Abstract
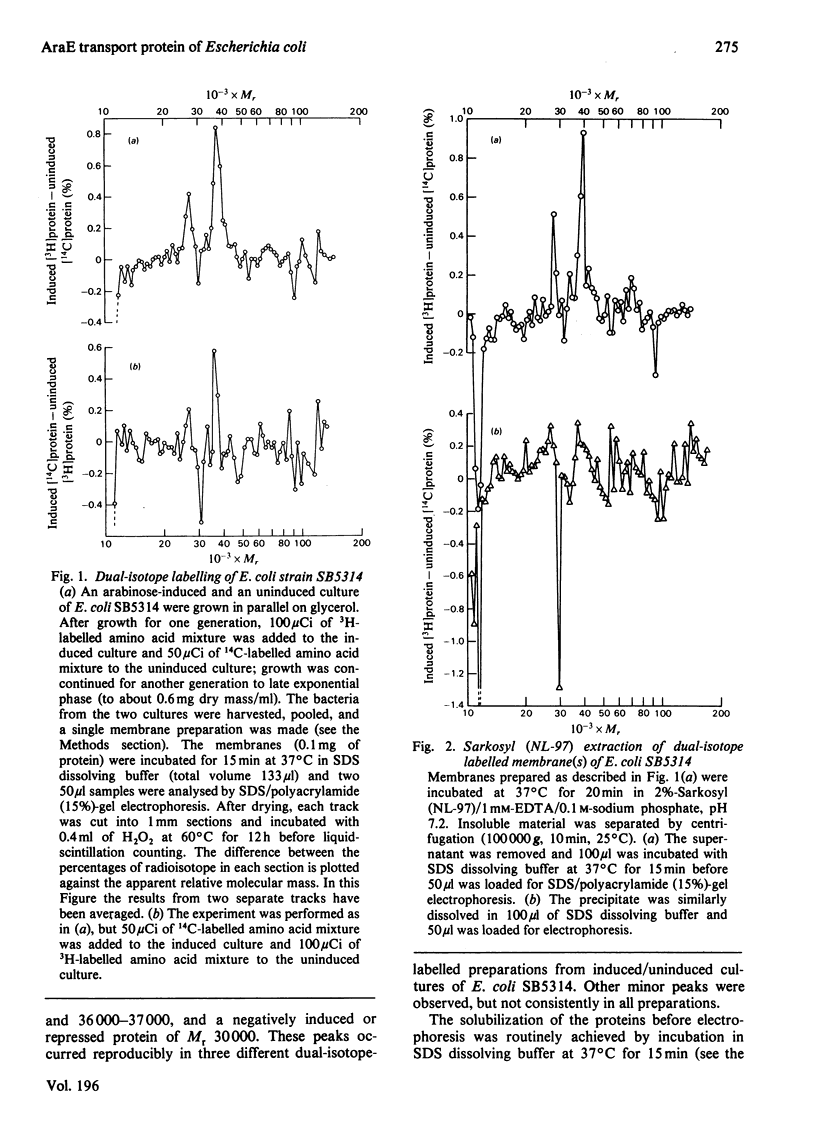
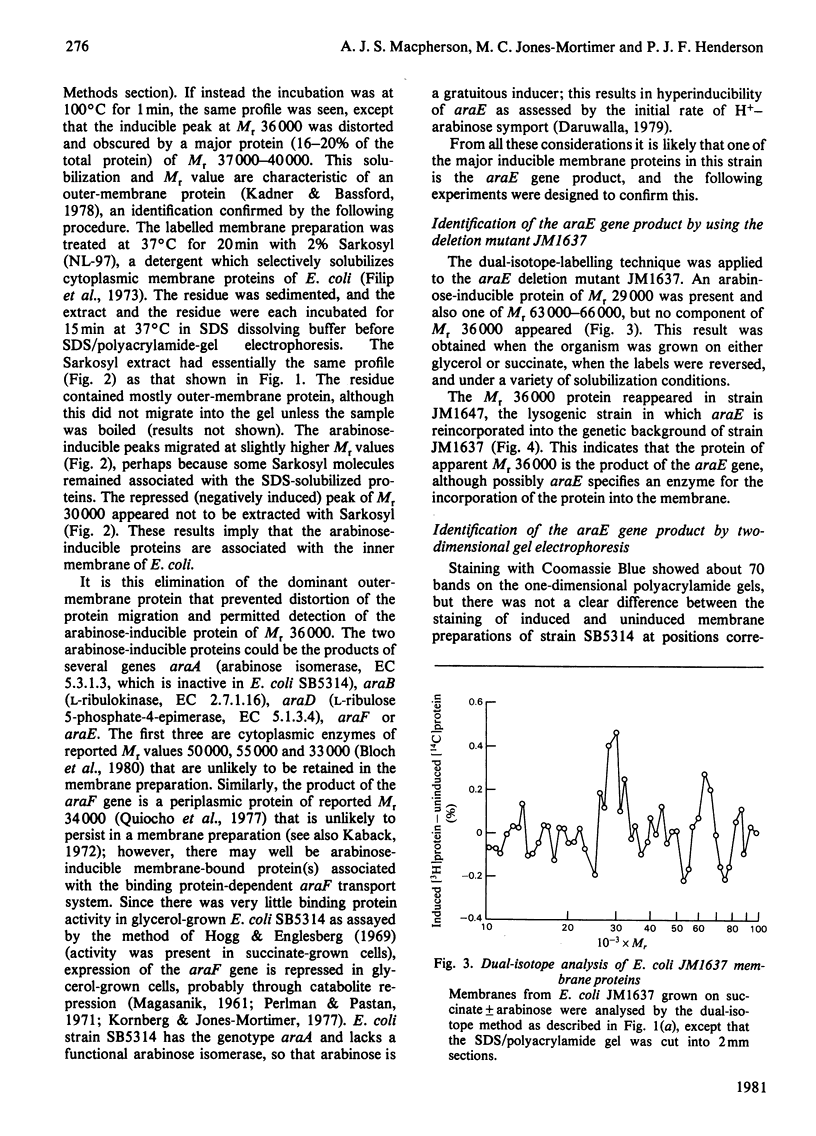
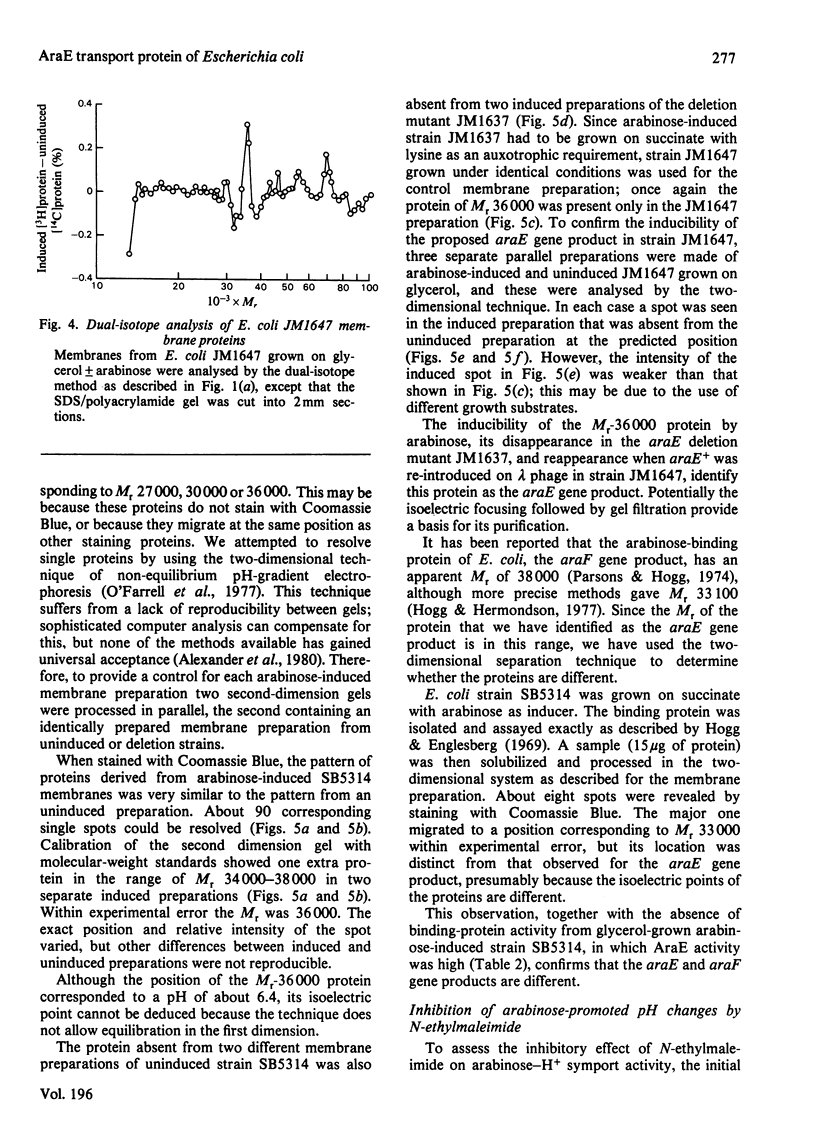
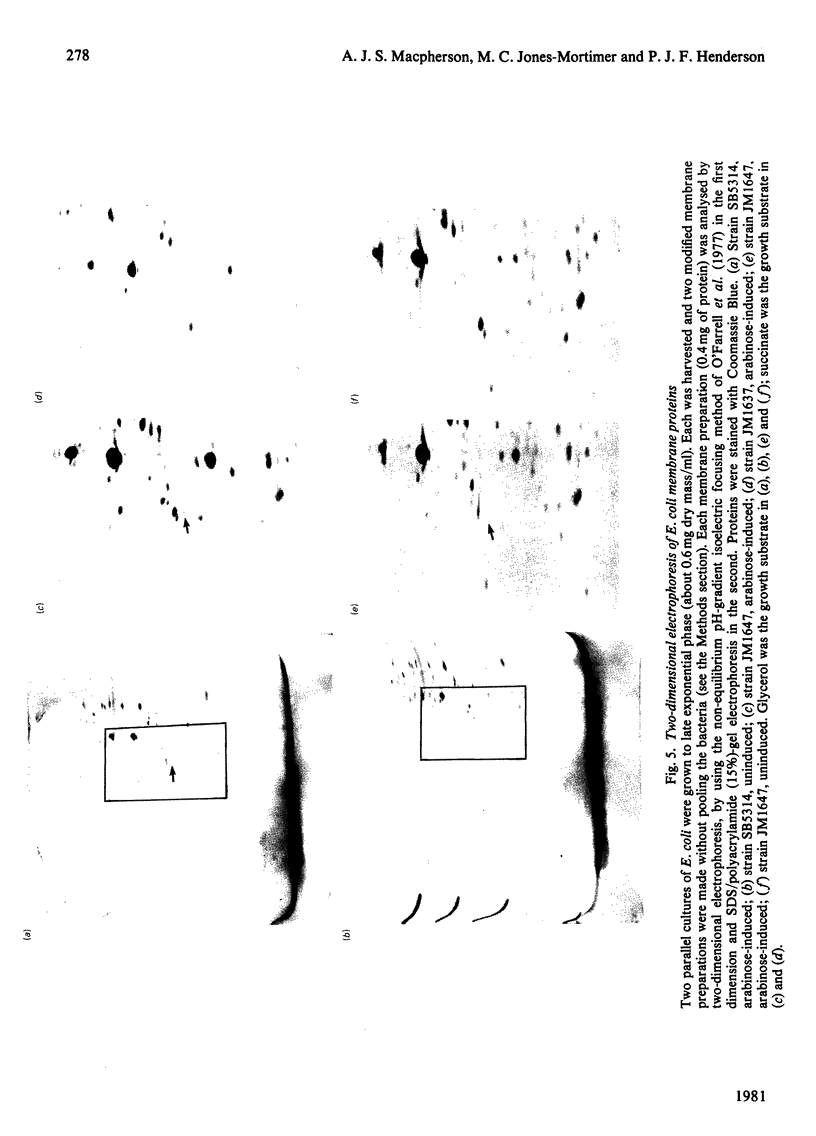
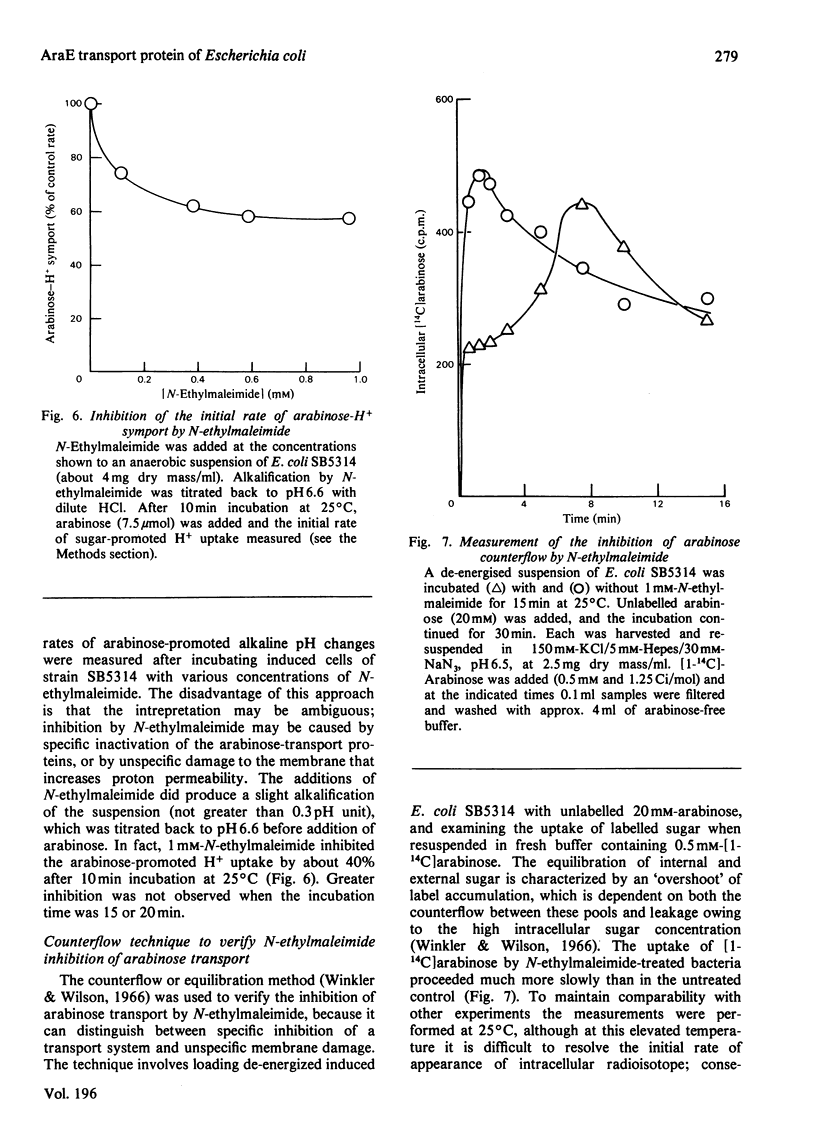
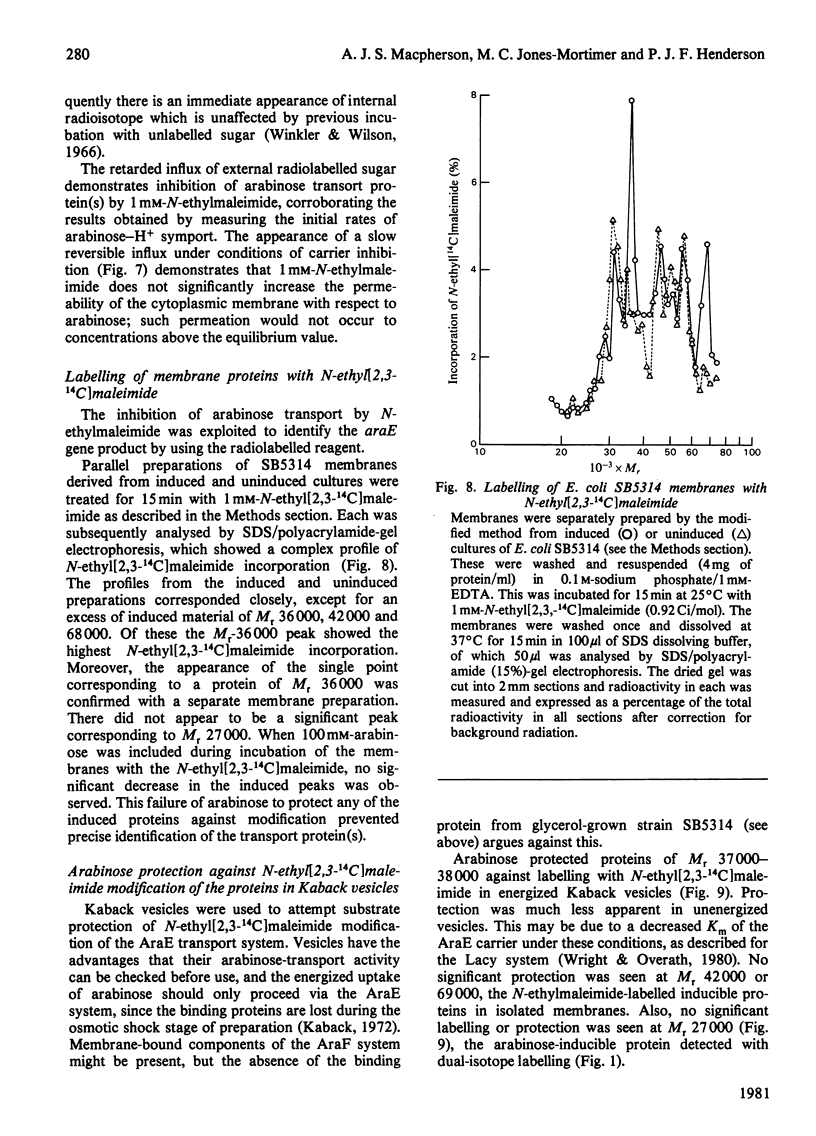
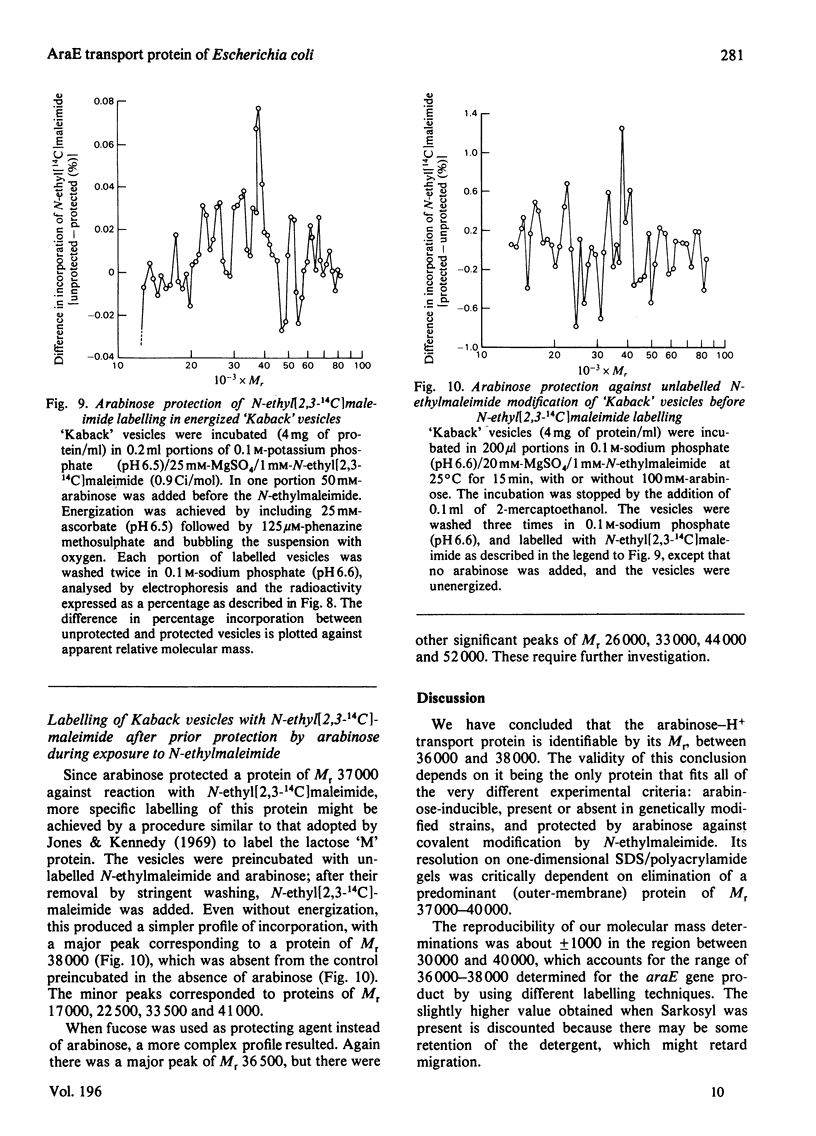
1. Two arabinose-inducible proteins are detected in membrane preparations from strains of Escherichia coli containing arabinose-H+ (or fucose-H+) transport activity; one protein has an apparent subunit relative molecular mass (Mr) of 36 000-37 000 and the other has Mr 27 000. 2. An araE deletion mutant was isolated and characterized; it has lost arabinose-H+ symport activity and the arabinose-inducible protein of Mr 36 000, but not the protein of Mr 27 000. 3. An araE+ specialized transducing phage was characterized and used to re-introduce the araE+ gene into the deletion strain, a procedure that restores both arabinose-H+ symport activity and the protein of Mr 36,000. 4. N-Ethylmaleimide inhibits arabinose transport and partially inhibits arabinose-H+ symport activity. 5. N-Ethylmaleimide modifies an arabinose-inducible protein of Mr 36 000-38 000, and arabinose protects the protein against the reagent. 