2.
(a) The results of meta-analysis regarding TBUT when comparing OSA patients to non-OSA patients. (b) The results of meta-analysis regarding Schirmer test when comparing OSA patients to non-OSA patients. (c) The results of meta-analysis regarding OSDI when comparing OSA patients to non-OSA patients. (d) The results of meta-analysis regarding CFS when comparing OSA patients to non-OSA patients.
Sensitivity Analysis, Subgroup Analysis & Publication Bias
The leave-one-out sensitivity analysis generally showed no changes to the main results. Subgroup analysis based on OSA severity (Supplementary Figures 1- 4a-c) revealed that in all of the dry eye parameters when compared with non-OSA patients, those with severe OSA had the worst ocular performance. Specifically, in patients with mild OSA, parameters including TBUT (p = 0.33), Schirmer test (p = 0.25), OSDI (p = 0.22), and CFS (p = 0.49) were not significantly different from non-OSA patients; on the other hand, patients with severe OSA had substantially poorer TBUT (mean difference (95% CI): −4.13 (−6.11, −2.14); p < 0.01), Schirmer test (mean difference (95% CI): −4.18 (−7.18, −1.19); p < 0.01), OSDI (mean difference (95% CI): 19.68 (0.96, 38.40); p = 0.04), and CFS (mean difference (95% CI): 0.45 (0.03, 0.87); p = 0.04) compared to those not diagnosed with OSA. Regarding patients with moderate OSA, only TBUT (mean difference (95% CI): −3.40 (−5.82, −0.99); p < 0.01) and OSDI (mean difference (95% CI): 15.77 (0.94, 30.61); p = 0.04) were significantly poorer than non-OSA patients.
Among all the above results of subgroup analyses, we found that heterogeneity dropped significantly when comparing mild-OSA patients to non-OSA patients in TBUT (I2 from 87% to 60%) and Schirmer test (I2 from 88% to 42%). As for the subgroup analysis based on study design (Supplementary Figures 1- 4d-e) for determining the heterogeneity changes (in comparing dry eye profiles in patients with OSA to non-OSA), statistical synthesis stratified by prospective cohort studies had substantial decrease in heterogeneity in TBUT (I2 from 87% to 0%) and Schirmer test (I2 from 88% to 73%), while cross-sectional studies had significant decrease in heterogeneity in TBUT (I2 from 87% to 0%) and OSDI (I2 from 98% to 0%). Publication bias was not further analyzed since only nine studies were eligible for the quantitative synthesis.
Risk of Bias & Quality of Evidence
The included studies generally did not have high risk of bias according to the assessment (Supplementary Table 2). The quality of evidence was inevitably low due to the baseline rating resulting from the observational study design; however, the rest of the subjects did not show serious concerns based on the evaluation (Supplementary Table 3).
The Impact of CPAP on DED
General Results
Three studies, including two prospective cohort studies33,41 and one randomized controlled trial (RCT),40 with 180 patients were included for studying the effect of CPAP on DED (Table 3). The qualitative analysis showed that TBUT was used to evaluate the dry eye condition in all of the studies, while Schirmer test and CFS were used in two,40,41 respectively. OSDI was used in only one study.40 As for the duration of CPAP use, patients in all of the studies received CPAP treatment for at least 3 months; moreover, in the study by Acar et al40 and Kadyan et al,33 patients with OSA had worn CPAP for more than 1 year. Among the dry eye parameters, significant improvement of TBUT, Schirmer test, and OSDI was shown in all of the reported studies after wearing CPAP. Nonetheless, CFS improved after CPAP treatment in only one of the two studies.40
Table 3.
