Abstract
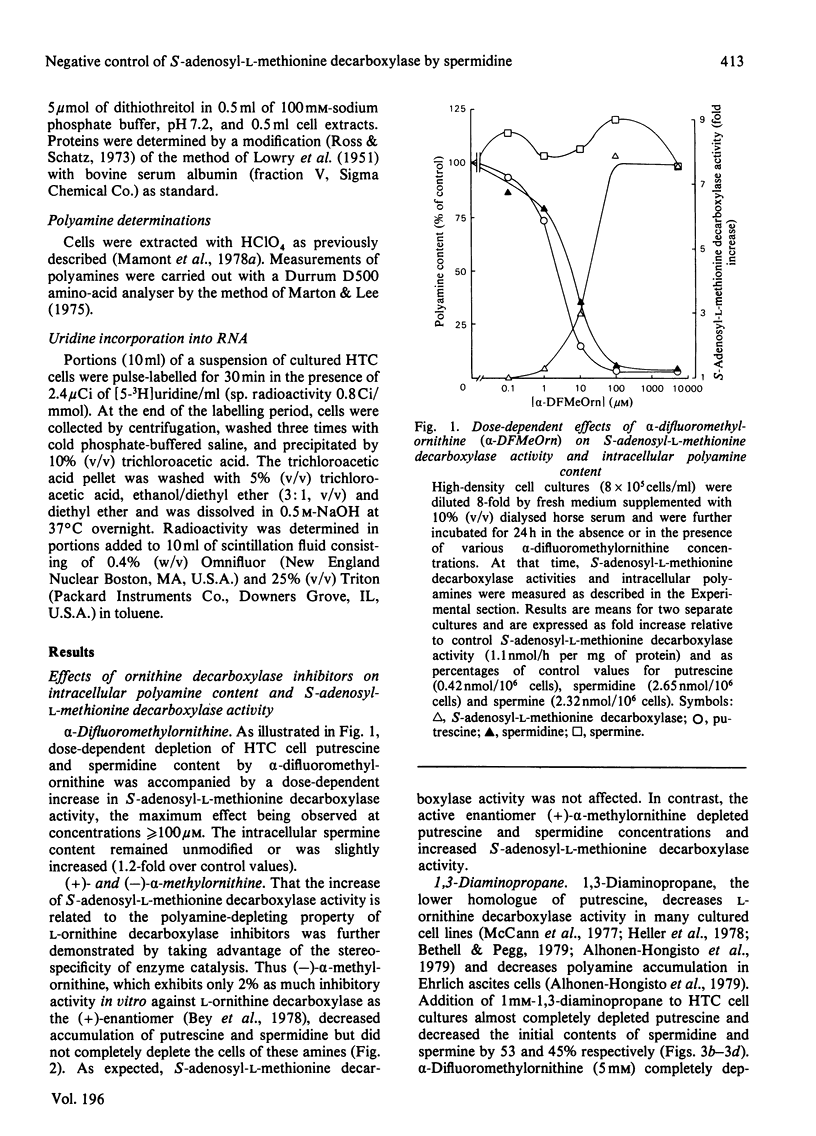
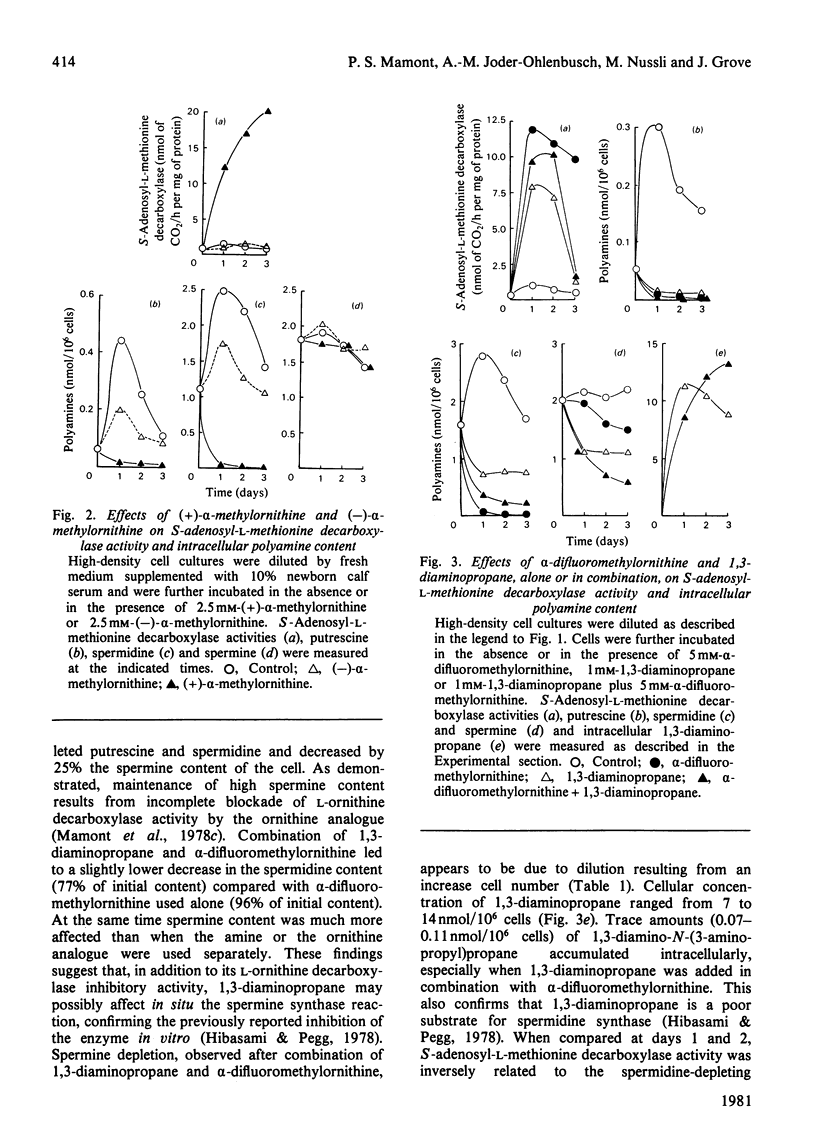
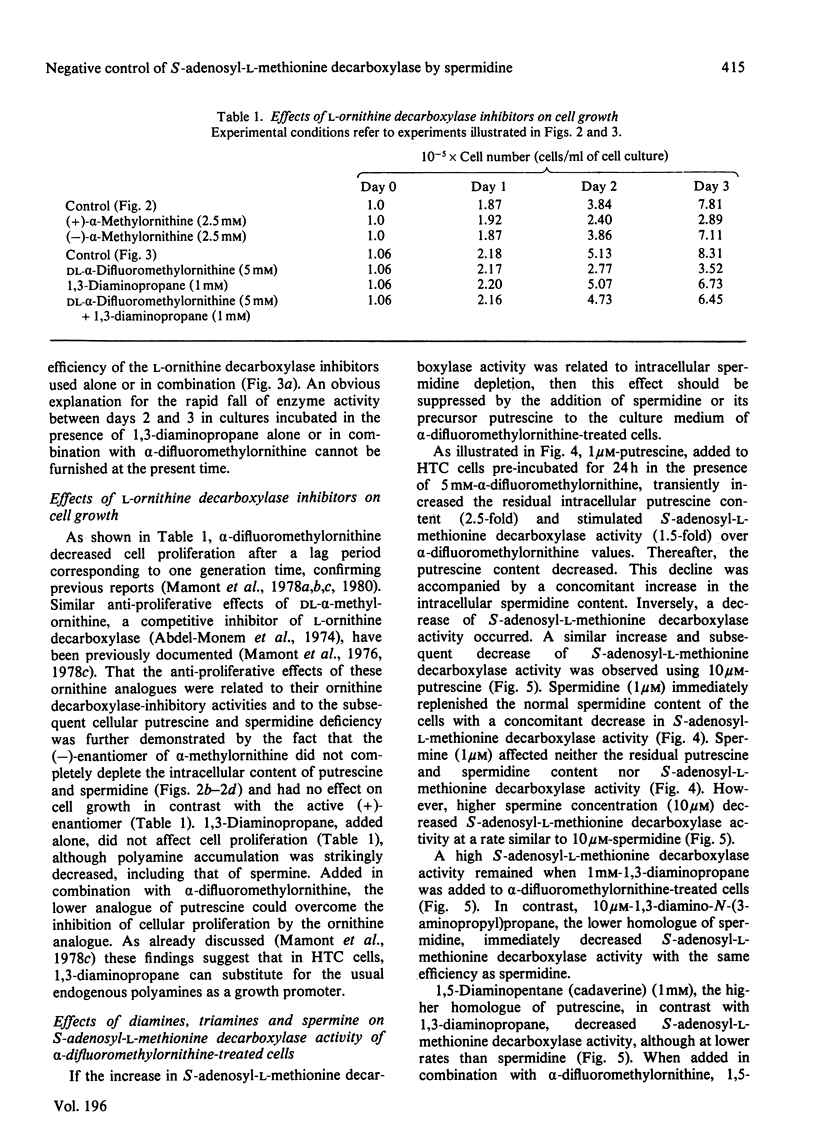
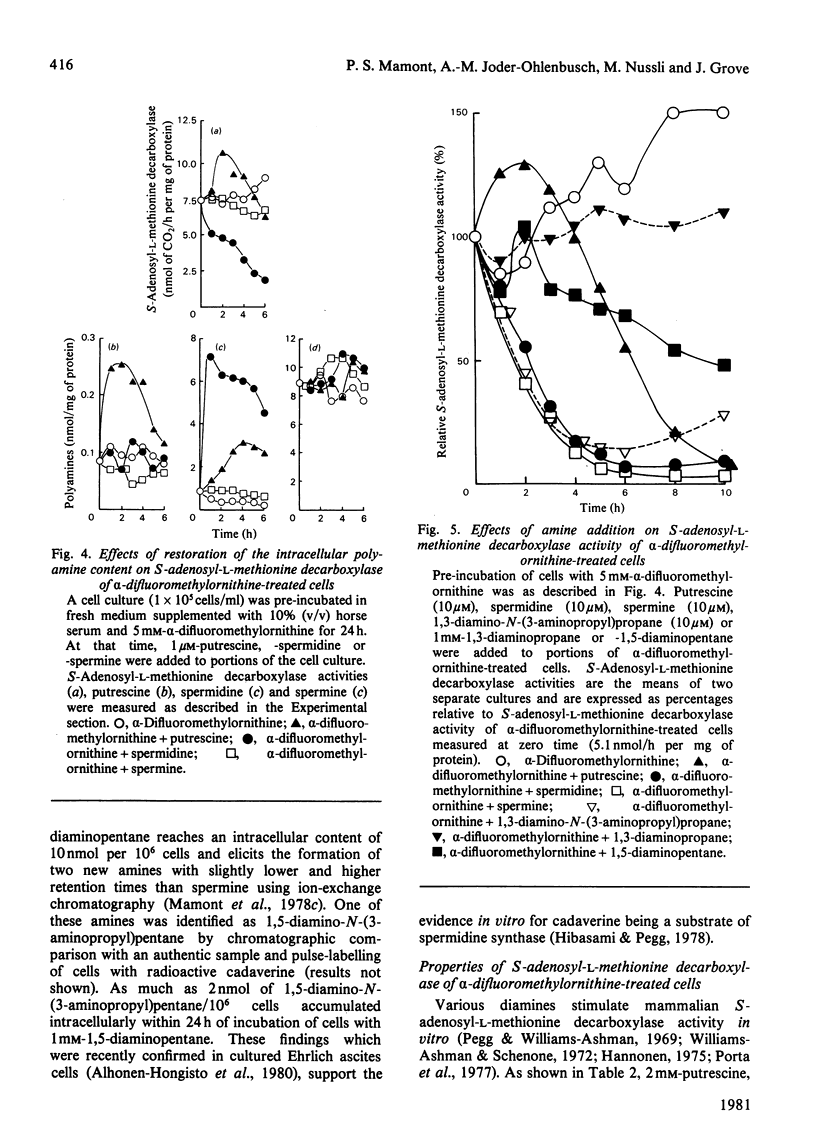
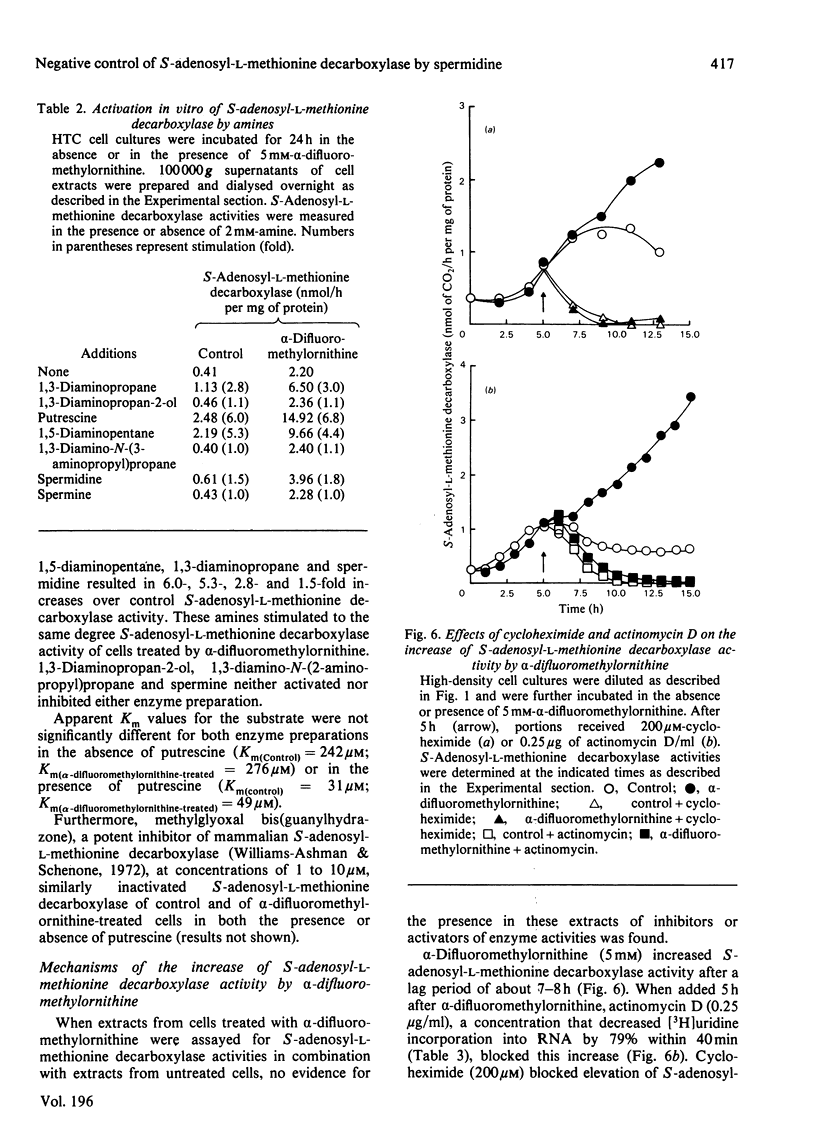
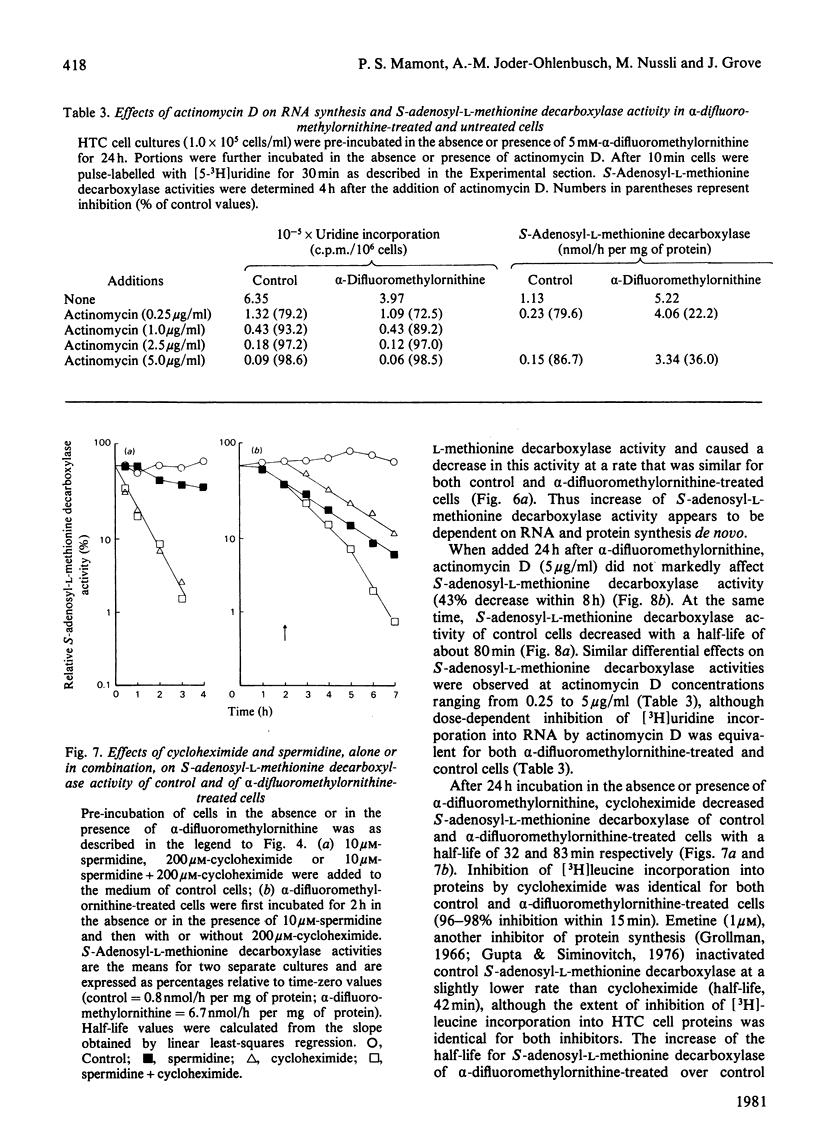
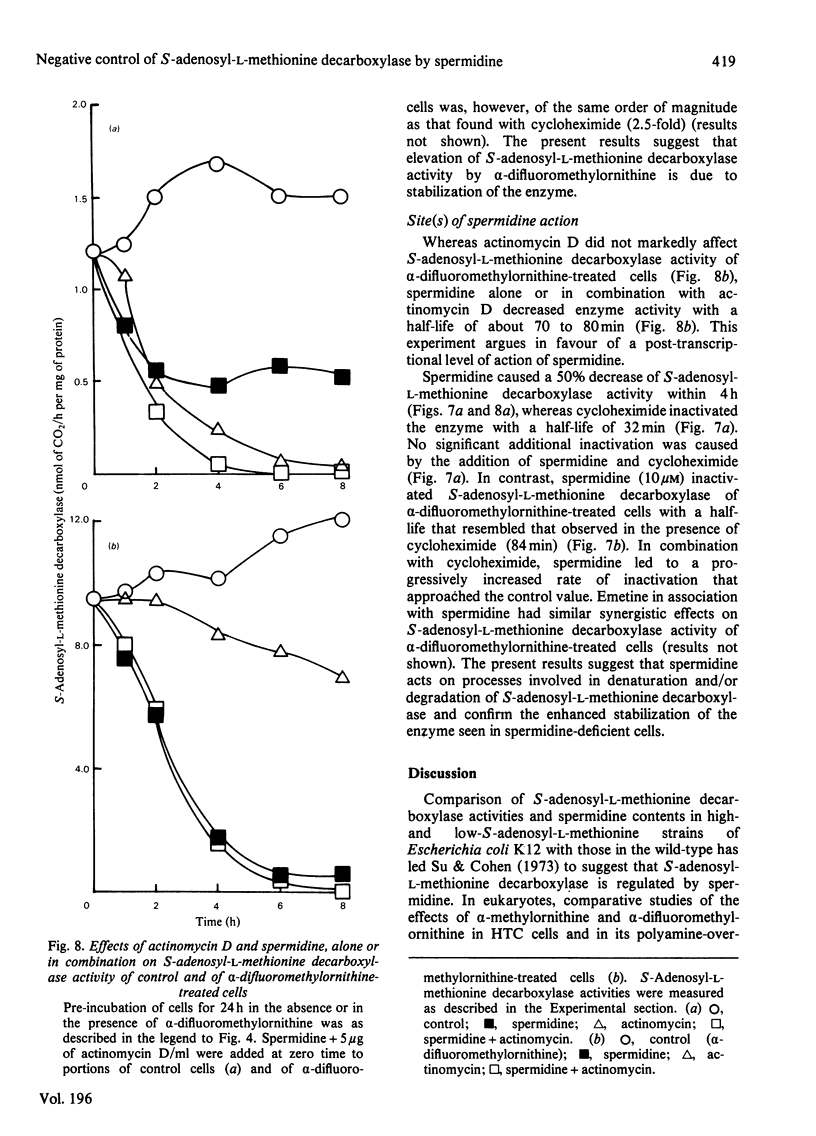
1. Direct or indirect inhibitors of l-ornithine decarboxylase (EC 4.1.1.17), structurally related or unrelated to l-ornithine, including dl-α-difluoromethylornithine, α-methylornithine and 1,3-diaminopropane, used alone or in combination, decreased polyamine concentrations in rat hepatoma tissue culture (HTC) cells and increased S-adenosyl-l-methionine decarboxylase activity (EC 4.1.1.50). 2. Comparison of the catalytic properties of S-adenosyl-l-methionine from cells with elevated and normal activities revealed no apparent modification of the catalytic site as judged by affinity for the substrate, stimulation by di- and tri-amines and inhibition by methylglyoxal bis-(guanylhydrazone). 3. Actinomycin D and cycloheximide, and RNA and a proteinsynthesis inhibitor respectively, blocked the increase of S-adenosyl-l-methionine decarboxylase activity elicited by α-difluoromethylornithine. In polyamine-depleted cells the apparent half-life of elevated S-adenosyl-l-methionine decarboxylase activity, determined by inhibition of protein synthesis, was 2.5-fold longer than in control cells. The present results suggest that elevation of S-adenosyl-l-methionine decarboxylase activity by α-difluoromethylornithine is due to stabilization of the enzyme. 