Abstract
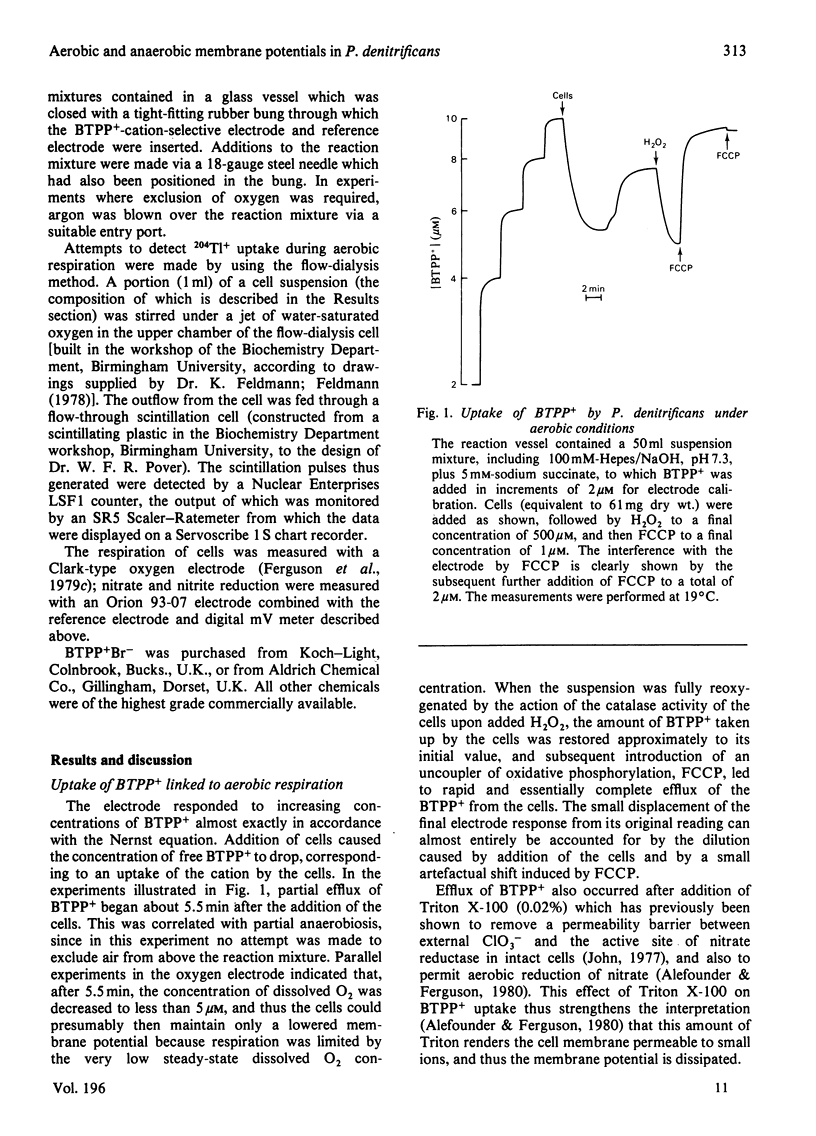
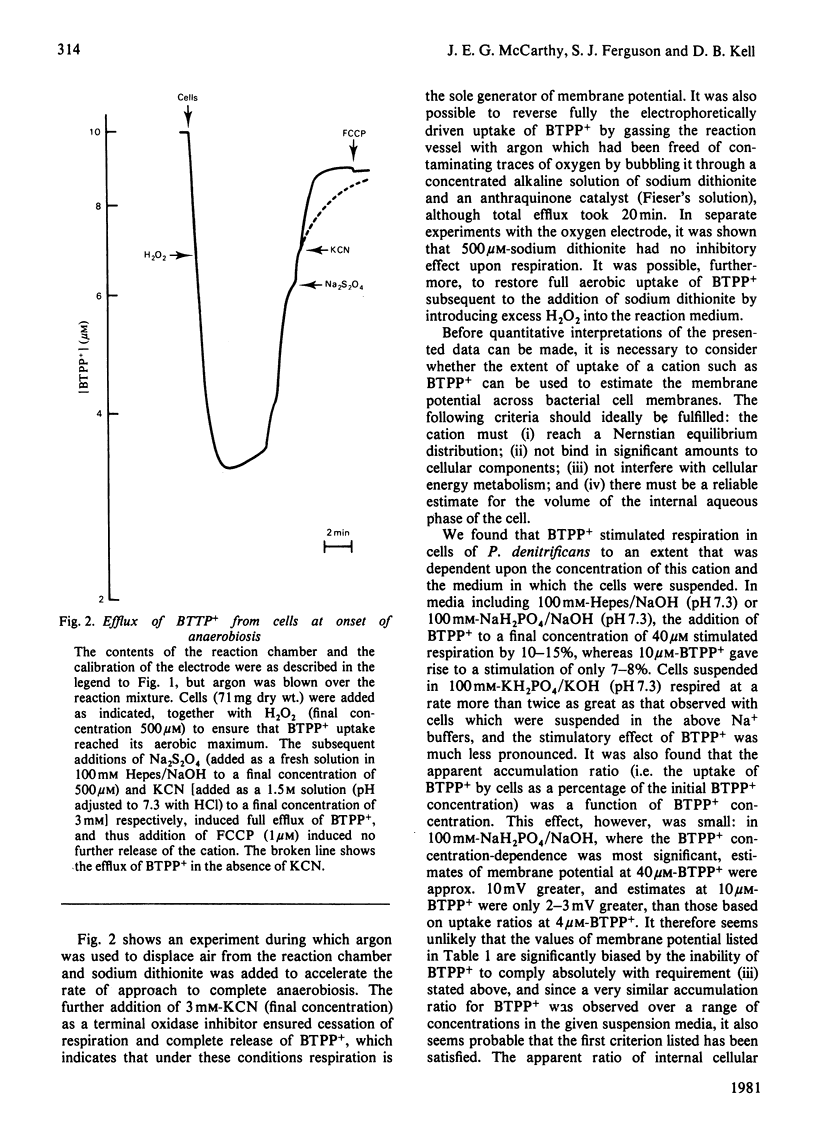
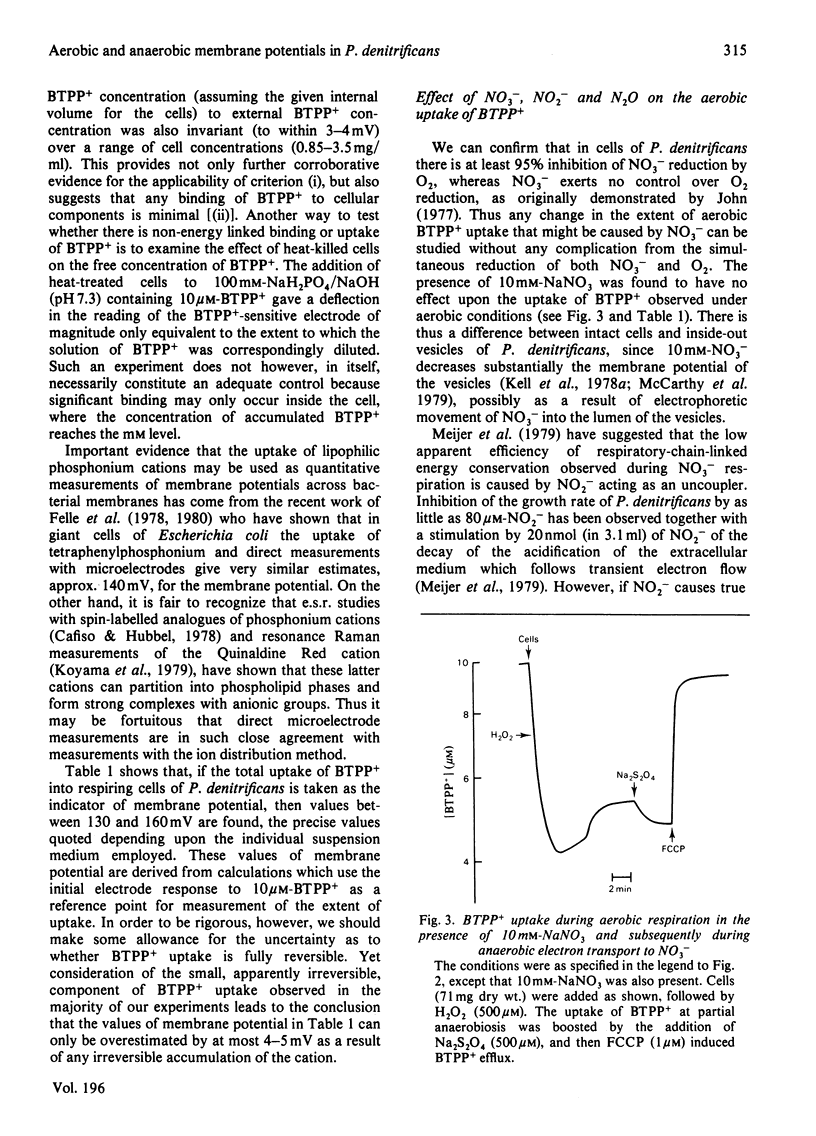
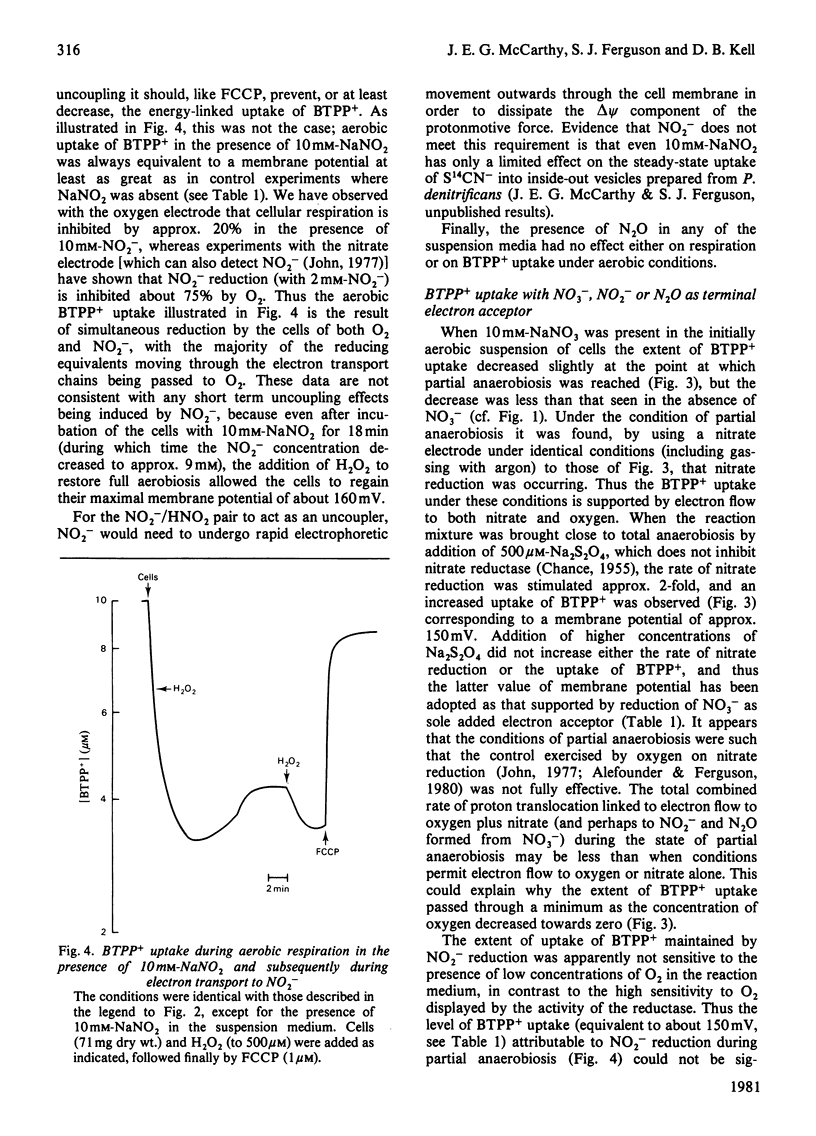
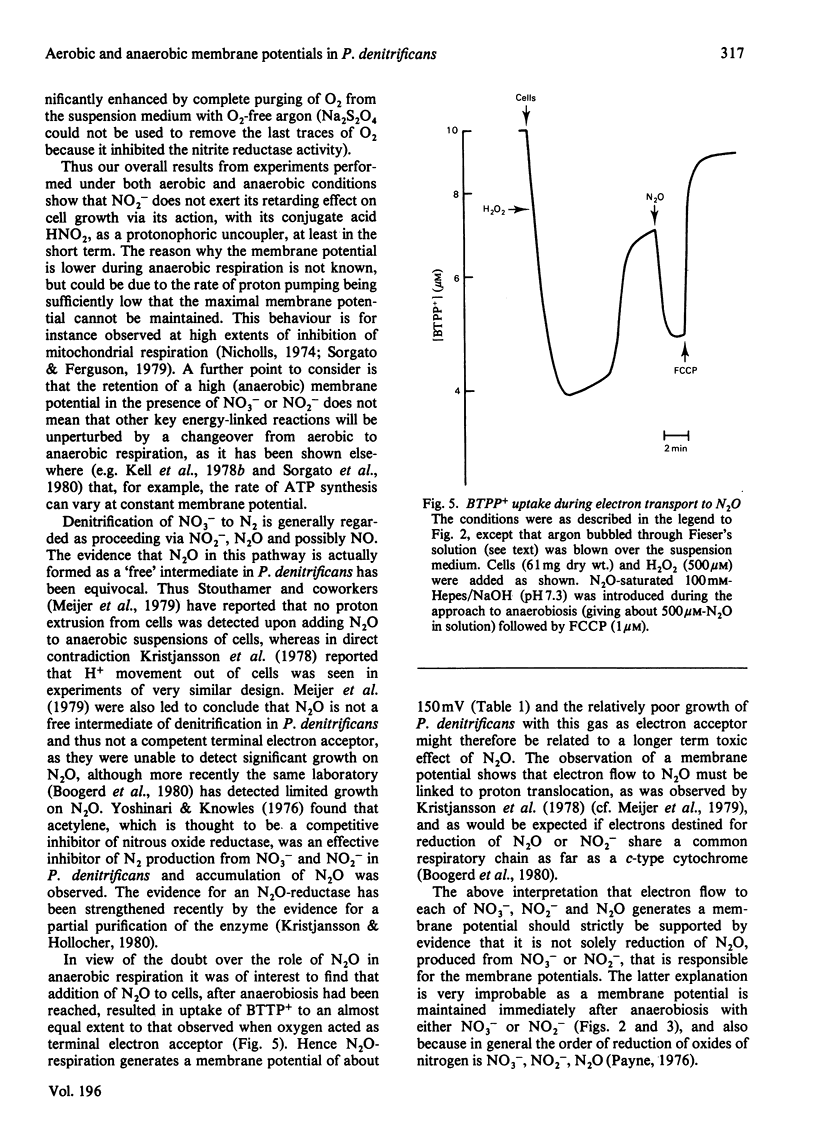
1. Aerobic respiration by cells of Paracoccus dentrificans drives the uptake of the lipophilic cation butyltriphenylphosphonium. Anaerobiosis or addition of an uncoupler of oxidative phosphorylation (carbonyl cyanide p-trifluoromethoxyphenylhydrazone) results in efflux of the cation. Changes in the concentration of butyltriphenylphosphonium in the suspension medium were measured by using an ion-selective electrode, the construction of which is described. 2. If the uptake of butyltriphenylphosphonium is used as an indicator of membrane potential, then at pH 7.3 an estimate of about 160 mV is obtained for cells of P. dentrificans respiring aerobically in 100 mM-Hepes [4-(2-hydroxyethyl)-1-piperazine-ethanesulphonic acid/NaOH or 100mM-NaH2PO4/NaOH. This potential, however, is decreased by more than 20 mV in reaction media containing a high concentration of phosphate (100 mM) together with at least 1 mM-K+. 3. Anaerobic electron transport with NO3-, NO2- or N2O as terminal electron acceptor generates a membrane potential of about 150mV in described suspension media. The presence of these species under aerobic conditions, moreover, has negligible effect upon the extent of uptake of butyltriphenylphosphonium normally driven by aerobic respiration. These data indicate that none of these molecules exert a significant uncoupling effect on the protonmotive force. 