Abstract
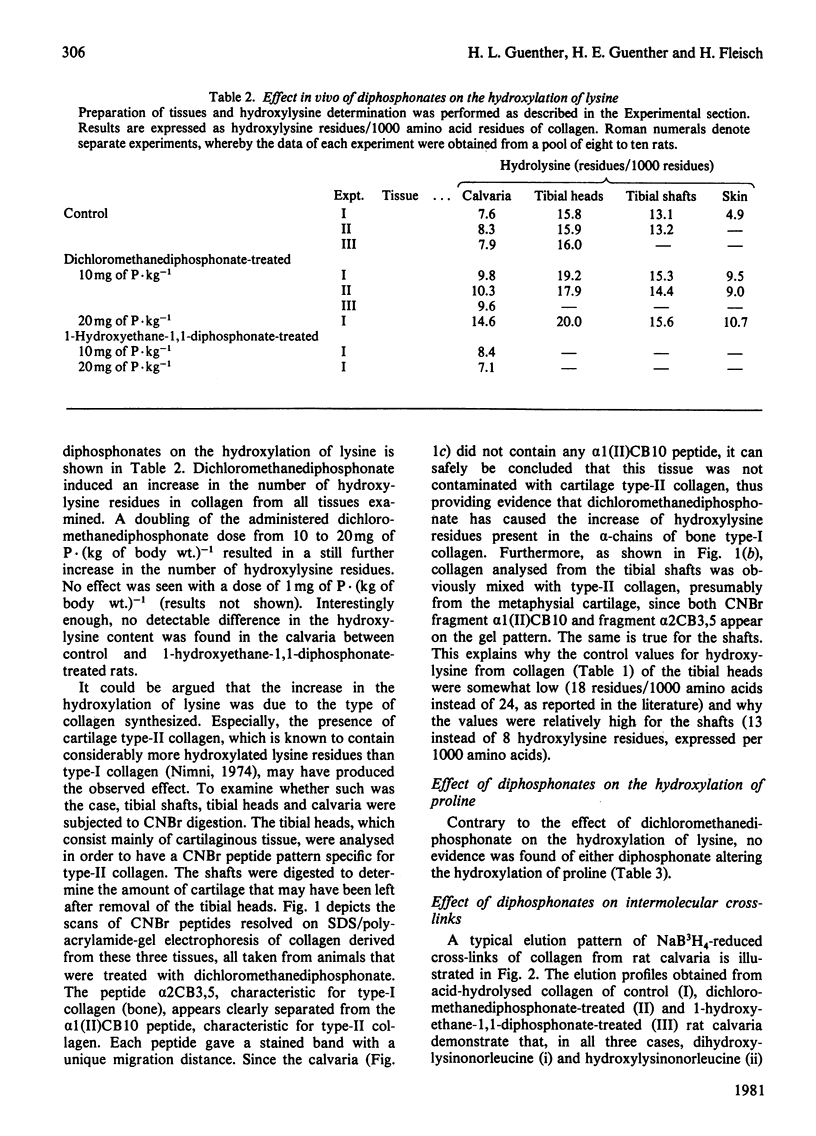
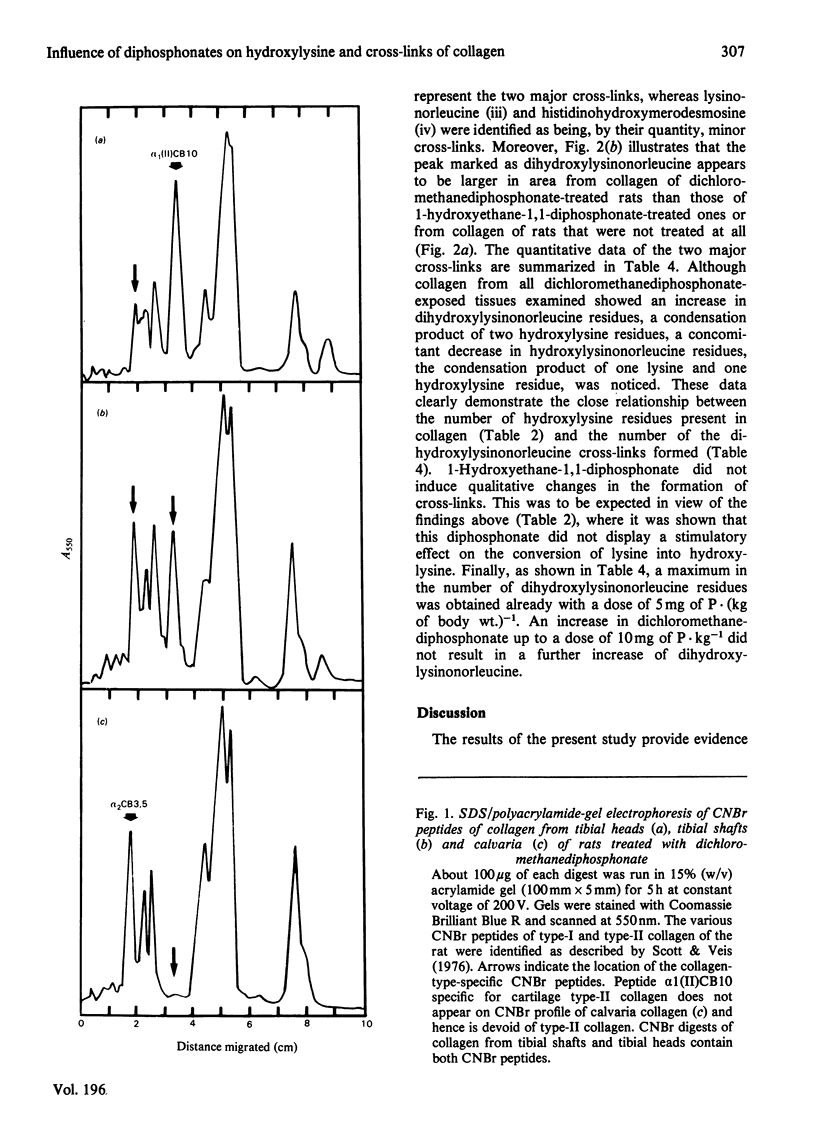
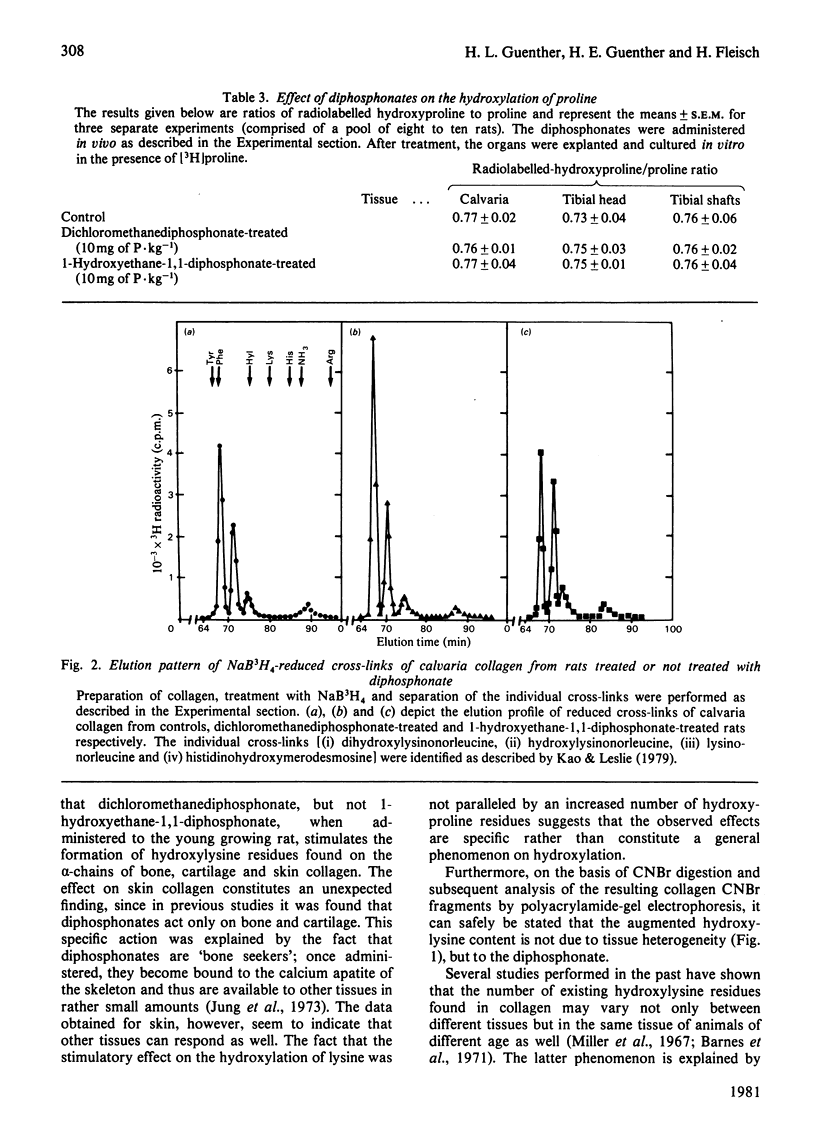
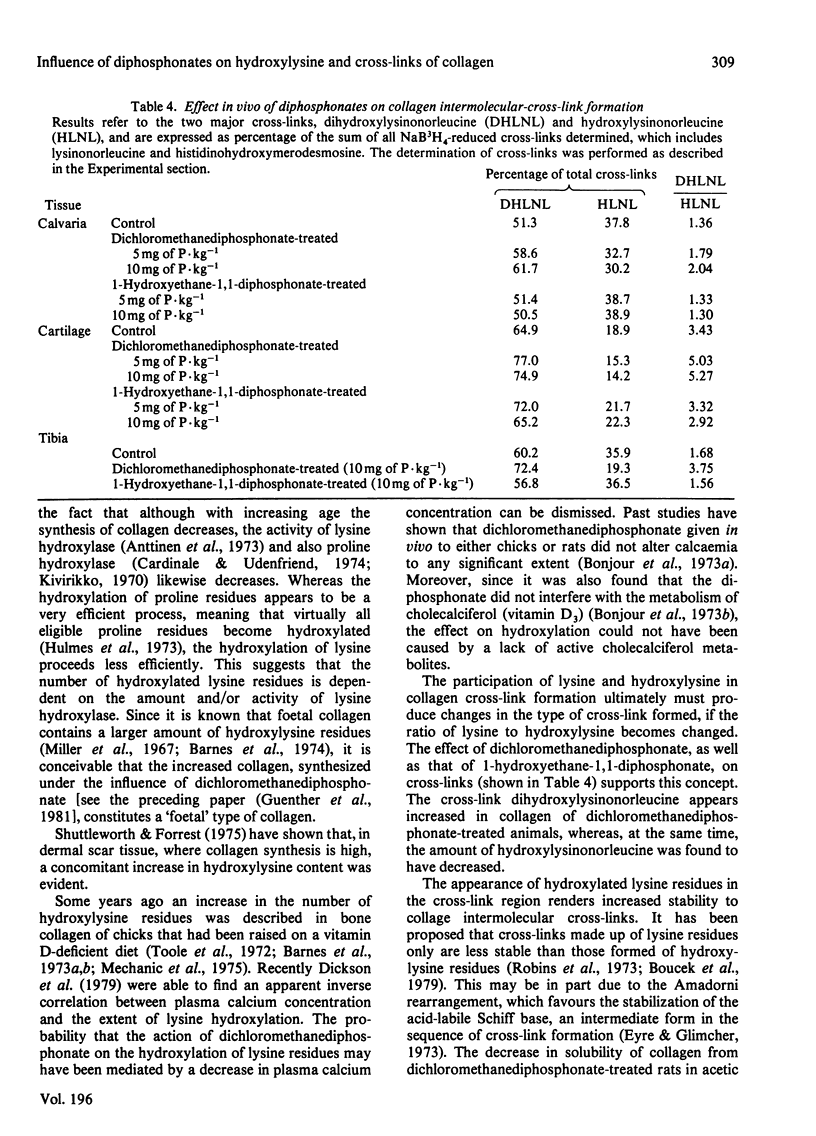
The effects in vivo of dichloromethanediphosphonate and 1-hydroxyethane 1,1-diphosphonate on collagen solubility, hydroxylation of lysine and proline and on the formation of collagen intermolecular cross-links were studied by using rat bone, cartilage and skin tissues. Dichloromethanediphosphonate decreased bone collagen solubility both in acetic acid and after pepsin treatment. Although none of the diphosphonates had any effect on the hydroxylation of proline, dichloromethane-diphosphonate, but not 1-hydroxyethane-1,1-diphosphonate, increased the number of hydroxylysine residues in the alpha-chains of bone, skin and cartilage collagen. The stimulatory effect was dose-dependent. The dichloromethanediphosphonate-mediated increase in hydroxylysine residues in bone and cartilage was manifested in an increase of dihydroxylysinonorleucine, the cross-link that is formed by the condensation of two hydroxylysine residues. The cross-link hydroxylysinonorleucine, a condensation product of hydroxylysine and lysine, on the other hand, was decreased. The total number of intermolecular cross-links was not changed by the diphosphonate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anttinen H., Orava S., Ryhänen L., Kivirikko K. I. Assay of protocollagen lysyl hydroxylase activity in the skin of human subjects and changes in the activity with age. Clin Chim Acta. 1973 Aug 30;47(2):289–294. doi: 10.1016/0009-8981(73)90326-4. [DOI] [PubMed] [Google Scholar]
