Abstract
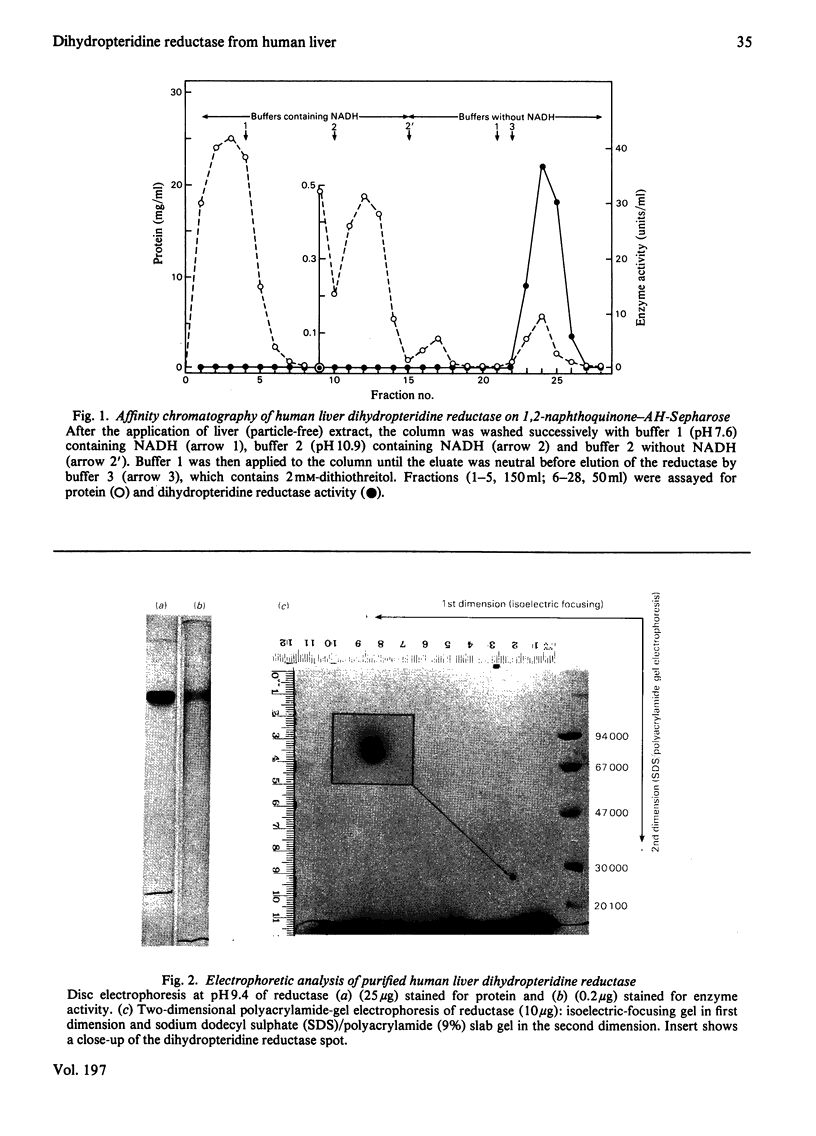
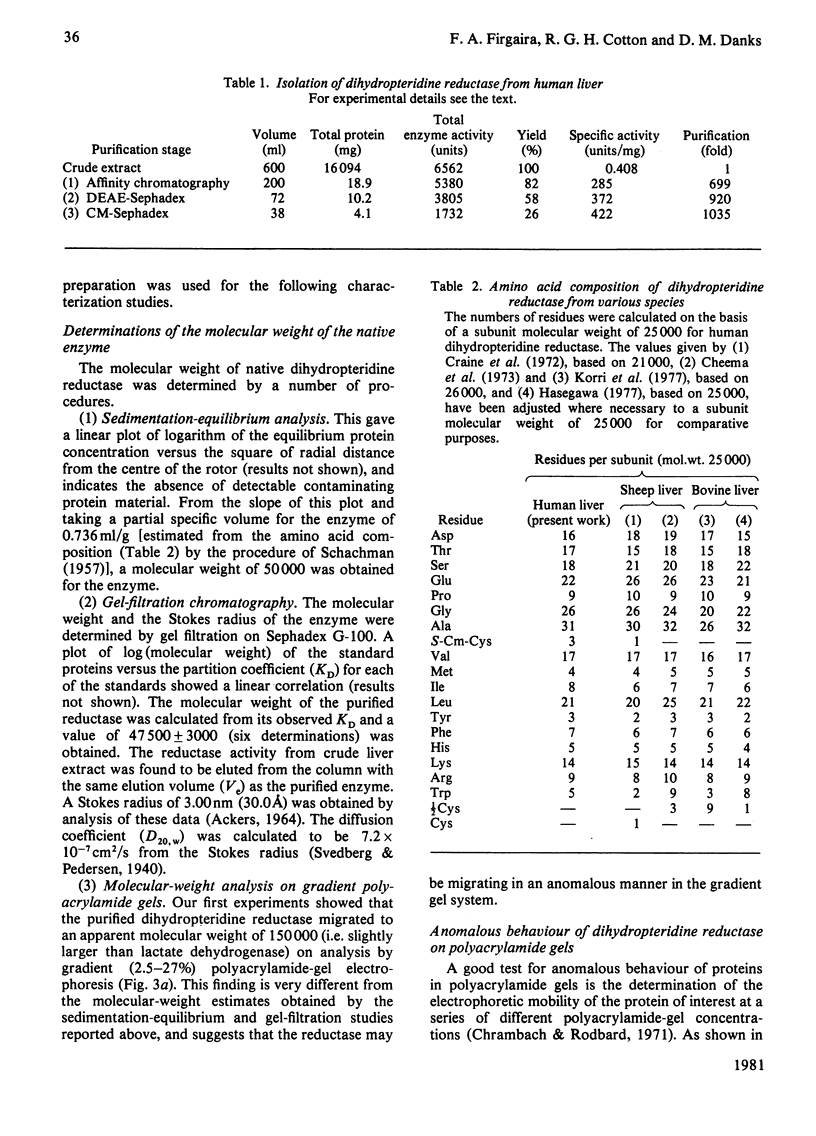
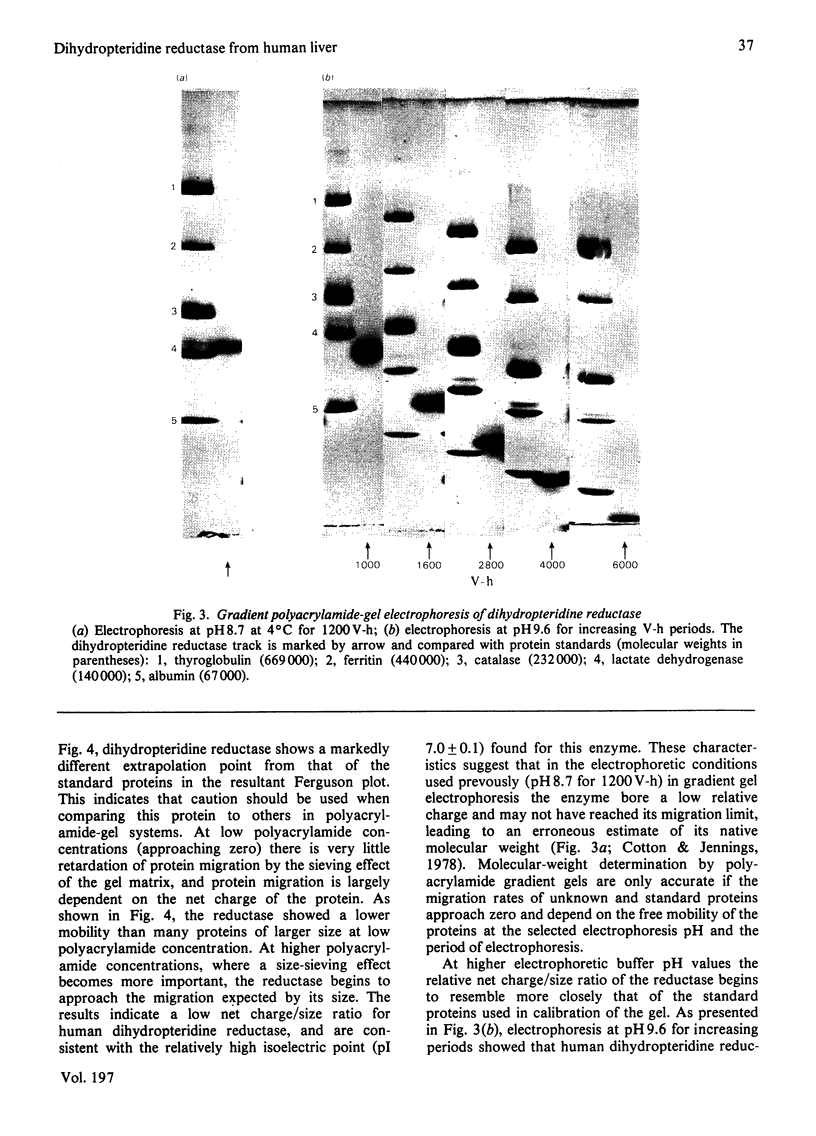
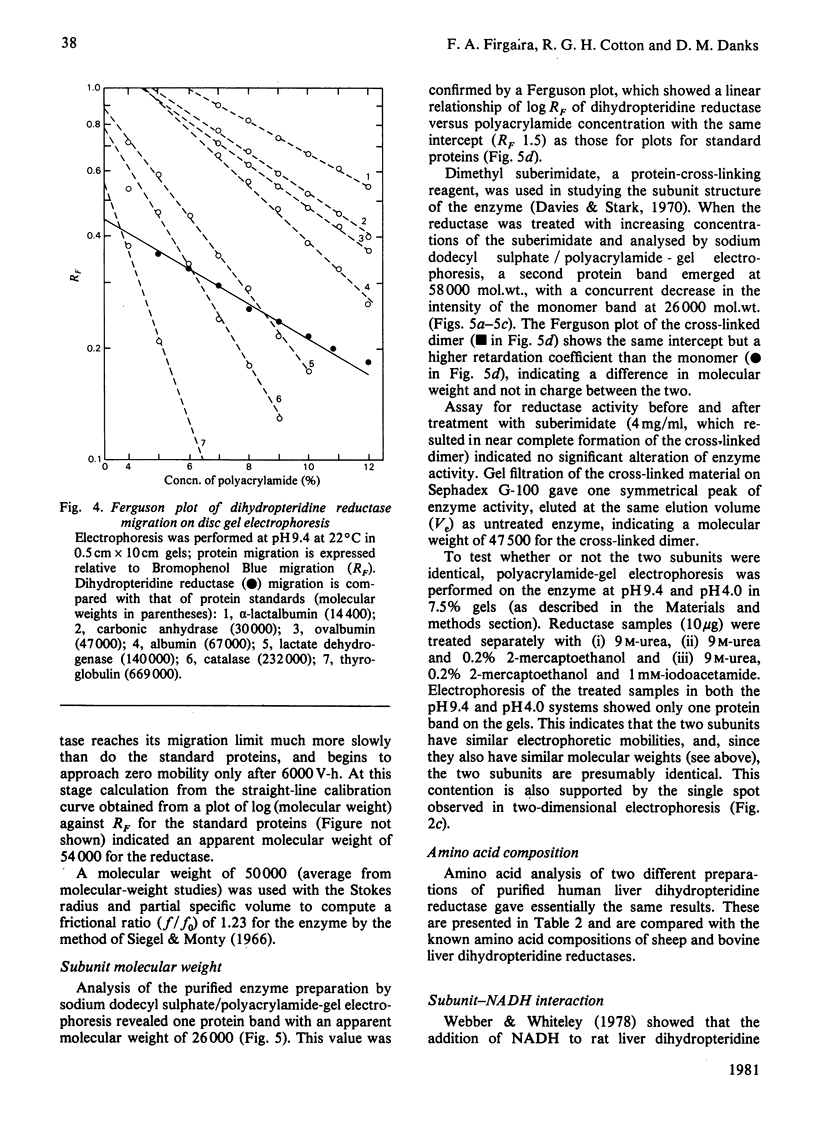
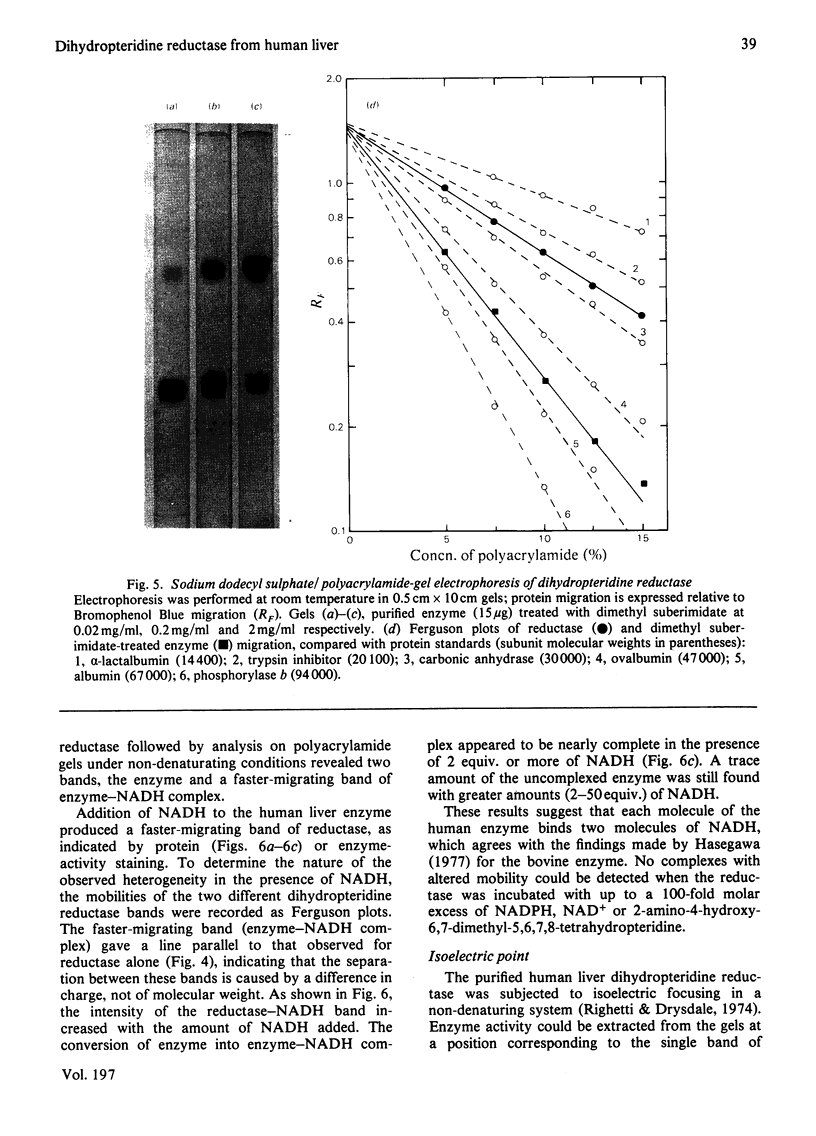
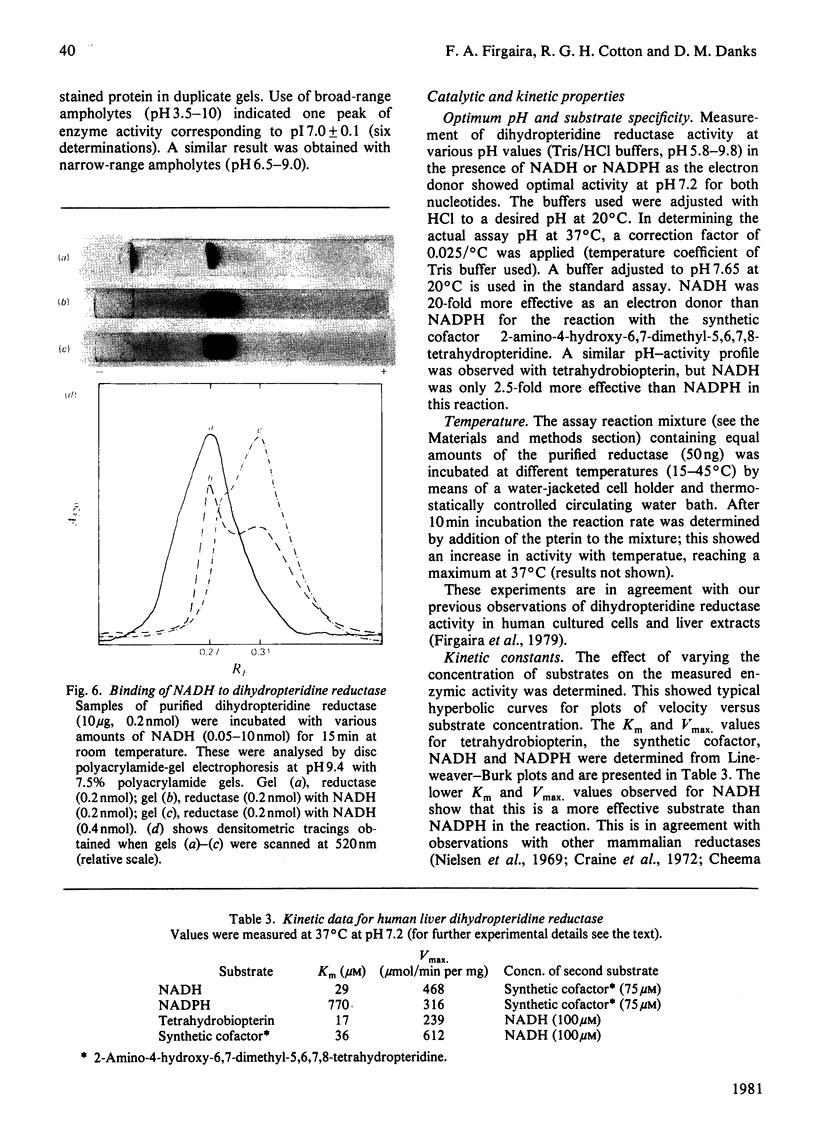
Dihydropteridine reductase (EC 1.6.99.7) was purified from human liver obtained at autopsy by a three-step chromatographic procedure with the use of (1) a naphthoquinone affinity adsorbent, (2) DEAE-Sephadex and (3) CM-Sephadex. The enzyme was typically purified 1000-fold with a yield of 25%. It gave a single band on non-denaturing and sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, and showed one spot on two-dimensional gel electrophoresis. The molecular weight of the enzyme was determined to be 50000 by sedimentation-equilibrium analysis and 47500 by gel filtration. On sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, a single subunit with mol.wt. 26000 was observed. A complex of dihydropteridine reductase with NADH was observed on gel electrophoresis. The isoelectric point of the enzyme was estimated to be pH 7.0. Amino acid analysis showed a residue composition similar to that seen for the sheep and bovine liver enzymes. The enzyme showed