6. These observations identify an arabinose-transport protein of Escherichia coli as the product of the araE+ gene. 7. The protein was recognized as a single spot staining with Coomassie Blue after two-dimensional gel electrophoresis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander A., Cullen B., Emigholz K., Norgard M. V., Monahan J. J. A computer program for displaying two-dimensional gel electrophoresis data. Anal Biochem. 1980 Mar 15;103(1):176–183. doi: 10.1016/0003-2697(80)90253-5. [DOI] [PubMed] [Google Scholar]
- Archer D. B., Rodwell A. W., Rodwell E. S. The nature and location of Acholeplasma laidlawii membrane proteins investigated by two-dimensional gel electrophoresis. Biochim Biophys Acta. 1978 Nov 2;513(2):268–283. doi: 10.1016/0005-2736(78)90179-7. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch P. L., Phillips T. A., Neidhardt F. C. Protein identifications of O'Farrell two-dimensional gels: locations of 81 Escherichia coli proteins. J Bacteriol. 1980 Mar;141(3):1409–1420. doi: 10.1128/jb.141.3.1409-1420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown C. E., Hogg R. W. A second transport system for L-arabinose in Escherichia coli B-r controlled by the araC gene. J Bacteriol. 1972 Aug;111(2):606–613. doi: 10.1128/jb.111.2.606-613.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Taylor A. L. Mutants of Escherichia coli with a growth requirement for either lysine or pyridoxine. J Bacteriol. 1971 Mar;105(3):988–998. doi: 10.1128/jb.105.3.988-998.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Isolation of a Mutant of Escherichia coli with a Temperature-sensitive Fructose-1,6-Diphosphate Aldolase Activity. J Bacteriol. 1966 Aug;92(2):464–469. doi: 10.1128/jb.92.2.464-469.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Carter J. R., Fox C. F., Kennedy E. P. Interaction of sugars with the membrane protein component of the lactose transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Jun;60(2):725–732. doi: 10.1073/pnas.60.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring R., Beyreuther K., Wright J. K., Overath P. In vitro and in vivo products of E. coli lactose permease gene are identical. Nature. 1980 Feb 7;283(5747):537–540. doi: 10.1038/283537a0. [DOI] [PubMed] [Google Scholar]