4. Restoration of the normal intracellular putrescine content, by addition of putrescine to the medium of polyamine-deficient cells, transiently increased S-adenosyl-l-methionine decarboxylase activity. Thereafter, intracellular conversion of putrescine into spermidine was accompanied by inactivation of the enzyme at a rate that was similar to that found on addition of spermidine itself. No relationship between total intracellular spermine content and S-adenosyl-l-methionine decarboxylase activity could be established. 5. Addition of 1mm-1,3-diaminopropane to polyamine-deficient cells did not cause a decrease in the activity of S-adenosyl-l-methionine decarboxylase, whereas addition of 1,5-diaminopentane (cadaverine) did. 1,3-Diamino-N-(3-aminopropyl)propane did not accumulate in cells treated with α-difluoromethylornithine and 1,3-diaminopropane, whereas addition of 1,5-diaminopentane led to the accumulation of 1,5-diamino-N-(3-aminopropyl)pentane. 1,3-Diamino-N-(3-aminopropyl)propane (10μm) was as effective as spermidine in decreasing S-adenosyl-l-methionine decarboxylase activity. Thus effectiveness of a diamine in decreasing enzyme activity is related to its capability of being converted into a closely structurally related homologue of spermidine by spermidine synthase. 6. The spermidine site of action appears to be post-translational since (a) the spermidine-induced decrease of S-adenosyl-l-methionine activity was not prevented by actinomycin D and (b) spermidine in the presence of cycloheximide led to a synergistic inactivation of the enzyme with a decay rate that progressively approached control values. Altogether these results are indirect evidence for a strict negative control of S-adenosyl-l-methionine decarboxylase by spermidine and substantiate previous findings [Mamont, Duchesne, Grove & Tardif (1978) Exp. Cell Res. 