Abstract
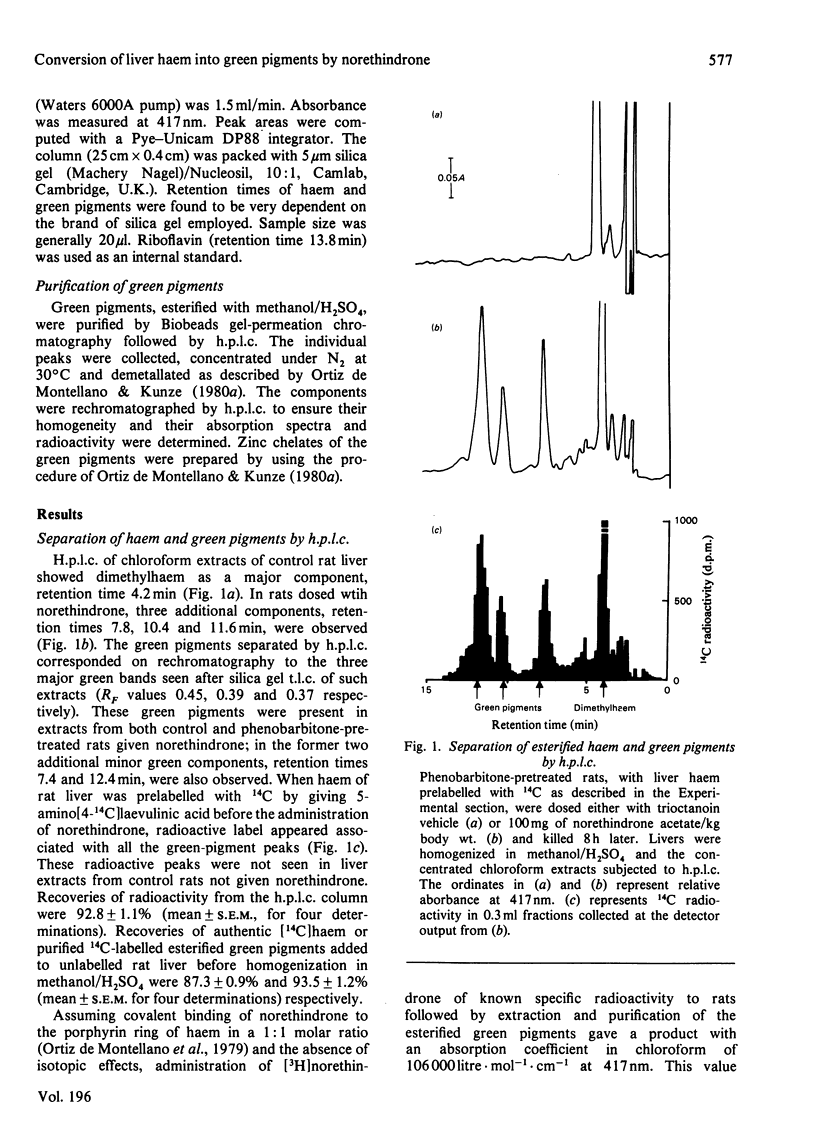
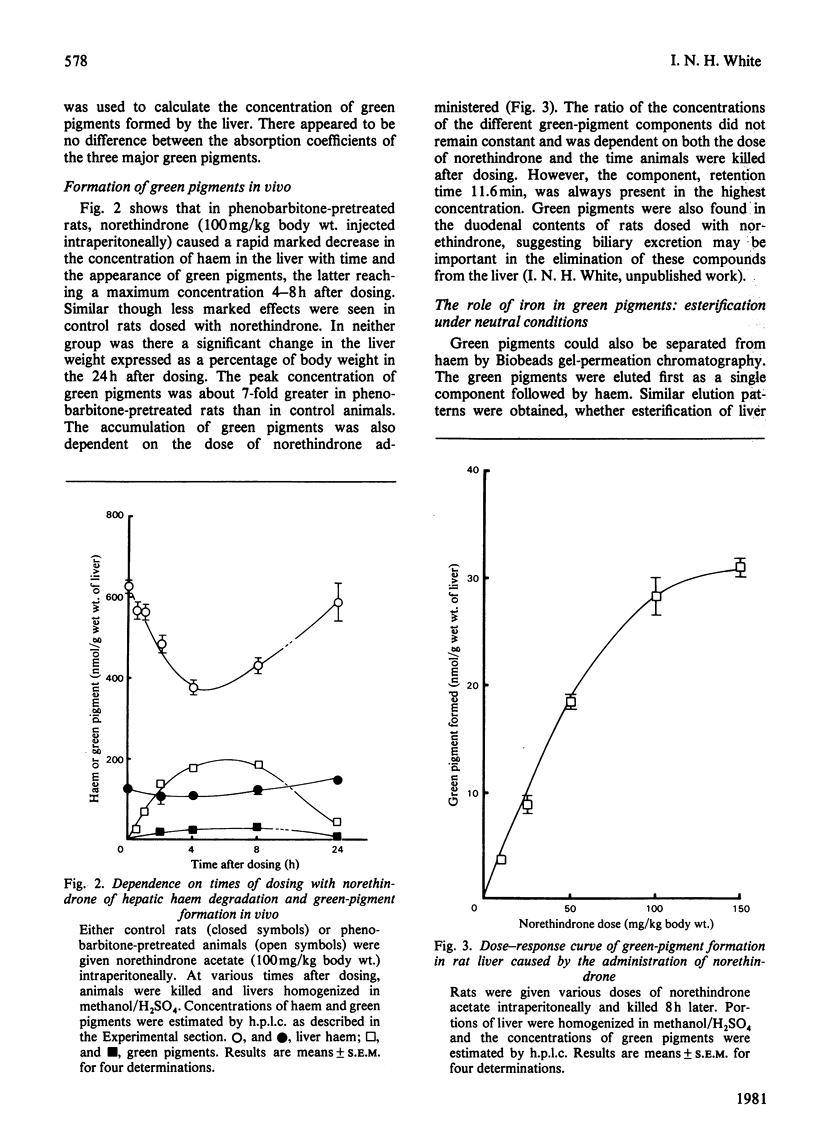
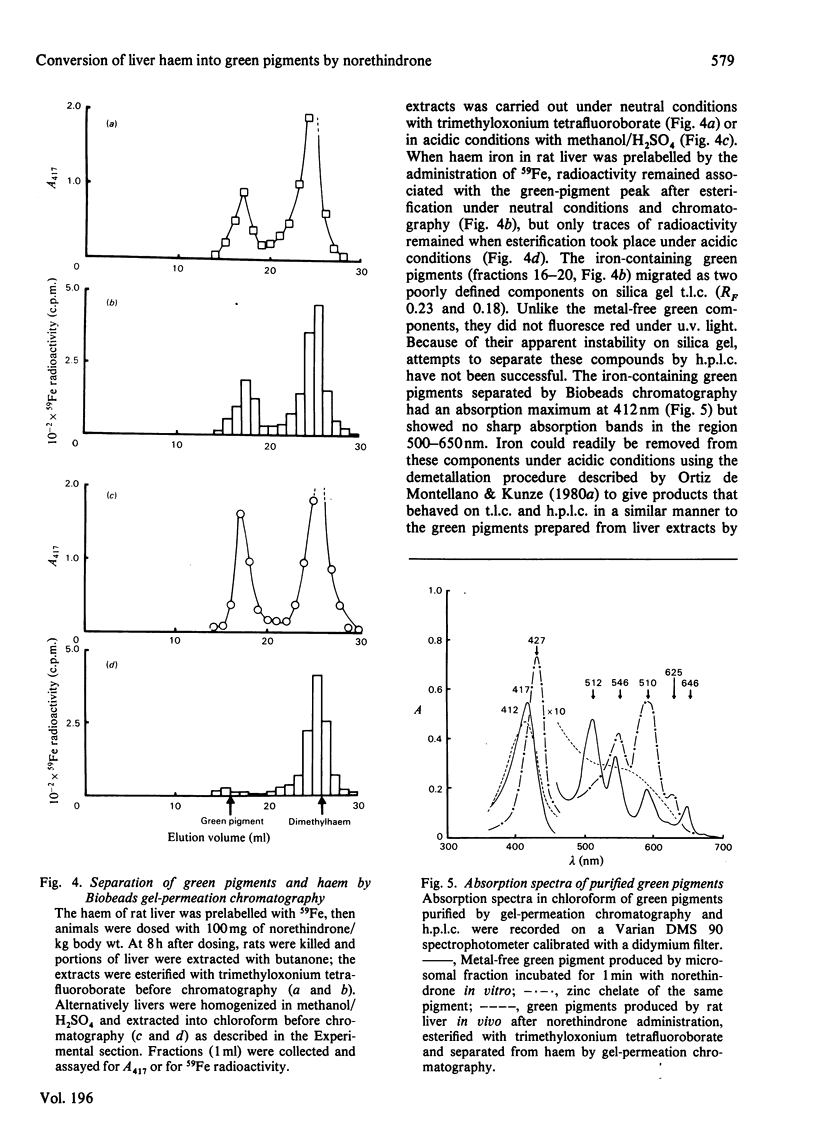
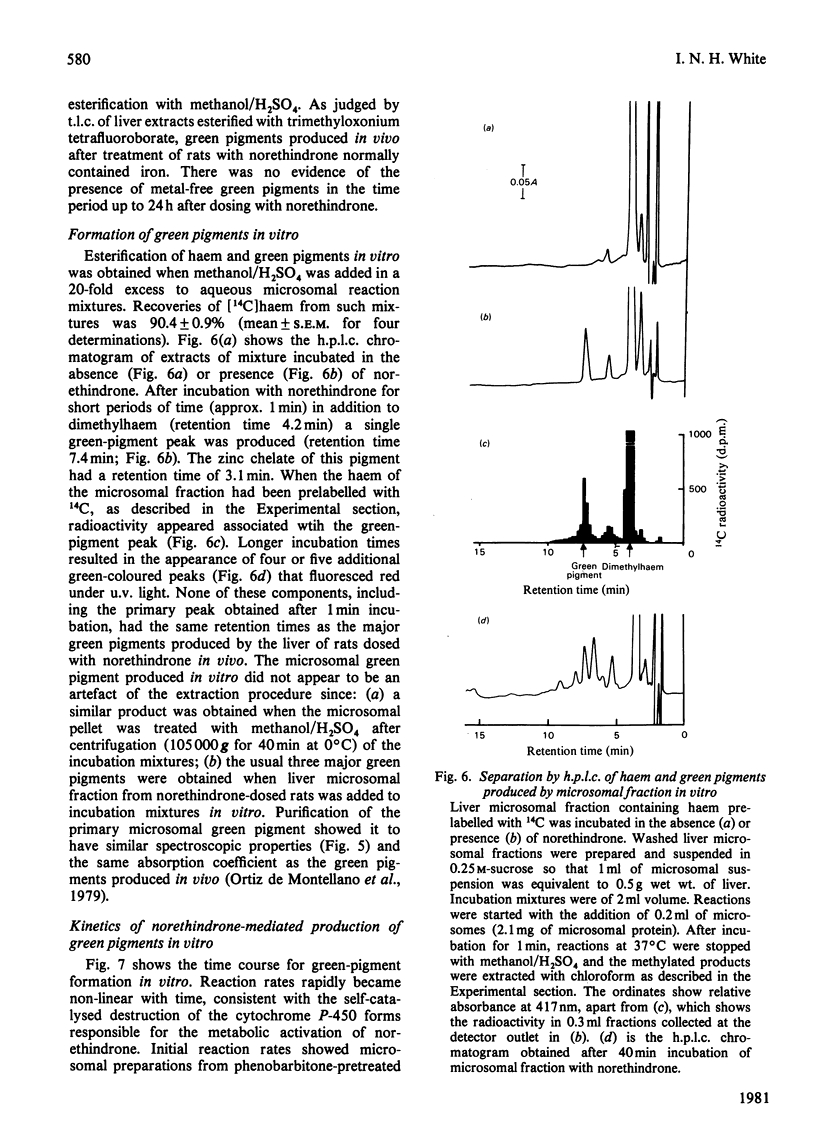
1. Factors affecting the norethindrone-mediated conversion of hepatic haem into green pigments have been studied in the rat. Concentrations of haem and green pigments were estimated spectrophotometrically after esterification and separation by silica gel high-pressure liquid chromatography (h.p.l.c.). 2. Accumulation of green pigments in the liver was dependent on the dose of steroid and the time after dosing, maximum values being reached after 4–8h. Phenobarbitone pretreatment of rats resulted in an 8-fold increase in the concentration of green pigments at these times. 3. In microsomal systems in vitro, the formation of green pigments in the presence of NADPH and norethindrone was also dependent on the concentration of steroid and incubation times. Reaction rates very rapidly became non-linear with time, consistent with the self-catalysed destruction of the form(s) of cytochrome P-450 responsible for the metabolic activation of norethindrone. Microsomal mixtures incubated for a short period of time (1min) with norethindrone gave only one green-pigment peak after h.p.l.c. Longer incubation times gave four or five additional green pigments. Results suggested that multiple green pigments may arise by metabolic transformation of a single precursor. 