Abstract
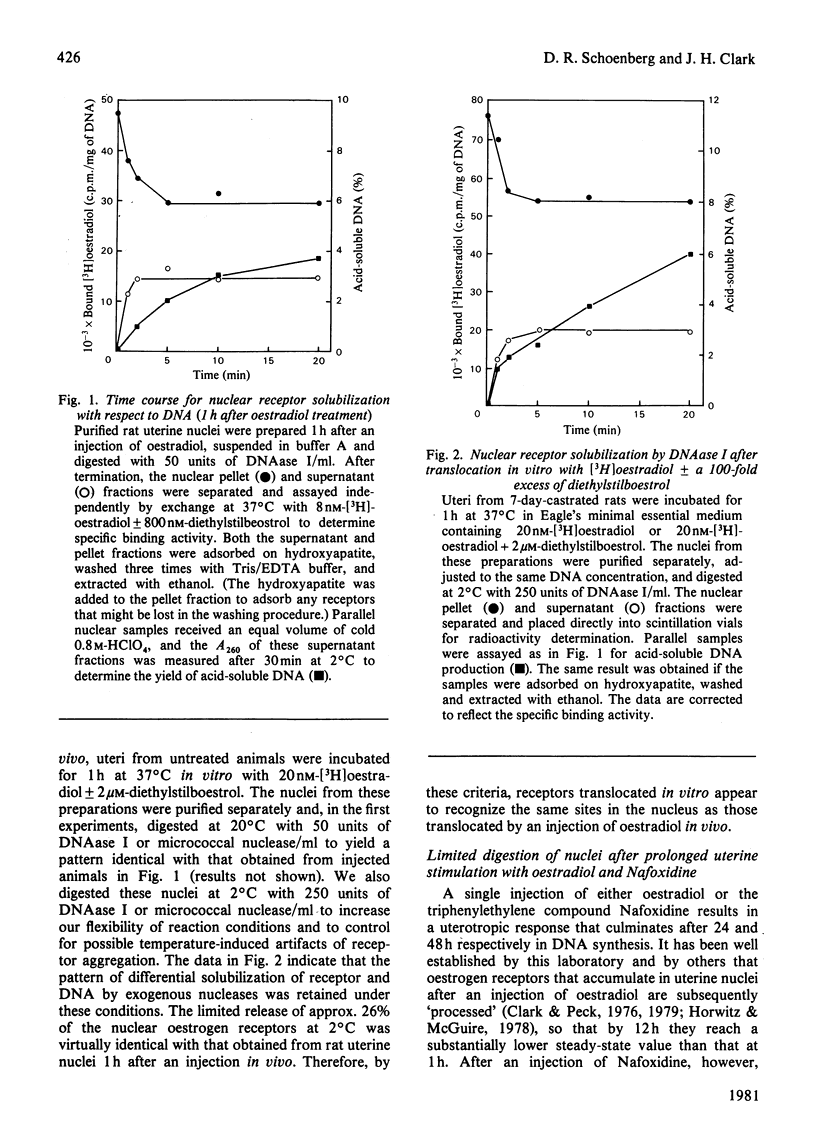
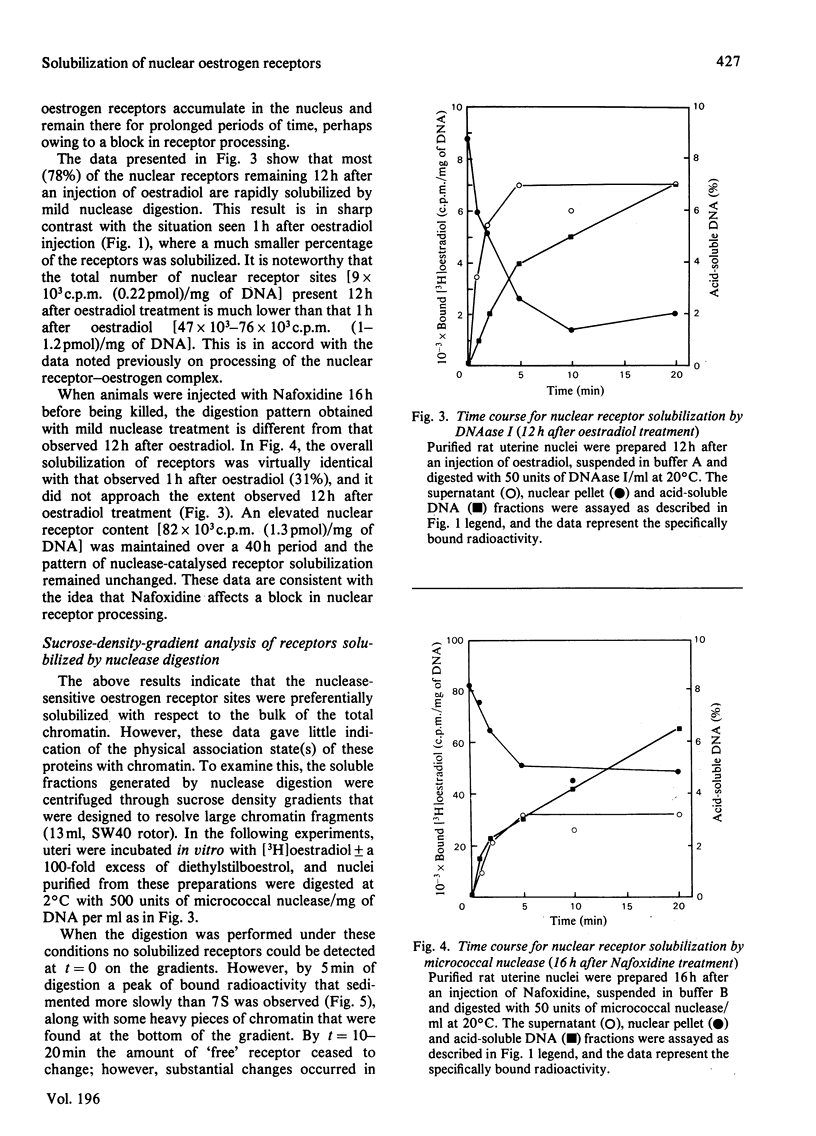
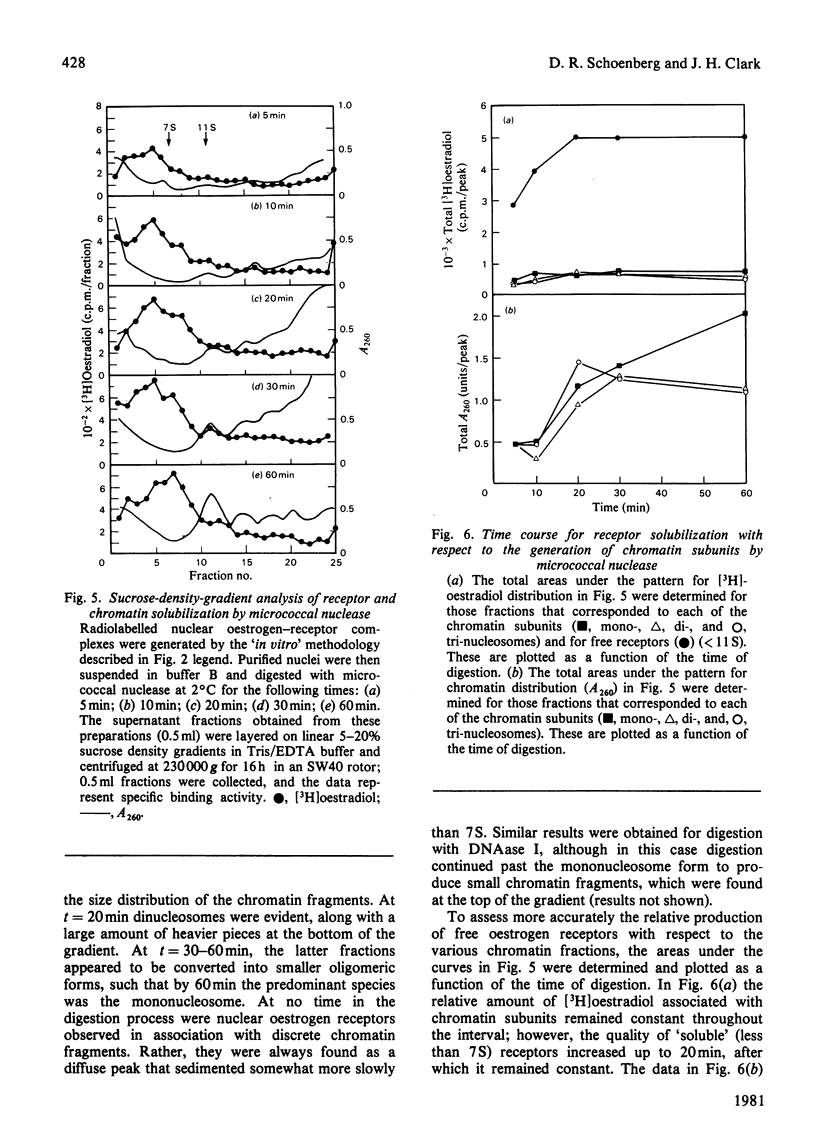
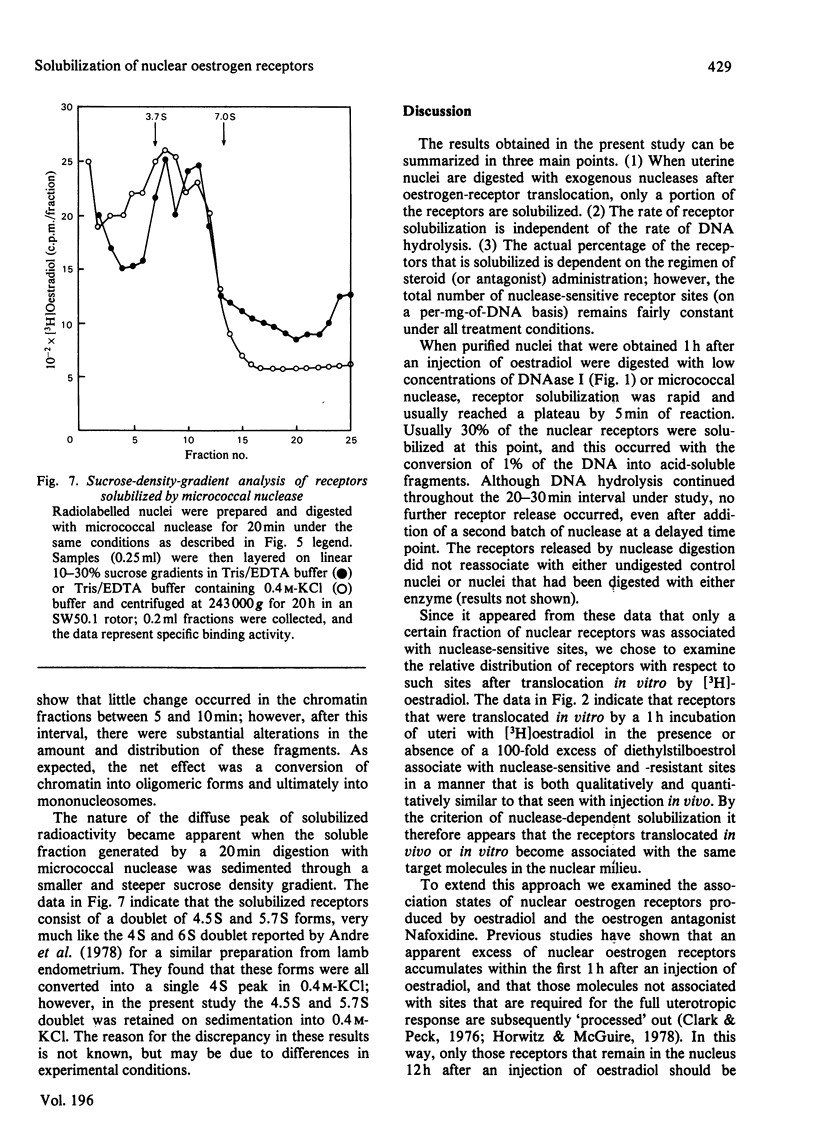
The solubilization of oestrogen receptors from uterine nuclei by micrococcal nuclease and deoxyribonuclease I was examined after the injection of oestradiol or Nafoxidine into castrated female rats. At 1h after an injection of oestradiol, 30% (0.18pmol/mg of DNA) of the nuclear oestrogen receptors was solubilized by 5 min of mild digestion with either nuclease. No further receptor release occurred, although DNA hydrolysis continued throughout a 20min interval. The limitation in receptor solubilization was not due to an artifact of digestion conditions or insufficient nuclease concentrations. Similar patterns of receptor solubilization and DNA hydrolysis were obtained with both nucleases whether the animals had been injected with oestradiol 1h before death or if the uteri from uninjected animals were incubated with [3H]oestradiol for 1h in vitro. When uterine nuclei were digested with these enzymes 12h after the animal was injected with oestradiol there was little change in the quantity of nuclease-sensitive sites (0.11pmol/mg of DNA); however, the quantity of nuclease-resistant sites decreased 10-fold. These values correspond quantitatively to the changes in salt-resistant and salt-extractable sites observed over a 12h interval after oestradiol treatment. Nuclease digestion of uterine nuclei obtained 16h after Nafoxidine