Abstract
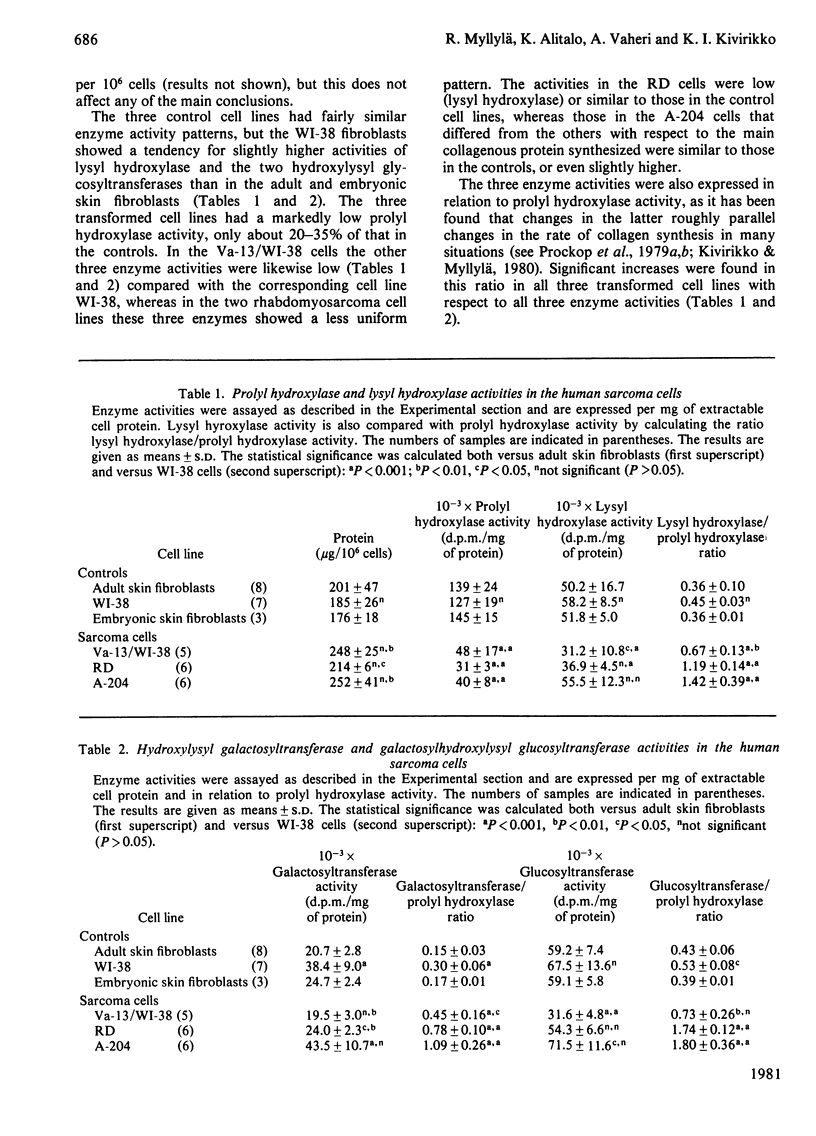
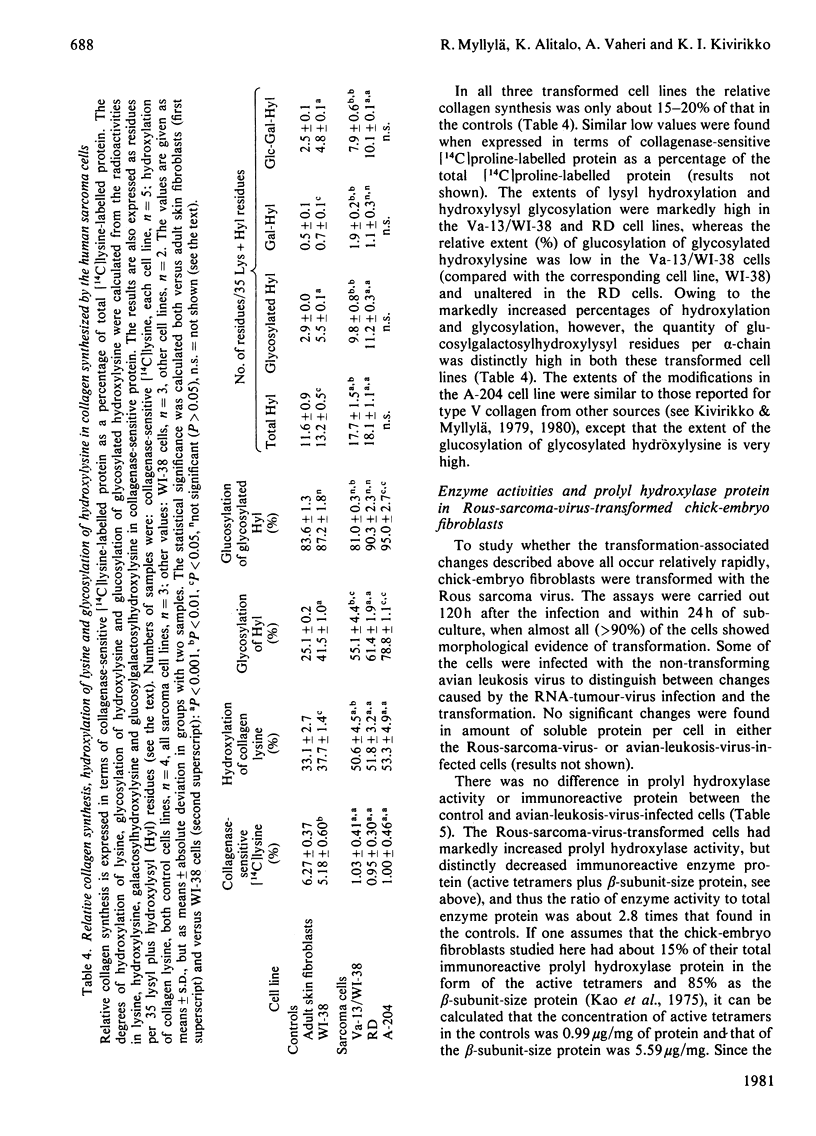
Changes in the regulation of collagen post-translational modification in transformed cells were studied in three established human sarcoma cell lines and in chick-embryo fibroblasts freshly transformed by Rous sarcoma virus. The collagens synthesized by all but one of these and by all the control human and chick-embryo cell lines were almost exclusively of types I and/or III. The relative rate of collagen synthesis and the amounts of prolyl hydroxylase activity and immunoreactive protein were markedly low in all the transformed human cell lines. The other enzymes studied, lysyl hydroxylase, hydroxylysyl galactosyltransferase and galactosylhydroxylysyl glucosyltransferase, never showed as large a decrease in activity as did prolyl hydroxylase, suggesting a more efficient regulation of the last enzyme than of the three others. The chick-embryo fibroblasts freshly transformed by Rous sarcoma virus differed from the human sarcoma cells in that prolyl hydroxylase activity was distinctly increased, whereas the decreases in immunoreactive prolyl hydroxylase protein and the three other enzyme activities were very similar to those in the simian-virus-40-transformed human fibroblasts. It seems possible that this increased prolyl hydroxylase activity is only a temporary phenomenon occurring shortly after the transformation, and may be followed by a decrease in activity later. The newly synthesized collagens of all the transformed cells that produced almost exclusively collagen types I and/or III had high extents of lysyl hydroxylation, and there was also an increase in the ratio of glycosylated to non-glycosylated hydroxylysine. The data suggest that one critical factor affecting modification is the rate of collagen synthesis, which affects the ratio of enzyme to substrate in the cell.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Alwine J. C., de Crombrugghe B., Pastan I. Use of recombinant plasmids to characterize collagen RNAs in normal and transformed chick embryo fibroblasts. J Biol Chem. 1979 Jun 25;254(12):4935–4938. [PubMed] [Google Scholar]
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K. Production of both interstitial and basement membrane procollagens by fibroblastic WI-38 cells from human embryonic lung. Biochem Biophys Res Commun. 1980 Apr 14;93(3):873–880. doi: 10.1016/0006-291x(80)91157-2. [DOI] [PubMed] [Google Scholar]