- Banes A. J., Bernstein P. H., Smith R. E., Mechanic G. L. Collagen biochemistry of osteopetrotic bone: I. Quantitative changes in bone collagen cross-links in virus-induced avian osteopetrosis. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1390–1397. doi: 10.1016/0006-291x(78)91290-1. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Morton L. F., Kodicek E. Bone collagen metabolism in vitamin D deficiency. Biochem J. 1973 Jan;132(1):113–115. doi: 10.1042/bj1320113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Morton L. F., Kodicek E. Hydroxylysine in the N-terminal regions of the 1 - and 2 -chains of various collagens. Biochem J. 1971 Nov;125(2):433–437. doi: 10.1042/bj1250433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Morton L. F., Kodicek E. The influence of dietary calcium deficiency and parathyroidectomy on bone collagen structure. Biochim Biophys Acta. 1973 Dec 6;328(2):373–382. doi: 10.1016/0005-2795(73)90271-7. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Morton L. F., Royce P. M. Age-related variations in hydroxylation of lysine and proline in collagen. Biochem J. 1974 May;139(2):461–468. doi: 10.1042/bj1390461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun. 1973 May 1;52(1):115–120. doi: 10.1016/0006-291x(73)90961-3. [DOI] [PubMed] [Google Scholar]
- Bonjour J. P., DeLuca H. F., Fleisch H., Trechsel U., Matejowec L. A., Omdahl J. L. Reversal of the EHDP inhibition of calcium absorption by 1,25-dihydroxycholecalciferol. Eur J Clin Invest. 1973 Jan;3(1):44–48. doi: 10.1111/j.1365-2362.1973.tb00328.x. [DOI] [PubMed] [Google Scholar]
- Bonjour J. P., Fleisch H., Copp D. H. Influence of a diphosphonate on serum calcium homeostasis. Proc Soc Exp Biol Med. 1973 Jun;143(2):404–407. doi: 10.3181/00379727-143-37331. [DOI] [PubMed] [Google Scholar]
- Boucek R. J., Noble N. L., Gunja-Smith Z. A possible role for dehydrodihydroxylysinonorleucine in collagen fibre and bundle formation. Biochem J. 1979 Mar 1;177(3):853–860. doi: 10.1042/bj1770853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale G. J., Udenfriend S. Prolyl hydroxylase. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):245–300. doi: 10.1002/9780470122860.ch6. [DOI] [PubMed] [Google Scholar]