4. When liver haem was prelabelled with 14C by using 5-amino[4-14C]laevulinic acid, subsequent dosing with norethindrone in vivo gave rise to three major 14C-labelled-green-pigment peaks on h.p.l.c. None of these components had the same retention times as the green pigments produced by microsomal fractions in vitro. 5. When liver haem was prelabelled with 59Fe by using 59FeCl3, norethindrone administration resulted in the detection of 59Fe-labelled green pigments if subsequent esterification was carried out under neutral conditions with trimethyloxonium tetrafluoroborate, but not when carried out under acidic conditions with methanol/H2SO4. These results suggested that green pigments normally contain chelated iron and that metal-free green pigments are not produced by the liver.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonkowsky H. L., Healey J. F., Sinclair P. R., Mayer Y. P., Erny R. Metabolism of hepatic haem and 'green pigments' in rats given 2-allyl-2-isopropylacetamide and ferric citrate. A new model for hepatic haem turnover. Biochem J. 1980 May 15;188(2):289–295. doi: 10.1042/bj1880289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia M. A., Farrell G. C., Schmid R., Ortiz de Montellano P. R., Yost G. S., Mico B. A. Incorporation of exogenous heme into hepatic cytochrome P-450 in vivo. J Biol Chem. 1979 Jan 10;254(1):15–17. [PubMed] [Google Scholar]