4. No 204Tl+ uptake into respiring cells was detected. This adds to the evidence that 204Tl+ is not a freely permeable cation in bacterial cells and therefore not an indicator of membrane potential as has been proposed. The absence of respiration-driven 204Tl+ uptake indicates that P. denitrificans cells grown under the conditions specified in the present work do not possess K+-transport systems of either the Kdp or TrkA types that have been described in Escherichia coli.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alefounder P. R., Ferguson S. J. The location of dissimilatory nitrite reductase and the control of dissimilatory nitrate reductase by oxygen in Paracoccus denitrificans. Biochem J. 1980 Oct 15;192(1):231–240. doi: 10.1042/bj1920231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker E. P. Accumulation of thallous ions (Tl+) as a measure of the electrical potential difference across the cytoplasmic membrane of bacteria. Biochemistry. 1978 Jul 11;17(14):2899–2904. doi: 10.1021/bi00607a031. [DOI] [PubMed] [Google Scholar]
- Bakker E. P., Harold F. M. Energy coupling to potassium transport in Streptococcus faecalis. Interplay of ATP and the protonmotive force. J Biol Chem. 1980 Jan 25;255(2):433–440. [PubMed] [Google Scholar]
- Boogerd F. C., van Verseveld H. W., Stouthamer A. H. Electron transport to nitrous oxide in Paracoccus denitrificans. FEBS Lett. 1980 May 5;113(2):279–284. doi: 10.1016/0014-5793(80)80609-0. [DOI] [PubMed] [Google Scholar]
- Booth I. R., Mitchell W. J., Hamilton W. A. Quantitative analysis of proton-linked transport systems. The lactose permease of Escherichia coli. Biochem J. 1979 Sep 15;182(3):687–696. doi: 10.1042/bj1820687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun I. I., Glagolev A. N., Grinius L. L., Skulachev V. P., Chetkauskaite A. V. Na+/K+-gradient kak faktor, stabiliziruiushchii énergizovannoe sostoianie membrany. Dokl Akad Nauk SSSR. 1979;247(4):971–974. [PubMed] [Google Scholar]