Characteristics of the included studies for studying the effect of CPAP use on DED
| Study | Country & Region | Study type | Mean Age (Year) | Gender (Male;%) | BMI (kg/m2) | Samples | OSA Patients Using CPAP | OSA Patients NOT Using or Haven’t Yet Using CPAP | Ocular profiles | Length of Use (for Patients Using CPAP) | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acar, 201440 | Turkey | RCT | 48.12 ± 10.54 | 62.8 | - | 51a (moderate OSA: 17; severe OSA: 34) | 51 | 51 | TBUT, Schirmer test, OSDI, CFS | 18 months | TBUT: 7.11 ± 1.82 (sec) before CPAP and 8.68 ± 1.76 (sec) after CPAP (p<0.01) Mean difference (sec)=1.57 (95%CI: [0.88, 2.66]) Schirmer test: 7.23 ± 1.95 (mm) before CPAP and 8.49 ± 1.79 (mm) after CPAP (p<0.01) Mean difference (mm)=1.26 (95%CI: [0.53, 1.99]) OSDI: 47.79 ± 21.04 before CPAP and 42.17 ± 19.97 after CPAP (p<0.01) Mean difference=-5.62 (95%CI: [-13.58, 2.34]) CFS: 1.05 ± 0.75 before CPAP and 0.68 ± 0.54 after CPAP (p<0.01) Mean difference=-0.37 (95%CI: [-0.62, -0.12]) |
| Hayirci, 201241 | Turkey | Prospective cohort | 52.48 ± 9.5 | 80.0 | 34.48 ± 7.51 | 40a (not reporting patient distribution based on OSA severity) | 40 | 40 | TBUT, Schirmer test, CFS | 4 months | TBUT: 7.63 ± 4.03 (sec) before CPAP and 6.75 ± 3.59 (sec) after CPAP (p=0.036) Mean difference (sec)=-0.88 (95%CI: [-2.55, 0.79]) Schirmer test: 14.66 ± 6.54 (mm) before CPAP and 16.80 ± 8.36 (mm) after CPAP (p=0.007) Mean difference (mm)=2.14 (95%CI: [-1.15, 5.43]) CFS: 1.28 ± 0.50 before CPAP and 1.36 ± 0.25 after CPAP (p=0.210) Mean difference=0.08 (95%CI: [-0.09, 0.25]) |
| Kadyan, 201033 | UK | Prospective cohort | 55.8 ± 11.0 | 83.1 | Non-CPAP: 36.55 ± 8.7; CPAP: 34.5 ± 6.59 | 89 (not reporting patient distribution based on OSA severity) | 67 | 22 | TBUT | 19.6 ± 15.3 months | TBUT: 6.38 ± 4.20 (sec) for not using CPAP and 9.23 ± 7.39 (sec) for using CPAP (p=0.029) Mean difference (sec)=2.85 (95%CI: [0.36, 5.34]) |
Notes: aSame patients evaluated for dry eye before and after using CPAP.
Abbreviations: OSA, obstructive sleep apnea; DED, dry eye disease; PAP, continuous positive airway pressure; RCT, randomized controlled trial; TBUT, tear breakup time; OSDI, ocular surface disease index; CFS, corneal fluorescence-staining score (Oxford grading system); CI, confidence interval.
Critical Appraisal of the Literature
Among the three included studies, two investigated the effect of CPAP use on dry eye profiles in the same group of patients (pre- and post-treatment),40,41 and one study compared the impact of CPAP use between those having used CPAP for a long period and not using.33 The RCT conducted by Acar et al40 analyzed 51 consecutive patients, including 17 moderate OSA patients and 34 severe OSA patients, and the effect of CPAP use for 18 months on dry eye status. Their results turned out that patients wearing CPAP had improvement in TBUT (mean difference (sec; 95% CI): 1.57 (0.88, 2.66); p < 0.01), Schirmer test (mean difference (mm; 95% CI): 1.26 (0.53, 1.99); p < 0.01), OSDI (mean difference (95% CI): −5.62 (−13.58, 2.34); p < 0.01), and CFS (mean difference (95% CI): −0.37 (−0.62, −0.12); p < 0.01). However, no further comparisons based on OSA severity were mentioned. Another study by Kadyan et al33 studied the effect of CPAP use for an average of 19.6 ± 15.3 months on dry eye status in 67 CPAP users, which was compared to 22 patients without using CPAP. Both groups shared similar baseline demographic data, and the results indeed showed significant improvement in TBUT (mean difference (sec; 95% CI): 2.85 (0.36, 5.34); p = 0.029) for patients wearing CPAP compared to those who did not.