- Englesberg E., Irr J., Power J., Lee N. Positive control of enzyme synthesis by gene C in the L-arabinose system. J Bacteriol. 1965 Oct;90(4):946–957. doi: 10.1128/jb.90.4.946-957.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier R. E., Pardee A. B. Evidence for inducible, L-malate binding proteins in the membrane of Bacillus subtilis. Identification of presumptive components of the C4-dicarboxylate transport systems. J Biol Chem. 1974 Sep 25;249(18):5948–5954. [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. Integration-negative mutants of bacteriophage lambda. J Mol Biol. 1968 Feb 14;31(3):487–505. doi: 10.1016/0022-2836(68)90423-3. [DOI] [PubMed] [Google Scholar]
- Heffernan L., Bass R., Englesberg E. Mutations affecting catabolite repression of the L-arabinose regulon in Escherichia coli B/r. J Bacteriol. 1976 Jun;126(3):1119–1131. doi: 10.1128/jb.126.3.1119-1131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J., Giddens R. A. 2-Deoxy-D-galactose, a substrate for the galactose-transport system of Escherichia coli. Biochem J. 1977 Oct 15;168(1):15–22. doi: 10.1042/bj1680015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J., Giddens R. A., Jones-Mortimer M. C. Transport of galactose, glucose and their molecular analogues by Escherichia coli K12. Biochem J. 1977 Feb 15;162(2):309–320. doi: 10.1042/bj1620309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R. W., Englesberg E. L-arabinose binding protein from Escherichia coli B-r. J Bacteriol. 1969 Oct;100(1):423–432. doi: 10.1128/jb.100.1.423-432.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R. W., Hermodson M. A. Amino acid sequence of the L-arabinose-binding protein from Escherichia coli B/r. J Biol Chem. 1977 Jul 25;252(14):5135–5141. [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Kornberg H. L. Order of genes adjacent to ptsX on the E. coli genome. Proc R Soc Lond B Biol Sci. 1976 May 18;193(1112):313–315. doi: 10.1098/rspb.1976.0049. [DOI] [PubMed] [Google Scholar]
- Jones T. H., Kennedy E. P. Characterization of the membrane protein component of the lactose transport system of Escherichia coli. J Biol Chem. 1969 Nov 10;244(21):5981–5987. [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Kaiser A. D., Masuda T. Specificity in curing by heteroimmune superinfection. Virology. 