- Burnell J. N., John P., Whatley F. R. The reversibility of active sulphate transport in membrane vesicles of Paracoccus denitrificans. Biochem J. 1975 Sep;150(3):527–536. doi: 10.1042/bj1500527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafiso D. S., Hubbell W. L. Estimation of transmembrane potentials from phase equilibria of hydrophobic paramagnetic ions. Biochemistry. 1978 Jan 10;17(1):187–195. doi: 10.1021/bi00594a028. [DOI] [PubMed] [Google Scholar]
- Damper P. D., Epstein W., Rosen B. P., Sorensen E. N. Thallous ion is accumulated by potassium transport systems in Escherichia coli. Biochemistry. 1979 Sep 18;18(19):4165–4169. doi: 10.1021/bi00586a018. [DOI] [PubMed] [Google Scholar]
- Deutsch C. J., Kula T. Transmembrane electrical and pH gradients of Paracoccus denitrificans and their relationship to oxidative phosphorylation. FEBS Lett. 1978 Mar 1;87(1):145–151. doi: 10.1016/0014-5793(78)80154-9. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Kula T., Wilson D. F. Regulation of energy metabolism: evidence against a primary role of adenine mucleotide translocase. FEBS Lett. 1978 Mar 1;87(1):139–144. doi: 10.1016/0014-5793(78)80153-7. [DOI] [PubMed] [Google Scholar]
- Feldmann K. New devices for flow dialysis and ultrafiltration for the study of protein--ligand interactions. Anal Biochem. 1978 Jul 15;88(1):225–235. doi: 10.1016/0003-2697(78)90414-1. [DOI] [PubMed] [Google Scholar]
- Felle H., Porter J. S., Slayman C. L., Kaback H. R. Quantitative measurements of membrane potential in Escherichia coli. Biochemistry. 1980 Jul 22;19(15):3585–3590. doi: 10.1021/bi00556a026. [DOI] [PubMed] [Google Scholar]
- Ferguson S. J., Gadian D. G., Kell D. B. Evidence from 31P nuclear magnetic resonance that polyphosphate synthesis is a slip reaction in Paracoccus denitrificans [proceedings]. Biochem Soc Trans. 1979 Feb;7(1):176–179. doi: 10.1042/bst0070176. [DOI] [PubMed] [Google Scholar]