On the other hand, the study by Hayirci et al41 analyzed 40 consecutive OSA patients and the effect of CPAP use for a relatively shorter 4 months on dry eye status. High mean BMI values (34.48 ± 7.51 kg/m2) and mean neck circumference (43.05 ± 3.80 cm) were demonstrated in their demographic data, but the authors did not provide the distribution of baseline OSA severity. The results disclosed that patients after wearing CPAP had only improvement in Schirmer test (mean difference (mm; 95% CI): 2.14 (−1.15, 5.43); p = 0.007); nevertheless, TBUT (mean difference (sec; 95% CI): −0.88 (−2.55, 0.79); p = 0.036) showed significant deterioration, and no substantial improvement in CFS was found (mean difference (95% CI): 0.08 (−0.09, 0.25); p = 0.210).
Risk of Bias & Quality Assessment
All of the studies had no serious risk of bias according to the assessment (Supplementary Table 2). On the other hand, quality assessment revealed very low quality due to serious concerns regarding the potential imprecision of results; this was primarily generated by the (majorly) observational study design, the limited study number, and the characteristics of the studied population among studies. Despite this, the rest of the subjects had no serious concerns that may lead to further downgrading of the evidence (Supplementary Table 3).
Discussion
The present study demonstrated that patients with OSA generally had poorer dry eye profiles when compared to those without OSA. Besides, when using these common dry eye measurements for assessment, DED deteriorated along with the severity of OSA. As for the impact of CPAP use on DED, the current literature generally revealed benefits on DED alleviation when wearing CPAP for a long-term period (more than 1 year); on the other hand, from the current limited evidence, it seems that a shorter period of CPAP use (less than half a year) had no enough protective effects against dry eye status.
To the best of our knowledge, this is the first systematic review analyzing the impact of CPAP on DED yet not the first regarding the impact of OSA on DED. A recent meta-analysis study by Sun et al42 has demonstrated that most of the dry eye parameters were indeed poorer in OSA patients compared to healthy controls, and the findings could verify ours. However, one major limitation of that study is that the diversity of OSA patients and use of database statistics for diagnosis (containing studies using ICD codes for enrolling patients) may potentially generate some selection bias of patients that were not exactly diagnosed as OSA. To minimize this effect, we only enrolled clinical studies using PSG for OSA diagnosis to provide more precise results. Together with the information of the effect of CPAP use on DED, the findings could help tailor the clinical evaluation for future clinicians.
The Impact of OSA on DED
The potential mechanisms underlying OSA and DED have been hypothesized previously, and the present study supports the relationship between OSA and DED. This could be partially verified by one of the largest population-based database studies to date by Galor et al,43 in which 16,862 patients (including patients receiving ocular therapy) were recruited, and ICD codes were used for determining the risk factors for dry eye status. Their results indeed showed that sleep apnea may be one of the risk factors increasing the risk of dry eye, yet was adjusted by age and gender only.
Contrarily, in the present study, CFS (unlike the rest of the parameters) was not significantly different between patients with and without OSA; furthermore, two of the studies revealed no difference among OSA subgroups regarding subjective dry eye parameters (OSDI and CFS). The study by Fox et al30 combined CFS and papillary conjunctivitis together for evaluating ocular surface score among OSA subgroups and claimed that ocular surface score was associated with male sex; based on this finding, their relatively lower male sex ratio compared to other studies may potentially explain the insignificant results. Lin et al35 also reported no significant difference of OSDI and CFS based on OSA severity; however, the non-parametrical statistical methods and the relatively lower BMI values compared to other studies may potentially influence the findings to some extent. The above findings may imply that using non-objective measurements for dry eye evaluation may be more easily influenced by diverse medical situations, which is consistent with the statement by Kourukmas et al.44 On the other hand, the study by Muhafiz et al36 revealed no significant difference in the required objective dry eye parameter (Schirmer test) between patients with and without OSA. Despite no further demographic data (except age) were provided for analysis, they also indicated substantially poorer Meibogland performance in OSA patients; the changes probably resulted in the subsequent instability of tear film and easy evaporation even when the “clinical dry eye” was not severe enough to be detected, which might be important for the early DED assessment in OSA patients.