115, 387–393]. Spermidine appears to act on some processes involved in denaturation and/or degradation of the enzyme protein. Putrescine appears to decrease the rate of these processes. The physiological significance of the regulatory control of S-adenosyl-l-methionine decarboxylase is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Monem M. M., Newton N. E., Weeks C. E. Inhibitors of polyamine biosynthesis. 1. Alpha-methyl-(plus or minus)-ornithine, an inhibitor of ornithine decarboxylase. J Med Chem. 1974 Apr;17(4):447–451. doi: 10.1021/jm00250a016. [DOI] [PubMed] [Google Scholar]
- Alhonen-Hongisto L., Pösö H., Jänne J. Inhibition by derivatives of diguanidines of cell proliferation in Ehrlich ascites cells grown in cultures. Biochem J. 1980 May 15;188(2):491–501. doi: 10.1042/bj1880491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhonen-Hongisto L., Pösö H., Jänne J. Inhibition of polyamine accumulation and cell proliferation by derivatives of diaminopropane in Ehrlich ascites cells grown in culture. Biochim Biophys Acta. 1979 Oct 25;564(3):473–487. doi: 10.1016/0005-2787(79)90037-6. [DOI] [PubMed] [Google Scholar]
- Alhonen-Hongisto L. Regulation of S-adenosylmethionine decarboxylase by polyamines in Ehrlich ascites-carcinoma cells grown in culture. Biochem J. 1980 Sep 15;190(3):747–754. doi: 10.1042/bj1900747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auricchio F., Martin D., Jr, Tompkins G. Control of degradation and synthesis of induced tyrosine aminotransferase studied in hepatoma cells in culture. Nature. 1969 Nov 22;224(5221):806–808. doi: 10.1038/224806b0. [DOI] [PubMed] [Google Scholar]