- Dickson I. R., Eyre D. R., Kodicek E. Influence of plasma calcium and vitamin D on bone collagen. Effects on lysine hydroxylation and crosslink formation. Biochim Biophys Acta. 1979 Nov 15;588(1):169–173. doi: 10.1016/0304-4165(79)90381-7. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Glimcher M. J. Analysis of a crosslinked peptide from calf bone collagen: evidence that hydroxylysyl glycoside participates in the crosslink. Biochem Biophys Res Commun. 1973 May 15;52(2):663–671. doi: 10.1016/0006-291x(73)90764-x. [DOI] [PubMed] [Google Scholar]
- Fleisch H., Russell R. G., Francis M. D. Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo. Science. 1969 Sep 19;165(3899):1262–1264. doi: 10.1126/science.165.3899.1262. [DOI] [PubMed] [Google Scholar]
- Guenther H. L., Croissant R. D., Schonfeld S. E., Slavkin H. C. Identification of four extracellular-matrix enamel proteins during embryonic-rabbit tooth-organ development. Biochem J. 1977 Jun 1;163(3):591–603. doi: 10.1042/bj1630591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther H. L., Guenther H. E., Fleisch H. The effects of 1-hydroxyethane-1,1-diphosphonate and dichloromethanediphosphonate on collagen synthesis by rabbit articular chondrocytes and rat bone cells. Biochem J. 1981 Apr 15;196(1):293–301. doi: 10.1042/bj1960293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, McCroskery P. A. The influence of temperature and fibril stability on degradation of cartilage collagen by rheumatoid synovial collagenase. N Engl J Med. 1974 Jan 3;290(1):1–6. doi: 10.1056/NEJM197401032900101. [DOI] [PubMed] [Google Scholar]