According to the previous literature, high BMI may serve as one of the most important risk factors for OSA.33 In our study, from the available data of the enrolled studies (for quantitative analysis purpose), BMI indeed seems relatively higher in OSA patients but may not reach statistical significance due to overlapping of the confidence interval in all of the studies; besides, no effect of high BMI or obesity on DED has been proved by the meta-analysis conducted by Tang et al.45 Together with the above evidence, we deemed that the pooled results of the impact of OSA on DED in our study may not be seriously confounded by this important risk factor. Additionally, from the perspective of statistics, heterogeneity of each dry eye parameter indeed dropped in certain situations when studies were grouped by OSA severity and study design. Regarding OSA severity, decreased heterogeneity was only seen in patients with mild OSA (compared to non-OSA patients) but remained high in those with moderate or severe OSA. Since patients with mild OSA had statistically similar results as non-OSA patients in dry eye parameters based on our analysis (Table 2), which is the same as the concept for grouping patients by Karaca et al38 (combining non-OSA and mild-OSA patients as a single group), this could probably imply that the ocular surface status was less influenced by this “less severe” hypopnea/apnea circumstance,42 and therefore unsurprisingly generated better statistical homogeneity than those with moderate or severe OSA.
The Impact of CPAP on DED
From the current available three studies, two of them33,40 revealed that OSA patients treated with CPAP (compared to “treatment-naïve” OSA patients) had significant improvement in all of the required DED parameters; both of the studies shared similar demographic baseline data and had a longer follow-up (at least 1 year). On the other hand, the study by Hayirci et al41 with a 4-month CPAP use demonstrated that most of the detected parameters showed no improvement (TBUT, CFS).
All of the three studies adopted TBUT; from evaluating this index, the results generally turned out that sustained use of CPAP may have benefits in improving TBUT compared to using CPAP for only a short period. In other words, not treated by CPAP for a long enough period may have greater short-term ocular irritation problems; this may be due to air leakage probably led by CPAP dislocation related to one-sided sleep position, especially in the very beginning of CPAP titration.41,46 Therefore, this impact may outweigh the benefits of CPAP use in improving OSA in a short-term CPAP use.47,48 It is worth noting that air leak is also associated with poor adherence to CPAP, especially in patients who have tried wearing for only a short period.46,49 Not used to tolerate CPAP and poor adherence could contribute to decreased treating benefits for OSA as well, and therefore lead to limited improvement in dry eye.
On the other hand, using CPAP for a relatively longer period may have significant benefits for dry eye improvement, and this could be related to healing of tear function tests and ocular surface stability along with OSA improvement.35,40 Wearing CPAP correctly, high compliance, and using CPAP for long enough time to reach therapeutic effect are essential to reach better improvement of OSA when compared to a relatively short-term use. Though some studies claimed that one-year duration may provide reliable long-term adherence,50 interestingly, it has also been revealed that wearing CPAP for more than 2 years may be associated with decreased adherence instead.51 The issue of compliance has also been addressed in a recent study using ICD codes to study the association between OSA, CPAP and DED (though this study was not enrolled in the present study for analysis based on the exclusion criteria).52 Although they reported that patients wearing CPAP may have poorer DED, the duration of CPAP use was failed to be recorded and analyzed due to the retrospective design and difficulty in estimating the compliance rate of wearing CPAP. In light of this, we deem that more studies may be needed to further investigate the use of CPAP with longer durations to understand the exact impact of CPAP on OSA and dry eye.
Some limitations should be considered in this study. First, we only analyzed the impact on dry eye for both aims. Since DED is a clinical diagnosis and is multifactorial, it may not be easy to identify the exact etiology in all of the cases in each single study. However, the most commonly used and validated assessment tools were adopted in this study to make the medical situations comparable. The results were expected to provide insights into future dry eye evaluation. Second, for the impact of CPAP on DED, due to the lack of available studies for generating statistical results with enough power, only narrative synthesis was conducted. More future studies focusing on this issue are still warranted to understand the impact of CPAP use on DED with further statistical evidence, especially the difference between a short-term and long-term use. Finally, since CPAP is the first-line conservative treatment for OSA, it remains understudied whether those unable to tolerate CPAP may also benefit from invasive OSA treatment (eg surgery) for DED, which should be investigated in depth in the future.