- Bethell D. R., Pegg A. E. Effects of diamines on ornithine decarboxylase activity in control and virally transformed mouse fibroblasts. Biochem J. 1979 Apr 15;180(1):87–94. doi: 10.1042/bj1800087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey P., Danzin C., Van Dorsselaer V., Mamont P., Jung M., Tardif C. Analogues of ornithine as inhibitors of ornithine decarboxylase. New deductions concerning the topography of the enzyme's active site. J Med Chem. 1978 Jan;21(1):50–55. doi: 10.1021/jm00199a009. [DOI] [PubMed] [Google Scholar]
- Demetriou A. A., Cohn M. S., Tabor C. W., Tabor H. Identification of pyruvate in S-adenosylmethionine decarboxylase from rat liver. J Biol Chem. 1978 Mar 10;253(5):1684–1686. [PubMed] [Google Scholar]
- Eloranta T. O., Raina A. M. Formation of CO2 from the carboxyl group of S-adenosylmethionine by liver membrane-associated enzymes involves the demethylation-transsulphuration pathway. Biochem Biophys Res Commun. 1978 Sep 14;84(1):23–30. doi: 10.1016/0006-291x(78)90257-7. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H., Morris D. R. S-adenosyl-L-methionine decarboxylase during lymphocyte transformation: decreased degradation in the presence of a specific inhibitor. Biochem Biophys Res Commun. 1973 Jun 8;52(3):1020–1025. doi: 10.1016/0006-291x(73)91039-5. [DOI] [PubMed] [Google Scholar]
- Fozard J. R., Part M. L., Prakash N. J., Grove J., Schechter P. J., Sjoerdsma A., Koch-Weser J. L-Ornithine decarboxylase:an essential role in early mammalian embryogenesis. Science. 1980 May 2;208(4443):505–508. doi: 10.1126/science.6768132. [DOI] [PubMed] [Google Scholar]