treatment gave a pattern qualitatively and quantitatively similar to that observed 1h after oestradiol treatment, a result consistent with the agonist/antagonist action of this compound. An analysis by sucrose-density-gradient centrifugation of the time course of nuclease-dependent receptor solubilization indicated that the solubilized receptors were not associated with discrete nucleosomal fragments. We believe that these data indicate that only a portion of the receptors translocated to the nucleus become associated with chromatin, and this association may occur on regions of chromatin that are preferentially susceptible to nucleolytic cleavage.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J., Clark J. H., Peck E. J., Jr Oestrogen and nuclear binding sites. Determination of specific sites by ( 3 H)oestradiol exchange. Biochem J. 1972 Feb;126(3):561–567. doi: 10.1042/bj1260561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre J., Raynaud A., Rochefort H. Characterization of the estradiol receptor extracted from nuclei by micrococcal nuclease. Biochemistry. 1978 Aug 22;17(17):3619–3626. doi: 10.1021/bi00610a031. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrack E. R., Hawkins E. F., Allen S. L., Hicks L. L., Coffey D. S. Concepts related to salt resistant estradiol receptors in rat uterine nuclei: nuclear matrix. Biochem Biophys Res Commun. 1977 Dec 7;79(3):829–836. doi: 10.1016/0006-291x(77)91186-x. [DOI] [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Fractionation of hen oviduct chromatin into transcriptionally active and inactive regions after selective micrococcal nuclease digestion. Cell. 1978 Sep;15(1):141–150. doi: 10.1016/0092-8674(78)90090-9. [DOI] [PubMed] [Google Scholar]