1970 Mar;40(3):522–529. doi: 10.1016/0042-6822(70)90195-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Switzer R. C., Van Keuren M. L. Trace polypeptides in cellular extracts and human body fluids detected by two-dimensional electrophoresis and a highly sensitive silver stain. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4335–4339. doi: 10.1073/pnas.76.9.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C. P., Englesberg E. The L-arabinose permease system in Escherichia coli B/r. Biochim Biophys Acta. 1966 Mar 28;117(1):217–230. doi: 10.1016/0304-4165(66)90169-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Parsons R. G., Hogg R. W. Crystallization and characterization of the L-arabinose-binding protein of Escherichia coli B-r. J Biol Chem. 1974 Jun 10;249(11):3602–3607. [PubMed] [Google Scholar]
- Quiocho F. A., Gilliland G. L., Phillips G. N., Jr The 2.8-A resolution structure of the L-arabinose-binding protein from Escherichia coli. Polypeptide chain folding, domain similarity, and probable location of sugar-binding site. J Biol Chem. 1977 Jul 25;252(14):5142–5149. [PubMed] [Google Scholar]
- Roberton A. M., Sullivan P. A., Jones-Mortimer M. C., Kornberg H. L. Two genes affecting glucarate utilization in Escherichia coli K12. J Gen Microbiol. 1980 Apr;117(2):377–382. doi: 10.1099/00221287-117-2-377. [DOI] [PubMed] [Google Scholar]
- Rotman B., Ganesan A. K., Guzman R. Transport systems for galactose and galactosides in Escherichia coli. II. Substrate and inducer specificities. J Mol Biol. 1968 Sep 14;36(2):247–260. doi: 10.1016/0022-2836(68)90379-3. [DOI] [PubMed] [Google Scholar]
- Schleif R. An L-arabinose binding protein and arabinose permeation in Escherichia coli. J Mol Biol. 1969 Nov 28;46(1):185–196. doi: 10.1016/0022-2836(69)90065-5. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Müller-Hill B., Abrutsch U., Aichele G., Overath P. Amplification of the lactose carrier protein in Escherichia coli using a plasmid vector. Mol Gen Genet. 1978 Feb 27;159(3):239–248. doi: 10.1007/BF00268260. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Overath P. Lactose transport in Escherichia coli: effect of transmembrane potential difference on apparent substrate affinity. Biochem Soc Trans. 1980 Jun;8(3):279–281. doi: 10.1042/bst0080279. [DOI] [PubMed] [Google Scholar]