- Grollman A. P. Structural basis for inhibition of protein synthesis by emetine and cycloheximide based on an analogy between ipecac alkaloids and glutarimide antibiotics. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1867–1874. doi: 10.1073/pnas.56.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. The isolation and preliminary characterization of somatic cell mutants resistant to the protein synthesis inhibitor-emetine. Cell. 1976 Oct;9(2):213–219. doi: 10.1016/0092-8674(76)90112-4. [DOI] [PubMed] [Google Scholar]
- Hannonen P. Enzymic decarboxylation of s-adenosyl-l-methionine in rat liver: possible interaction of putrescine with the prosthetic group. Acta Chem Scand B. 1975;29(3):295–299. doi: 10.3891/acta.chem.scand.29b-0295. [DOI] [PubMed] [Google Scholar]
- Hannonen P., Raina A., Jänne J. Polyamine synthesis in the regenerating rat liver: stimulation of S-adenosyl methionine decarboxylase, and spermidine and spermine synthases after partial hepatectomy. Biochim Biophys Acta. 1972 Jun 26;273(1):84–90. doi: 10.1016/0304-4165(72)90194-8. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Chen K. Y., Kyriakidis D. A., Fong W. F., Canellakis E. S. The modulation of the induction of ornithine decarboxylase by spermine, spermidine and diamines. J Cell Physiol. 1978 Aug;96(2):225–234. doi: 10.1002/jcp.1040960211. [DOI] [PubMed] [Google Scholar]
- Hershko A., Tomkins G. M. Studies on the degradation of tyrosine aminotransferase in hepatoma cells in culture. Influence of the composition of the medium and adenosine triphosphate dependence. J Biol Chem. 1971 Feb 10;246(3):710–714. [PubMed] [Google Scholar]
- Hibasami H., Pegg A. E. Differential inhibition of mammalian aminopropyltransferase activities. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1398–1405. doi: 10.1016/0006-291x(78)91291-3. [DOI] [PubMed] [Google Scholar]