- Arbogast B. W., Yoshimura M., Kefalides N. A., Holtzer H., Kaji A. Failure of cultured chick embryo fibroblasts to incorporate collagen into their extracellular matrix when transformed by Rous sarcoma virus. An effect of transformation but not of virus production. J Biol Chem. 1977 Dec 25;252(24):8863–8868. [PubMed] [Google Scholar]
- Berg R. A., Kao W. W., Kedersha N. L. The assembly of tetrameric prolyl hydroxylase in tendon fibroblasts from newly synthesized alpha-subunits and from preformed cross-reacting protein. Biochem J. 1980 Sep 1;189(3):491–499. doi: 10.1042/bj1890491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. Collagen-galactosyl transferase: subcellular localization and distribution in fibroblasts transformed by oncogenic viruses. Life Sci. 1969 Jul 15;8(14):737–746. doi: 10.1016/0024-3205(69)90010-1. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B., Eylar E. H. Collagen-glucosyl transferase in fibriblasts transformed by oncogenic viruses. Nature. 1968 May 11;218(5141):582–583. doi: 10.1038/218582a0. [DOI] [PubMed] [Google Scholar]
- Chen-Kiang S., Cardinale G. J., Udenfriend S. Homology between a prolyl hydroxylase subunit and a tissue protein that crossreacts immunologically with the enzyme. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4420–4424. doi: 10.1073/pnas.74.10.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- Green H., Todaro G. J., Goldberg B. Collagen synthesis in fibroblasts transformed by oncogenic viruses. Nature. 1966 Feb 26;209(5026):916–917. doi: 10.1038/209916a0. [DOI] [PubMed] [Google Scholar]
- Hata R. I., Peterkofsky B. Retention of transformant specific type III collagen in dibutyryl cAMP treated Kirsten sarcoma virus transformed BALB 3T3 cells and in a flat revertant. J Cell Physiol. 1978 Jun;95(3):343–352. doi: 10.1002/jcp.1040950312. [DOI] [PubMed] [Google Scholar]
- Hata R. I., Peterkofsky B. Specific changes in the collagen phenotype of BALB 3T3 cells as a result of transformation by sarcoma viruses or a chemical carcinogen. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2933–2937. doi: 10.1073/pnas.74.7.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. H., Adams S. L., Sobel M. E., Pastan I., de Crombrugghe B. Decreased levels of collagen mRNA in rous sarcoma virus-transformed chick embryo fibroblasts. J Biol Chem. 1978 Aug 25;253(16):5869–5874. [PubMed] [Google Scholar]
- Kamine J., Rubin H. Coordinate control of collagen synthesis and cell growth in chick embryo fibroblasts and the effect of viral transformation on collagen synthesis. J Cell Physiol. 1977 Jul;92(1):1–11. doi: 10.1002/jcp.1040920102. [DOI] [PubMed] [Google Scholar]