anomalous migration in polyacrylamide-gel electrophoresis. A Ferguson plot indicated that this behaviour is due to a low net charge/size ratio of the enzyme under the electrophoretic conditions used. The kinetic properties of the enzyme with tetrahydrobiopterin, 2-amino-4-hydroxy-6,7-dimethyl-5,6,7,8-tetrahydropteridine, NADH and NADPH are compared, and the effects of pH, temperature and a number of different compounds on catalytic activity are presented.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- Aksnes A., Skotland T., Flatmark T., Ljones T. Bovine dihydropteridine reductase: purification by affinity chromatography and comparison of enzymes from liver and adrenal medulla. Neurochem Res. 1979 Jun;4(3):385–398. doi: 10.1007/BF00963808. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleile D. M., Foster M., Brady J. W., Harrison J. H. Identification of essential arginyl residues in cytoplasmic malate dehydrogenase with butanedione. J Biol Chem. 1975 Aug 25;250(16):6222–6227. [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Chauvin M. M., Korri K. K., Tirpak A., Simpson R. C., Scrimgeour K. G. Purification of dihydropterin reductase using immobilized Cibacron Blue. Can J Biochem. 1979 Feb;57(2):178–187. doi: 10.1139/o79-022. [DOI] [PubMed] [Google Scholar]