- Hopkins D., Manchester K. L. Control of activity of S-adenosylmethionine decarboxylase in muscle by spermidine. FEBS Lett. 1980 Jan 14;109(2):299–302. doi: 10.1016/0014-5793(80)81109-4. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Lindsay V. J. Control of ornithine decarboxylase activity in stimulated human lymphocytes by putrescine and spermidine. Biochem J. 1973 Apr;132(4):791–796. doi: 10.1042/bj1320791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney F. T., Lee K. L., Stiles C. D., Fritz J. E. Further evidence against post-transcriptional control of inducible tyrosine aminotransferase synthesis in cultured hepatoma cells. Nat New Biol. 1973 Dec 19;246(155):208–210. doi: 10.1038/newbio246208a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mamont P. S., Böhlen P., McCann P. P., Bey P., Schuber F., Tardif C. Alpha-methyl ornithine, a potent competitive inhibitor of ornithine decarboxylase, blocks proliferation of rat hepatoma cells in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1626–1630. doi: 10.1073/pnas.73.5.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Tardif C. Initial characterization of a HTC cell variant partially resistant to the anti-proliferative effect of ornithine decarboxylase inhibitors. Exp Cell Res. 1978 Sep;115(2):387–393. doi: 10.1016/0014-4827(78)90292-6. [DOI] [PubMed] [Google Scholar]
- Marton L. J., Lee P. L. More sensitive automated detection of polyamines in physiological fluids and tissue extracts with omicron-phthalaldehyde. Clin Chem. 1975 Nov;21(12):1721–1724. [PubMed] [Google Scholar]