- Kao W. W., Berg R. A., Prockop D. J. Ascorbate increases the synthesis of procollagen hydroxyproline by cultured fibroblasts from chick embryo tendons without activation of prolyl hydroxyla. Biochim Biophys Acta. 1975 Dec 5;411(2):202–215. doi: 10.1016/0304-4165(75)90300-1. [DOI] [PubMed] [Google Scholar]
- Kao W. W., Prockop D. J., Berg R. A. Kinetics for the secretion of nonhelical procollagen by freshly isolated tendon cells. J Biol Chem. 1979 Apr 10;254(7):2234–2243. [PubMed] [Google Scholar]
- Kao W., Chou K. L. CRP, immunologically cross-reacting protein of prolyl hydroxylase. Its role in assembly of active pro-yl hydroxylase and cellular localization in L-929 fibroblasts. Arch Biochem Biophys. 1980 Jan;199(1):147–157. doi: 10.1016/0003-9861(80)90267-2. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Collagen glycosyltransferases. Int Rev Connect Tissue Res. 1979;8:23–72. doi: 10.1016/b978-0-12-363708-6.50008-4. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Prockop D. J. Partial purification and characterization of protocollagen lysine hydroxylase from chick embryos. Biochim Biophys Acta. 1972 Feb 28;258(2):366–379. doi: 10.1016/0005-2744(72)90228-8. [DOI] [PubMed] [Google Scholar]
- Krieg T., Aumailley M., Dessau W., Wiestner M., Müller P. Synthesis of collagen by human fibroblasts and their SV40 transformants. Exp Cell Res. 1980 Jan;125(1):23–30. doi: 10.1016/0014-4827(80)90184-6. [DOI] [PubMed] [Google Scholar]
- Krieg T., Timpl R., Alitalo K., Kurkinen M., Vaheri A. Type III procollagen is the major collageneous component produced by a continuous rhabdomyosarcoma cell line. FEBS Lett. 1979 Aug 15;104(2):405–409. doi: 10.1016/0014-5793(79)80863-7. [DOI] [PubMed] [Google Scholar]
- Kumamoto C. A., Fessler J. H. Biosynthesis of A,B procollagen. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6434–6438. doi: 10.1073/pnas.77.11.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuutti E. R., Tuderman L., Kivirikko K. I. Human prolyl hydroxylase. Purification, partial characterization and preparation of antiserum to the enzyme. Eur J Biochem. 1975 Sep 1;57(1):181–188. doi: 10.1111/j.1432-1033.1975.tb02289.x. [DOI] [PubMed] [Google Scholar]
- Levinson W., Bhatnagar R. S., Liu T. Z. Loss of ability to synthesize collagen in fibroblasts transformed by rous sarcoma virus. J Natl Cancer Inst. 1975 Oct;55(4):807–810. doi: 10.1093/jnci/55.4.807. [DOI] [PubMed] [Google Scholar]