- Ferguson S. J., John P., Lloyd W. J., Radda G. K., Whatley F. R. The ATPase as an irreversible component in electron transport linked ATP synthesis. FEBS Lett. 1976 Mar 1;62(3):272–275. doi: 10.1016/0014-5793(76)80073-7. [DOI] [PubMed] [Google Scholar]
- Ferguson S. J., John P. The inhibitor-sensitivity of the plasma-membrane adenosine triphosphatase of Paracoccus denitrificans: comparison with the mitochondrial adenosine triphosphatase [proceedings]. Biochem Soc Trans. 1977;5(5):1525–1527. doi: 10.1042/bst0051525. [DOI] [PubMed] [Google Scholar]
- Ferguson S. J., Jones O. T., Kell D. B., Sorgato M. C. Comparison of permeant ion uptake and carotenoid band shift as methods for determining the membrane potential in chromatophores from Rhodopseudomonas sphaeroides Ga. Biochem J. 1979 Apr 15;180(1):75–85. doi: 10.1042/bj1800075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. J., Sorgato M. C., Kell D. B., John P. Comparative aspects of the energetics of oxidative phosphorylation in bacteria and mitochondria. Biochem Soc Trans. 1979 Oct;7(5):870–874. doi: 10.1042/bst0070870. [DOI] [PubMed] [Google Scholar]
- John P., Whatley F. R. The bioenergetics of Paracoccus denitrificans. Biochim Biophys Acta. 1977 Oct 5;463(2):129–153. doi: 10.1016/0304-4173(77)90006-4. [DOI] [PubMed] [Google Scholar]
- Kamo N., Muratsugu M., Hongoh R., Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol. 1979 Aug;49(2):105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- Kashket E. R. Active transport of thallous ions by Streptococcus lactis. J Biol Chem. 1979 Sep 10;254(17):8129–8131. [PubMed] [Google Scholar]
- Kell D. B., John P., Ferguson S. J. On the current-voltage relationships of energy-transducing membranes: phosphorylating membrane vesicles from Paracoccus denitrificans [proceedings]. Biochem Soc Trans. 1978;6(6):1292–1295. doi: 10.1042/bst0061292. [DOI] [PubMed] [Google Scholar]