- Maudsley D. V. Regulation of polyamine biosynthesis. Biochem Pharmacol. 1979;28(2):153–161. doi: 10.1016/0006-2952(79)90496-9. [DOI] [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Duchesne M. C., Mamont P. S. Effect of alpha-methyl ornithine on ornithine decarboxylase activity of rat hepatoma cells in culture. Biochem Biophys Res Commun. 1977 Jun 6;76(3):893–899. doi: 10.1016/0006-291x(77)91585-6. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Schimke R. T. Regulation of protein synthesis in chick oviduct. 3. Mechanism of ovalbumin "superinduction" by actinomycin D. J Biol Chem. 1973 Mar 10;248(5):1502–1512. [PubMed] [Google Scholar]
- Pegg A. E. Investigation of the turnover of rat liver S-adenosylmethionine decarboxylase using a specific antibody. J Biol Chem. 1979 May 10;254(9):3249–3253. [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Porta R., Esposito C., Pietra G. D. S-adenosylmethionine decarboxylase from human placenta. Int J Biochem. 1977;8(5):347–352. doi: 10.1016/0020-711x(77)90003-9. [DOI] [PubMed] [Google Scholar]
- Prakash N. J., Schechter P. J., Mamont P. S., Grove J., Koch-Weser J., Sjoerdsma A. Inhibition of EMT6 tumor growth by interference with polyamine biosynthesis; effects of alpha-difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase. Life Sci. 1980 Jan 21;26(3):181–194. doi: 10.1016/0024-3205(80)90292-1. [DOI] [PubMed] [Google Scholar]
- Ross E., Schatz G. Assay of protein in the presence of high concentrations of sulfhydryl compounds. Anal Biochem. 1973 Jul;54(1):304–306. doi: 10.1016/0003-2697(73)90280-7. [DOI] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Hori C., Kano K., Oka T. Purification and characterization of S-adenosyl-L-methionine decarboxylase from mouse mammary gland and liver. Biochemistry. 1979 Dec 11;18(25):5541–5548. doi: 10.1021/bi00592a003. [DOI] [PubMed] [Google Scholar]