Conclusion
OSA patients may suffer from poorer dry eye condition compared to non-OSA patients, according to our statistical analysis. Besides, from the current available literature, it seems that a longer period use of CPAP may improve DED to a greater extent along with OSA improvement, while a shorter use of CPAP may be accompanied with more ocular irritation symptoms related to DED.
Funding Statement
The authors thank the research grant from the Chang Gung Memorial Hospital (CMRPG8L1343), Taiwan.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
This study was registered and approved by the International Prospective Register of Systematic Reviews (PROSPERO); the registration number was CRD42024503983.
Disclosure
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- 1.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su MC, Chen YC, Huang KT, Wang CC, Lin MC, Lin HC. Association of metabolic factors with high-sensitivity C-reactive protein in patients with sleep-disordered breathing. Eur Arch Otorhinolaryngol. 2013;270(2):749–754. doi: 10.1007/s00405-012-2191-4 [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323(14):1389–1400. doi: 10.1001/jama.2020.3514 [DOI] [PubMed] [Google Scholar]
- 4.Mashaqi S, Rangan P, Saleh AA, et al. Biomarkers of gut barrier dysfunction in obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2023;69:101774. doi: 10.1016/j.smrv.2023.101774 [DOI] [PubMed] [Google Scholar]
- 5.Vanek J, Prasko J, Genzor S, et al. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020;72:50–58. doi: 10.1016/j.sleep.2020.03.017 [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Kang R, Zhao S, et al. Sexual dysfunction in patients with obstructive sleep apnea: a systematic review and meta-analysis. J Sex Med. 2015;12(10):1992–2003. doi: 10.1111/jsm.12983 [DOI] [PubMed] [Google Scholar]
- 7.Lin PW, Lin HC, Chang CT, Lin MC, Friedman M, Salapatas AM. Decreased peripapillary and macular vascular densities in patients with moderate/severe obstructive sleep apnea/hypopnea syndrome. Nat Sci Sleep. 2023;15:1–12. doi: 10.2147/NSS.S384372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin PW, Lin HC, Friedman M, et al. Effects of CPAP for patients with OSA on visual sensitivity and retinal thickness. Sleep Med. 2020;67:156–163. doi: 10.1016/j.sleep.2019.10.019 [DOI] [PubMed] [Google Scholar]
- 9.Chiu LW, Lin CW, Lin PW, et al. Homocysteine levels in severe OSA patients before and after TORS-OSA surgery. Otolaryngol Head Neck Surg. 2023;168(5):1238–1244. doi: 10.1002/ohn.218 [DOI] [PubMed] [Google Scholar]
- 10.Lin CW, Lin PW, Chiu LW, et al. Inflammatory biomarkers of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in 563 severe OSA patients before and after surgery. J Otolaryngol Head Neck Surg. 2023;52(1):49. doi: 10.1186/s40463-023-00653-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang KL, Lin PW, Chang CT, et al. OSA treatment on cardio- and cerebrovascular comorbidities: a long-term nationwide cohort study. Otolaryngol Head Neck Surg. 2022;167(3):600–606. doi: 10.1177/01945998211065656 [DOI] [PubMed] [Google Scholar]
- 12.Chuang YC, Lin PW, Lin HC, et al. Effects of TORS-OSA surgery on lower urinary tract symptoms, overactive bladder symptoms, and nocturia in male patients with obstructive sleep apnea/hypopnea syndrome. Nat Sci Sleep. 2022;14:547–556. doi: 10.2147/NSS.S349807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin CH, Lin PW, Lin HC, Friedman M, Lin MC. Effects of OSA surgery on leptin and metabolic profiles. Otolaryngol Head Neck Surg. 2019;161(6):1048–1055. doi: 10.1177/0194599819877647 [DOI] [PubMed] [Google Scholar]
- 14.Rapoport DM, Garay SM, Goldring RM. Nasal CPAP in obstructive sleep apnea: mechanisms of action. Bull Eur Physiopathol Respir. 1983;19(6):616–620. [PubMed] [Google Scholar]
- 15.Lebret M, Arnol N, Martinot JB, et al. Determinants of unintentional leaks during CPAP treatment in OSA. Chest. 2018;153(4):834–842. doi: 10.1016/j.chest.2017.08.017 [DOI] [PubMed] [Google Scholar]