- Kell D. B., John P., Ferguson S. J. The protonmotive force in phosphorylating membrane vesicles from Paracoccus denitrificans. Magnitude, sites of generation and comparison with the phosphorylation potential. Biochem J. 1978 Jul 15;174(1):257–266. doi: 10.1042/bj1740257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell D. B., John P., Sorgato M. C., Ferguson S. J. Continuous monitoring of the electrical potential across energy-transducing membranes using ion-selective electrodes. Application to submitochondrial particles and chromatophores. FEBS Lett. 1978 Feb 15;86(2):294–298. doi: 10.1016/0014-5793(78)80583-3. [DOI] [PubMed] [Google Scholar]
- Kell D. B. On the functional proton current pathway of electron transport phosphorylation. An electrodic view. Biochim Biophys Acta. 1979 Jul 3;549(1):55–99. doi: 10.1016/0304-4173(79)90018-1. [DOI] [PubMed] [Google Scholar]
- Koyama Y., Carey P. R., Long R. A., Martin W. G., Schneider H. A resonance Raman and electronic absorption probe of membrane energization. Quinaldine red in cells of Streptococcus faecalis. J Biol Chem. 1979 Oct 25;254(20):10276–10285. [PubMed] [Google Scholar]
- Kristjansson J. K., Hollocher T. C. First practical assay for soluble nitrous oxide reductase of denitrifying bacteria and a partial kinetic characterization. J Biol Chem. 1980 Jan 25;255(2):704–707. [PubMed] [Google Scholar]
- Kristjansson J. K., Walter B., Hollocher T. C. Respiration-dependent proton translocation and the transport of nitrate and nitrite in Paracoccus denitrificans and other denitrifying bacteria. Biochemistry. 1978 Nov 14;17(23):5014–5019. doi: 10.1021/bi00616a024. [DOI] [PubMed] [Google Scholar]