- Hulmes D. J., Miller A., Parry D. A., Piez K. A., Woodhead-Galloway J. Analysis of the primary structure of collagen for the origins of molecular packing. J Mol Biol. 1973 Sep 5;79(1):137–148. doi: 10.1016/0022-2836(73)90275-1. [DOI] [PubMed] [Google Scholar]
- Jimenez S., Harsch M., Rosenbloom J. Hydroxyproline stabilizes the triple helix of chick tendon collagen. Biochem Biophys Res Commun. 1973 May 1;52(1):106–114. doi: 10.1016/0006-291x(73)90960-1. [DOI] [PubMed] [Google Scholar]
- Jung A., Bisaz S., Fleisch H. The binding of pyrophosphate and two diphosphonates by hydroxyapatite crystals. Calcif Tissue Res. 1973 Mar 30;11(4):269–280. doi: 10.1007/BF02547227. [DOI] [PubMed] [Google Scholar]
- Kao K. Y., Leslie J. G. Intermolecular cross-links in collagen of human placenta. Biochim Biophys Acta. 1979 Oct 24;580(2):366–371. doi: 10.1016/0005-2795(79)90148-x. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I. Urinary excretion of hydroxyproline in health and disease. Int Rev Connect Tissue Res. 1970;5:93–163. doi: 10.1016/b978-0-12-363705-5.50008-7. [DOI] [PubMed] [Google Scholar]
- Lane J. M., Miller E. J. Isolation and characterization of the peptides derived from the alpha 2 chain of chick bone collagen after cyanogen bromide cleavage. Biochemistry. 1969 May;8(5):2134–2139. doi: 10.1021/bi00833a053. [DOI] [PubMed] [Google Scholar]
- Mechanic G. L., Toverud S. U., Ramp W. K., Gonnerman W. A. The effect of vitamin D on the structural crosslinks and maturation of chick bone collagen. Biochim Biophys Acta. 1975 Jun 26;393(2):419–425. doi: 10.1016/0005-2795(75)90070-7. [DOI] [PubMed] [Google Scholar]
- Meunier P. J., Chapuy M. C., Alexandre C., Bressot C., Edouard C., Vignon C., Mathieu L., Trechsel U. Effects of disodium dichloromethylene diphosphonate on Paget's disease of bone. Lancet. 1979 Sep 8;2(8141):489–492. doi: 10.1016/s0140-6736(79)91551-4. [DOI] [PubMed] [Google Scholar]