- Cheema S., Soldin S. J., Knapp A., Hofmann K. T., Scrimgeour K. G. Properties of purifed quinonoid dihydropterin reductase. Can J Biochem. 1973 Sep;51(9):1229–1239. doi: 10.1139/o73-163. [DOI] [PubMed] [Google Scholar]
- Chrambach A., Rodbard D. Polyacrylamide gel electrophoresis. Science. 1971 Apr 30;172(3982):440–451. doi: 10.1126/science.172.3982.440. [DOI] [PubMed] [Google Scholar]
- Cotton R. G., Jennings I. A naphthoquinone adsorbent for affinity chromatography of human dihydropteridine reductase. Eur J Biochem. 1978 Feb 1;83(1):319–324. doi: 10.1111/j.1432-1033.1978.tb12097.x. [DOI] [PubMed] [Google Scholar]
- Craine J. E., Hall E. S., Kaufman S. The isolation and characterization of dihydropteridine reductase from sheep liver. J Biol Chem. 1972 Oct 10;247(19):6082–6091. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Danks D. M., Bartholomé K., Clayton B. E., Curtius H., Gröbe H., Kaufman S., Leeming R., Pfleiderer W., Rembold H., Rey F. Malignant hyperphenylalaninaemia--current status (June 1977). J Inherit Metab Dis. 1978;1(2):49–53. doi: 10.1007/BF01801843. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Firgaira F. A., Cotton R. G., Danks D. M. Human dihydropteridine reductase: a method for the measurement of activity in cultured cells, and its application to malignant hyperphenylalaninemia. Clin Chim Acta. 1979 Jul 2;95(1):47–59. doi: 10.1016/0009-8981(79)90335-8. [DOI] [PubMed] [Google Scholar]
- Hasegawa H. Dihydropteridine reductase from bovine liver. Purification, crystallization, and isolation of a binary complex with NADH. J Biochem. 1977 Jan;81(1):169–177. doi: 10.1093/oxfordjournals.jbchem.a131432. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Korri K. K., Chippel D., Chauvin M. M., Tirpak A., Scrimgeour K. G. Quinonoid dihydropterin reductase from beef liver. Can J Biochem. 1977 Nov;55(11):1145–1152. doi: 10.1139/o77-171. [DOI] [PubMed] [Google Scholar]
- Lange L. G., 3rd, Riordan J. F., Vallee B. L. Functional arginyl residues as NADH binding sites of alcohol dehydrogenases. Biochemistry. 1974 Oct 8;13(21):4361–4370. doi: 10.1021/bi00718a019. [DOI] [PubMed] [Google Scholar]
- Margolis J., Kenrick K. G. 2-dimensional resolution of plasma proteins by combination of polyacrylamide disc and gradient gel electrophoresis. Nature. 1969 Mar 15;221(5185):1056–1057. doi: 10.1038/2211056a0. [DOI] [PubMed] [Google Scholar]