- Sakai T., Perry J. W., Hori C., Oka T. Putrescine and the regulation of S-adenosyl-L-methionine decarboxylase in cultured mouse mammary gland. Biochim Biophys Acta. 1980 Aug 7;614(2):577–582. doi: 10.1016/0005-2744(80)90246-6. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Stability of HeLa cell mRNA in actinomycin. Nature. 1972 Nov 10;240(5376):100–102. doi: 10.1038/240100a0. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., Levinson B. B., Tomkins G. M. "Superinduction" of tyrosine aminotransferase by actinomycin D: a reevaluation. Cell. 1975 May;5(1):29–35. doi: 10.1016/0092-8674(75)90088-4. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Tomkins G. M., Curran J. F. Induction of tyrosine alpha-ketoglutarate transaminase by steroid hormones in a newly established tissue culture cell line. Proc Natl Acad Sci U S A. 1966 Jul;56(1):296–303. doi: 10.1073/pnas.56.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Canellakis Z. N. Polyamines in mammalian biology and medicine. Perspect Biol Med. 1979 Spring;22(3):421–453. doi: 10.1353/pbm.1979.0013. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Schenone A. Methyl glyoxal bis(guanylhydrazone) as a potent inhibitor of mammalian and yeast S-adenosylmethionine decarboxylases. Biochem Biophys Res Commun. 1972 Jan 14;46(1):288–295. doi: 10.1016/0006-291x(72)90661-4. [DOI] [PubMed] [Google Scholar]
- Wilson J., Corti A., Hawkins M., Williams-Ashman H. G., Pegg A. E. The decarboxylation of S-adenosylmethionine by detergent-treated extracts of rat liver. Biochem J. 1979 Jun 15;180(3):515–522. doi: 10.1042/bj1800515. [DOI] [PMC free article] [PubMed] [Google Scholar]