- Miller E. J. A review of biochemical studies on the genetically distinct collagens of the skeletal system. Clin Orthop Relat Res. 1973 May;(92):260–280. doi: 10.1097/00003086-197305000-00024. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Lane J. M., Piez K. A. Isolation and characterization of the peptides derived from the alpha-1 chain of chick bone collagen after cyanogen bromide cleavage. Biochemistry. 1969 Jan;8(1):30–39. doi: 10.1021/bi00829a006. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Martin G. R., Piez K. A., Powers M. J. Characterization of chick bone collagen and compositional changes associated with maturation. J Biol Chem. 1967 Dec 10;242(23):5481–5489. [PubMed] [Google Scholar]
- Miller E. J., Robertson P. B. The stability of collagen cross-links when derived from hydroxylsyl residues. Biochem Biophys Res Commun. 1973 Sep 5;54(1):432–439. doi: 10.1016/0006-291x(73)90940-6. [DOI] [PubMed] [Google Scholar]
- Nimni M. E. Collagen: Its structure and function in normal and pathological connective tissues. Semin Arthritis Rheum. 1974 Winter;4(2):95–150. doi: 10.1016/0049-0172(74)90001-8. [DOI] [PubMed] [Google Scholar]
- Reynolds J. J., Murphy H., Mühlbauer R. C., Morgan D. B., Fleisch H. Inhibition by diphosphonates of bone resorption in mice and comparison with grey-lethal osteopetrosis. Calcif Tissue Res. 1973;12(1):59–71. doi: 10.1007/BF02013722. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Shimokomaki M., Bailey A. J. The chemistry of the collagen cross-links. Age-related changes in the reducible components of intact bovine collagen fibres. Biochem J. 1973 Apr;131(4):771–780. doi: 10.1042/bj1310771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. Automated amino acid analysis with sensitive fluorescence detection. J Clin Chem Clin Biochem. 1976 Jul;14(7):361–364. doi: 10.1515/cclm.1976.14.1-12.361. [DOI] [PubMed] [Google Scholar]