- De Matteis F., Cantoni L. Alteration of the porphyrin nucleus of cytochrome P-450 caused in the liver by treatment with allyl-containing drugs. Is the modified porphyrin N-substituted? Biochem J. 1979 Oct 1;183(1):99–103. doi: 10.1042/bj1830099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H. Drug-induced conversion of liver haem into modified porphyrins. Evidence for two classes of products. Biochem J. 1980 Apr 1;187(1):285–288. doi: 10.1042/bj1870285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F. Loss of haem in rat liver caused by the porphyrogenic agent 2-allyl-2-isopropylacetamide. Biochem J. 1971 Oct;124(4):767–777. doi: 10.1042/bj1240767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Unseld A. Increased liver haem degradation caused by foreign chemicals: a comparison of the effects of 2-allyl-2-isopropylacetamide and cobaltous chloride. Biochem Soc Trans. 1976;4(2):205–209. doi: 10.1042/bst0040205. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Kunze K. L. Inactivation of hepatic cytochrome P-450 by allenic substrates. Biochem Biophys Res Commun. 1980 May 30;94(2):443–449. doi: 10.1016/0006-291x(80)91251-6. [DOI] [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Kunze K. L. Self-catalyzed inactivation of hepatic cytochrome P-450 by ethynyl substrates. J Biol Chem. 1980 Jun 25;255(12):5578–5585. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Kunze K. L., Yost G. S., Mico B. A. Self-catalyzed destruction of cytochrome P-450: covalent binding of ethynyl sterols to prosthetic heme. Proc Natl Acad Sci U S A. 1979 Feb;76(2):746–749. doi: 10.1073/pnas.76.2.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- White I. N. Metabolic activation of acetylenic substituents to derivatives in the rat causing the loss of hepatic cytochrome P-450 and haem. Biochem J. 1978 Sep 15;174(3):853–861. doi: 10.1042/bj1740853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White I. N., Muller-Eberhard U. Decreased liver cytochrome P-450 in rats caused by norethindrone or ethynyloestradiol. Biochem J. 1977 Jul 15;166(1):57–64. doi: 10.1042/bj1660057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White I. N. Structure-activity relationships in the destruction of cytochrome P-450 mediated by certain ethynyl-substituted compounds in rats. Biochem Pharmacol. 1980 Dec;29(24):3253–3255. doi: 10.1016/0006-2952(80)90299-3. [DOI] [PubMed] [Google Scholar]
- Ziman M. R., Bradshaw J. J., Ivanetich K. M. The effect of fluroxene [(2,2,2-trifluoroethoxy)ethane] on haem biosynthesis and degradation. Biochem J. 1980 Sep 15;190(3):571–580. doi: 10.1042/bj1900571. [DOI] [PMC free article] [PubMed] [Google Scholar]