- Majamaa K., Kuutti-Savolainen E. R., Tuderman L., Kivirikko K. I. Turnover of prolyl hydroxylase tetramers and the monomer-size protein in chick-embryo cartilaginous bone and lung in vivo. Biochem J. 1979 Feb 15;178(2):313–322. doi: 10.1042/bj1780313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllylä R., Risteli L., Kivirikko K. I. Assay of collagen-galactosyltransferase and collagen-glucosyltransferase activities and preliminary characterization of enzymic reactions with transferases from chick-embryo cartilage. Eur J Biochem. 1975 Apr 1;52(3):401–410. doi: 10.1111/j.1432-1033.1975.tb04008.x. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Risteli L., Kivirikko K. I. Collagen glucosyltransferase. Partial purification and characterization of the enzyme from whole chick embryos and chick-embryo cartilage. Eur J Biochem. 1976 Jan 2;61(1):59–67. doi: 10.1111/j.1432-1033.1976.tb09997.x. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Risteli L., Kivirikko K. I. Glucosylation of galactosylhydroxylysyl residues in collagen in vitro by collagen glucosyltransferase. Inhibition by triple-helical conformation of the substrate. Eur J Biochem. 1975 Oct 15;58(2):517–521. doi: 10.1111/j.1432-1033.1975.tb02400.x. [DOI] [PubMed] [Google Scholar]
- Oikarinen A., Anttinen H., Kivirikko K. I. Effect of L-azetidine-2-carboxylic acid on glycosylations of collagen in chick-embryo tendon cells. Biochem J. 1976 Dec 15;160(3):639–645. doi: 10.1042/bj1600639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikarinen A., Anttinen H., Kivirikko K. I. Further studies on the effect of the collagen triple-helix formation on the hydroxylation of lysine and the glycosylations of hydroxylysine in chick-embryo tendon and cartilage cells. Biochem J. 1977 Sep 15;166(3):357–362. doi: 10.1042/bj1660357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikarinen A., Anttinen H., Kivirikko K. I. Hydroxylation of lysine and glycosylation of hydroxylysine during collagen biosynthesis in isolated chick-embryo cartilage cells. Biochem J. 1976 Jun 15;156(3):545–551. doi: 10.1042/bj1560545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (first of two parts). N Engl J Med. 1979 Jul 5;301(1):13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (second of two parts). N Engl J Med. 1979 Jul 12;301(2):77–85. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- Risteli J., Kivirikko K. I. Intracellular enzymes of collagen biosynthesis in rat liver as a function of age and in hepatic injury induced by dimethylnitrosamine. Changes in prolyl hydroxylase, lysyl hydroxylase, collagen galactosyltransferase and collagen glucosyltransferase activities. Biochem J. 1976 Aug 15;158(2):361–367. doi: 10.1042/bj1580361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risteli J., Tuderman