- Russell R. G., Mühlbauer R. C., Bisaz S., Williams D. A., Fleisch H. The influence of pyrophosphate, condensed phosphates, phosphonates and other phosphate compounds on the dissolution of hydroxyapatite in vitro and on bone resorption induced by parathyroid hormone in tissue culture and in thyroparathyroidectomised rats. Calcif Tissue Res. 1970;6(3):183–196. doi: 10.1007/BF02196199. [DOI] [PubMed] [Google Scholar]
- Scott P. G., Veis A. The cyanogen bromide peptides of bovine soluble and insoluble collagens. I. Characterization of peptides from soluble type I collagen by sodium dodecylsulphate polyacrylamide gel electrophoresis. Connect Tissue Res. 1976;4(2):107–116. doi: 10.3109/03008207609152206. [DOI] [PubMed] [Google Scholar]
- Shuttleworth C. A., Forrest L. Changes in guinea-pig dermal collagen during development. Eur J Biochem. 1975 Jul 1;55(2):391–395. doi: 10.1111/j.1432-1033.1975.tb02174.x. [DOI] [PubMed] [Google Scholar]
- Siris E. S., Sherman W. H., Baquiran D. C., Schlatterer J. P., Osserman E. F., Canfield R. E. Effects of dichloromethylene diphosphonate on skeletal mobilization of calcium in multiple myeloma. N Engl J Med. 1980 Feb 7;302(6):310–315. doi: 10.1056/NEJM198002073020602. [DOI] [PubMed] [Google Scholar]
- Stegemann H., Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967 Nov;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Toole B. P., Kang A. H., Trelstad R. L., Gross J. Collagen heterogeneity within different growth regions of long bones of rachitic and non-rachitic chicks. Biochem J. 1972 May;127(4):715–720. doi: 10.1042/bj1270715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater C. A., Harris E. D., Jr, Siegel R. C. Native cross-links in collagen fibrils induce resistance to human synovial collagenase. Biochem J. 1979 Sep 1;181(3):639–645. doi: 10.1042/bj1810639. [DOI] [PMC free article] [PubMed] [Google Scholar]