- Capony F., Rochefort H. In vivo effect of anti-estrogens on the localisation and replenishment of estrogen receptor. Mol Cell Endocrinol. 1975 Sep;3(3):233–251. doi: 10.1016/0303-7207(75)90047-7. [DOI] [PubMed] [Google Scholar]
- Clark J. H., Peck E. J., Jr, Anderson J. N. Oestrogen receptors and antagonism of steroid hormone action. Nature. 1974 Oct 4;251(5474):446–448. doi: 10.1038/251446a0. [DOI] [PubMed] [Google Scholar]
- Clark J. H., Peck E. J. Nuclear retention of receptor-oestrogen complex and nuclear acceptor sites. Nature. 1976 Apr 15;260(5552):635–637. doi: 10.1038/260635a0. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. W., Clark J. H., Glasser S. R., Peck E. J., Jr RNA polymerase activity and uterine growth: Differential stimulation by estradiol, estriol, and nafoxidine. Biochemistry. 1976 Apr 6;15(7):1370–1374. doi: 10.1021/bi00652a003. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., McGuire W. L. Estrogen control of progesterone receptor in human breast cancer. Correlation with nuclear processing of estrogen receptor. J Biol Chem. 1978 Apr 10;253(7):2223–2228. [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Katzenellenbogen J. A., Ferguson E. R., Krauthammer N. Anti-estrogen interaction with uterine estrogen receptors. Studies with a radiolabeled anti-estrogen (CI-628). J Biol Chem. 1978 Feb 10;253(3):697–707. [PubMed] [Google Scholar]