L., Kivirikko K. I. Intracellular enzymes of collagen biosynthesis in rat liver as a function of age and in hepatic injury induced by dimethylnitrosamine. Purification of rat prolyl hydroxylase and comparison of changes in prolyl hydroxylase activity with changes in immunoreactive prolyl hydroxylase. Biochem J. 1976 Aug 15;158(2):369–376. doi: 10.1042/bj1580369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risteli J., Tuderman L., Tryggvason K., Kivirikko K. I. Effect of hepatic injury on prolyl 3-hydroxylase and 4-hydroxylase activities in rat liver and on immunoreactive prolyl 4-hydroxylase concentrations in the liver and serum. Biochem J. 1978 Jan 15;170(1):129–135. doi: 10.1042/bj1700129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. W., Moen R. C., Davidson J. M., Byers P. H., Bornstein P., Palmiter R. D. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry. 1978 May 2;17(9):1581–1590. doi: 10.1021/bi00602a001. [DOI] [PubMed] [Google Scholar]
- Sandmeyer S., Bornstein P. Declining procollagen mRNA sequences in chick embryo fibroblasts infected with rous sarcoma virus. Correlation with procollagen synthesis. J Biol Chem. 1979 Jun 25;254(12):4950–4953. [PubMed] [Google Scholar]
- Smith B. D., Biles D., Gonnerman W., Faris B., Levine A., Capparell N., Moolten F., Franzblau C. Collagen synthesis in normal BHK cells and temperature-sensitive chemically transformed BHK cells. In Vitro. 1979 Jun;15(6):455–462. doi: 10.1007/BF02618415. [DOI] [PubMed] [Google Scholar]
- Sundarraj N., Church R. L. Alterations of post-translational modifications of procollagen by SV40-transformed human fibroblasts. FEBS Lett. 1977 Dec 20;85(1):47–51. doi: 10.1016/0014-5793(78)81245-9. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Kuutti E. R., Kivirikko K. I. An affinity-column procedure using poly(L-proline) for the purification of prolyl hydroxylase. Purification of the enzyme from chick embryos. Eur J Biochem. 1975 Mar 3;52(1):9–16. doi: 10.1111/j.1432-1033.1975.tb03967.x. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Kuutti E. R., Kivirikko K. I. Radiommunoassay for human and chick prolyl hydroxylases. Eur J Biochem. 1975 Dec 15;60(2):399–405. doi: 10.1111/j.1432-1033.1975.tb21016.x. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Kurkinen M., Lehto V. P., Linder E., Timpl R. Codistribution of pericellular matrix proteins in cultured fibroblasts and loss in transformation: fibronectin and procollagen. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4944–4948. doi: 10.1073/pnas.75.10.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]