- Lawford H. G. Energy transduction in the mitochondrionlike bacterium Paracoccus denitrificans during carbon- or sulphate-limited aerobic growth in continuous culture. Can J Biochem. 1978 Jan;56(1):13–22. doi: 10.1139/o78-003. [DOI] [PubMed] [Google Scholar]
- Meijer E. M., van der Zwaan J. W., Wever R., Stouthamer A. H. Anaerobic respiration and energy conservation in Paracoccus denitrificans. Functioning of iron-sulfur centers and the uncoupling effect of nitrite. Eur J Biochem. 1979 May 2;96(1):69–76. doi: 10.1111/j.1432-1033.1979.tb13014.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4(3):399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- Muratsugu M., Kamo N., Kurihara K., Kobatake Y. Selective electrode for dibenzyl dimethyl ammonium cation as indicator of the membrane potential in biological systems. Biochim Biophys Acta. 1977 Feb 4;464(3):613–619. doi: 10.1016/0005-2736(77)90035-9. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Russell L. M., Rosenberg H. Linked transport of phosphate, potassium ions and protons in Escherichia coli. Biochem J. 1979 Oct 15;184(1):13–21. doi: 10.1042/bj1840013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. M., Rosenberg H. The nature of the link between potassium transport and phosphate transport in Escherichia coli. Biochem J. 1980 Jun 15;188(3):715–723. doi: 10.1042/bj1880715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes P. B., Smith L. The isolation and properties of the cytoplasmic membrane of Micrococcus denitrificans. Biochim Biophys Acta. 1968 Feb 12;153(2):350–362. doi: 10.1016/0005-2728(68)90080-7. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Acid-base titration across the plasma membrane of Micrococcus denitrificans: factors affecting the effective proton conductance and the respiratory rate. J Bioenerg. 1970 Jun;1(1):61–72. doi: 10.1007/BF01516089. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Respiration-driven proton translocation in Micrococcus denitrificans. J Bioenerg. 1971 Sep;1(3):309–323. doi: 10.1007/BF01516290. [DOI] [PubMed] [Google Scholar]