- Lawson G. M., Tsai M. J., O'Malley B. W. Deoxyribonuclease I sensitivity of the nontranscribed sequences flanking the 5' and 3' ends of the ovomucoid gene and the ovalbumin and its related X and Y genes in hen oviduct nuclei. Biochemistry. 1980 Sep 16;19(19):4403–4441. doi: 10.1021/bi00560a004. [DOI] [PubMed] [Google Scholar]
- Massol N., Lebeau M. C., Baulieu E. E. Estrogen receptor in hen oviduct chromatin, digested by micrococcal nuclease. Nucleic Acids Res. 1978 Mar;5(3):723–738. doi: 10.1093/nar/5.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie P. S. Binding of androgen receptor to prostatic chromatin requires intact linker DNA. J Biol Chem. 1979 May 25;254(10):3947–3952. [PubMed] [Google Scholar]
- Schoenberg D. R., Clark J. H. Effect of intercalating drugs on the release of uterine nuclear estrogen receptors. J Biol Chem. 1979 Sep 10;254(17):8270–8275. [PubMed] [Google Scholar]
- Scott R. W., Frankel F. R. Enrichment of estradiol-receptor complexes in a transcriptionally active fraction of chromatin from MCF-7 cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1291–1295. doi: 10.1073/pnas.77.3.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior M. B., Frankel F. R. Evidence for two kinds of chromatin binding sites for the estradiol-receptor complex. Cell. 1978 Aug;14(4):857–863. doi: 10.1016/0092-8674(78)90341-0. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]