- 16.Rotty MC, Suehs CM, Mallet JP, et al. Mask side-effects in long-term CPAP-patients impact adherence and sleepiness: the InterfaceVent real-life study. Respir Res. 2021;22(1):17. doi: 10.1186/s12931-021-01618-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matossian C, Song X, Chopra I, Sainski-Nguyen A, Ogundele A. The prevalence and incidence of dry eye disease among patients using continuous positive airway pressure or other nasal mask therapy devices to treat sleep apnea. Clin Ophthalmol. 2020;14:3371–3379. doi: 10.2147/OPTH.S274949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian L, Wei W. Identified risk factors for dry eye syndrome: a systematic review and meta-analysis. PLoS One. 2022;17(8):e0271267. doi: 10.1371/journal.pone.0271267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, He X, Li Q, et al. Obstructive sleep apnea affects lacrimal gland function. Invest Ophthalmol Vis Sci. 2022;63(3):3. doi: 10.1167/iovs.63.3.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pflugfelder SC, de Paiva CS. The pathophysiology of dry eye disease: what we know and future directions for research. Ophthalmology. 2017;124(11s):S4–s13. doi: 10.1016/j.ophtha.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang HB, Ku YH, Kim EC, Kim HS, Kim MS, Hwang HS. Easy and effective test to evaluate tear-film stability for self-diagnosis of dry eye syndrome: blinking tolerance time (BTT). BMC Ophthalmol. 2020;20(1):438. doi: 10.1186/s12886-020-01686-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalton JE, Bolen SD, Mascha EJ. Publication bias: the elephant in the review. Anesth Analg. 2016;123(4):812–813. doi: 10.1213/ANE.0000000000001596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 26.Herzog R, Álvarez-pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Á G. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 28.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acar M, Firat H, Acar U, Ardic S. Ocular surface assessment in patients with obstructive sleep apnea-hypopnea syndrome. Sleep Breath. 2013;17(2):583–588. doi: 10.1007/s11325-012-0724-0 [DOI] [PubMed] [Google Scholar]
- 30.Fox TP, Schwartz JA, Chang AC, et al. Association between eyelid laxity and obstructive sleep apnea. JAMA Ophthalmol. 2017;135(10):1055–1061. doi: 10.1001/jamaophthalmol.2017.3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunes I, Oltulu R, Oltulu P, Turk N, Yosunkaya S. Ocular surface in patients with obstructive sleep apnea syndrome: evaluation of clinical parameters and impression cytology. Eye Contact Lens. 2023;49(1):14–18. doi: 10.1097/ICL.0000000000000945 [DOI] [PubMed] [Google Scholar]
- 32.Hao L, Tian Q, Liu S, Xu Z, Yang L. Alterations of ocular surface parameters in patients with obstructive sleep apnea syndrome. Front Med Lausanne. 2023;10:1220104. doi: 10.3389/fmed.2023.1220104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadyan A, Asghar J, Dowson L, Sandramouli S. Ocular findings in sleep apnoea patients using continuous positive airway pressure. Eye. 2010;24(5):843–850. doi: 10.1038/eye.2009.212 [DOI] [PubMed] [Google Scholar]
- 34.Karaca EE, Akcam HT, Uzun F, Ozdek S, Ulukavak Ciftci T. Evaluation of ocular surface health in patients with obstructive sleep apnea syndrome. Turk J Ophthalmol. 2016;46(3):104–108. doi: 10.4274/tjo.57778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin PW, Lin HC, Chang CT, et al. Alterations of ocular surface and tear film in patients with obstructive sleep apnea/hypopnea syndrome. Nat Sci Sleep. 2022;14:277–290. doi: 10.2147/NSS.S340105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muhafiz E, Ölçen M, Erten R, Bozkurt E. Evaluation of meibomian glands in obstructive sleep apnea-hypopnea syndrome. Cornea. 2020;39(6):685–690. doi: 10.1097/ICO.0000000000002252 [DOI] [PubMed] [Google Scholar]