- Skulachev V. P. Membrane electricity as a convertible energy currency for the cell. Can J Biochem. 1980 Mar;58(3):161–175. doi: 10.1139/o80-023. [DOI] [PubMed] [Google Scholar]
- Skulachev V. P. Membrane-linked energy buffering as the biological function of Na+/K+ gradient. FEBS Lett. 1978 Mar 15;87(2):171–179. doi: 10.1016/0014-5793(78)80326-3. [DOI] [PubMed] [Google Scholar]
- Sorgato M. C., Branca D., Ferguson S. J. The rate of ATP synthesis by submitochondrial particles can be independent of the magnitude of the protonmotive force. Biochem J. 1980 Jun 15;188(3):945–948. doi: 10.1042/bj1880945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgato M. C., Ferguson S. J. Variable proton conductance of submitochondrial particles. Biochemistry. 1979 Dec 11;18(25):5737–5742. doi: 10.1021/bi00592a034. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Wagner G., Hartmann R., Oesterhelt D. Potassium uniport and ATP synthesis in Halobacterium halobium. Eur J Biochem. 1978 Aug 15;89(1):169–179. doi: 10.1111/j.1432-1033.1978.tb20909.x. [DOI] [PubMed] [Google Scholar]
- Yoshinari T., Knowles R. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem Biophys Res Commun. 1976 Apr 5;69(3):705–710. doi: 10.1016/0006-291x(76)90932-3. [DOI] [PubMed] [Google Scholar]
- van Verseveld H. W., Stouthamer A. H. Growth yields and the efficiency of oxidative phosphorylation during autotrophic growth of Paracoccus denitrificans on methanol and formate. Arch Microbiol. 1978 Jul;118(1):21–26. doi: 10.1007/BF00406069. [DOI] [PubMed] [Google Scholar]