- Nagradova N. K., Asryants R. A. Essential arginine residues in D-glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta. 1975 Mar 28;386(1):365–368. doi: 10.1016/0005-2795(75)90279-2. [DOI] [PubMed] [Google Scholar]
- Nielsen K. H., Simonsen V., Lind K. E. Dihydropteridine reductase. A method for the measurement of activity, and investigations of the specificity for NADH and NADPH. Eur J Biochem. 1969 Jul;9(4):497–502. doi: 10.1111/j.1432-1033.1969.tb00636.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Pajot P. Fluroescence of proteins in 6-M guanidine hydrochloride. A method for the quantitative determination of tryptophan. Eur J Biochem. 1976 Mar 16;63(1):263–269. doi: 10.1111/j.1432-1033.1976.tb10228.x. [DOI] [PubMed] [Google Scholar]
- Parish C. R., Marchalonis J. J. A simple and rapid acrylamide gel method for estimating the molecular weights of proteins and protein subunits. Anal Biochem. 1970 Apr;34(2):436–450. doi: 10.1016/0003-2697(70)90128-4. [DOI] [PubMed] [Google Scholar]
- Righetti P. G., Drysdale J. W. Isoelectric focusing in gels. J Chromatogr. 1974 Sep 25;98(2):271–321. doi: 10.1016/s0021-9673(00)92076-4. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Snady H., Musacchio J. M. Quinonoid dihydropterin reductase-I. Purification and characterization of the bovine brain enzyme. Biochem Pharmacol. 1978;27(15):1939–1945. doi: 10.1016/0006-2952(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Webber S., Deits T. L., Snyder W. R., Whiteley J. M. The purification of rat and sheep liver dihydropteridine reductases by affinity chromatography on methotrexate-sepharose. Anal Biochem. 1978 Feb;84(2):491–503. doi: 10.1016/0003-2697(78)90068-4. [DOI] [PubMed] [Google Scholar]
- Webber S., Whiteley J. M. Pyridine nucleotide interaction with rat liver dihydropteridine reductase. J Biol Chem. 1978 Oct 10;253(19):6724–6729. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Williams C. D., Dickens G., Letendre C. H., Guroff G., Haines C., Shiota T. Isolation and characterization of dihydropteridine reductase from Pseudomonas species. J Bacteriol. 1976 Sep;127(3):1197–1207. doi: 10.1128/jb.127.3.1197-1207.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodroofe M. N., Butterworth P. J. Evidence for the importance of arginine residues in pig kidney alkaline phosphatase. Biochem J. 1979 Jul 1;181(1):137–142. doi: 10.1042/bj1810137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]