- 37.Pu Q, Wu Z, Li AL, Guo HJJ XX, Li XY, Li X-Y. Association between poor sleep quality and an increased risk of dry eye disease in patients with obstructive sleep apnea syndrome. Front Med Lausanne. 2022;9:870391. doi: 10.3389/fmed.2022.870391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karaca I, Yagci A, Palamar M, Tasbakan MS, Basoglu OK. Ocular surface assessment and morphological alterations in meibomian glands with meibography in obstructive sleep apnea Syndrome. Ocul Surf. 2019;17(4):771–776. doi: 10.1016/j.jtos.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Li S, Li M, Zeng S, Chen B, Zhang L. Evaluation of the ocular surface and meibomian gland in obstructive sleep apnea hypopnea syndrome. Front Med Lausanne. 2022;9:832954. doi: 10.3389/fmed.2022.832954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Acar M, Firat H, Yuceege M, Ardic S. Long-term effects of PAP on ocular surface in obstructive sleep apnea syndrome. Can J Ophthalmol. 2014;49(2):217–221. doi: 10.1016/j.jcjo.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 41.Hayirci E, Yagci A, Palamar M, Basoglu OK, Veral A. The effect of continuous positive airway pressure treatment for obstructive sleep apnea syndrome on the ocular surface. Cornea. 2012;31(6):604–608. doi: 10.1097/ICO.0b013e31824a2040 [DOI] [PubMed] [Google Scholar]
- 42.Sun J, He J, Liang Z. Comparison of ocular surface assessment outcomes between healthy controls and patients with obstructive sleep apnea-hypopnea syndrome: a meta-analysis of the literature. Front Physiol. 2023;14:1163947. doi: 10.3389/fphys.2023.1163947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galor A, Feuer W, Lee DJ, et al. Prevalence and risk factors of dry eye syndrome in a United States veterans affairs population. Am J Ophthalmol. 2011;152(3):377–384e372. doi: 10.1016/j.ajo.2011.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kourukmas R, Roth M, Geerling G. Automated vs. human evaluation of corneal staining. Graefes Arch Clin Exp Ophthalmol. 2022;260(8):2605–2612. doi: 10.1007/s00417-022-05574-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang YL, Cheng YL, Ren YP, Yu XN, Shentu XC. Metabolic syndrome risk factors and dry eye syndrome: a Meta-analysis. Int J Ophthalmol. 2016;9(7):1038–1045. doi: 10.18240/ijo.2016.07.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heidari R, Shamsadini A, Najafi A, Erfanian R. Identifying affecting factors on acceptance with CPAP on the first night of PAP titration in sleep clinic on patients with obstructive sleep apnea. Iran J Otorhinolaryngol. 2023;35(131):303–309. doi: 10.22038/IJORL.2023.68296.3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eguchi K, Yabuuchi T, Nambu M, et al. Investigation on factors related to poor CPAP adherence using machine learning: a pilot study. Sci Rep. 2022;12(1):19563. doi: 10.1038/s41598-022-21932-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ercelik M, Balbay EG, Gulhan PY, et al. Factors affecting compliance with positive airway pressure therapy in obstructive sleep apnea. Sleep Breath. 2022;26(2):725–732. doi: 10.1007/s11325-021-02447-4 [DOI] [PubMed] [Google Scholar]
- 49.Valentin A, Subramanian S, Quan SF, Berry RB, Parthasarathy S. Air leak is associated with poor adherence to autoPAP therapy. Sleep. 2011;34(6):801–806. doi: 10.5665/SLEEP.1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morrone E, Giordano A, Carli S, et al. Something is changing in adherence to CPAP therapy: real world data after 1 year of treatment in patients with obstructive sleep apnoea. Eur Respir J. 2020;55(3). doi: 10.1183/13993003.01419-2019. [DOI] [PubMed] [Google Scholar]
- 51.La Piana GE, Scartabellati A, Chiesa L, et al. Long-term adherence to CPAP treatment in patients with obstructive sleep apnea: importance of educational program. Patient Prefer Adherence. 2011;5:555–562. doi: 10.2147/PPA.S24018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu YK, Sun CC, Chen KJ, et al. Associations between obstructive sleep apnea syndrome, dry eye disease, and CPAP usage among Taiwanese patients: a retrospective analysis. Nat Sci Sleep. 2024;16:1001–1009. doi: 10.2147/NSS.S458245 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.