Abstract
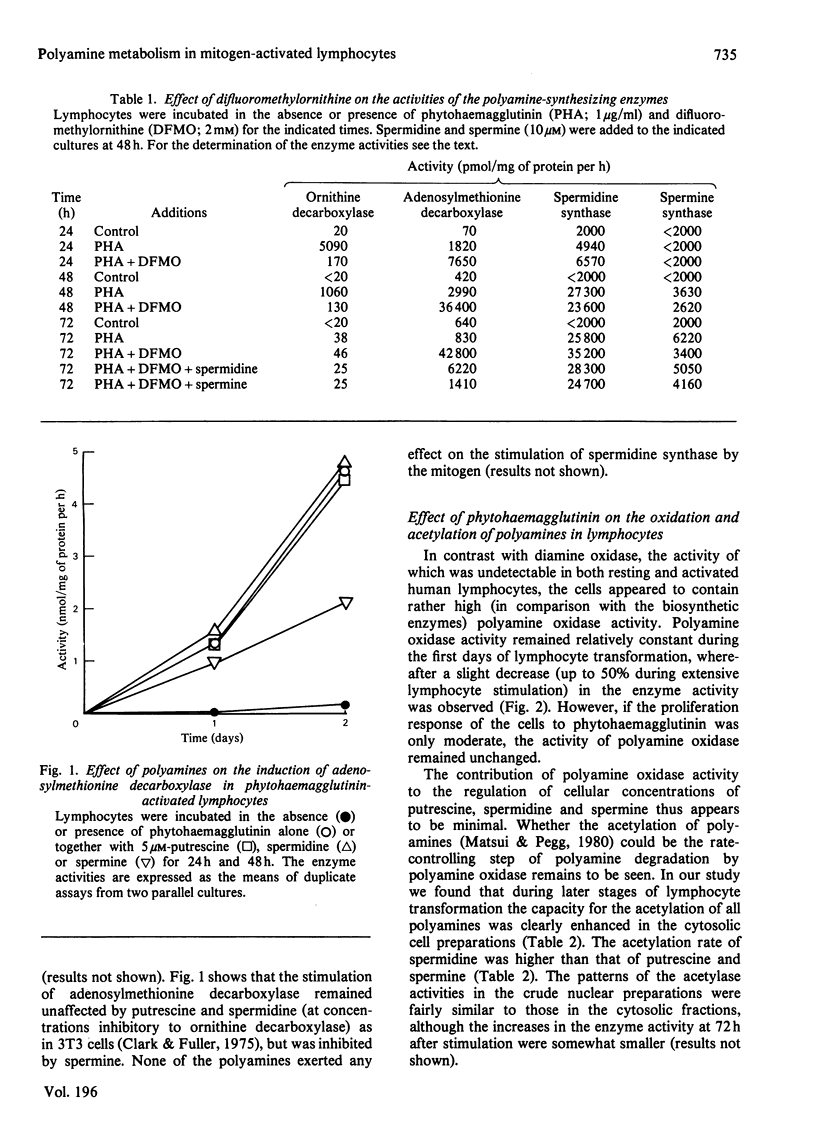
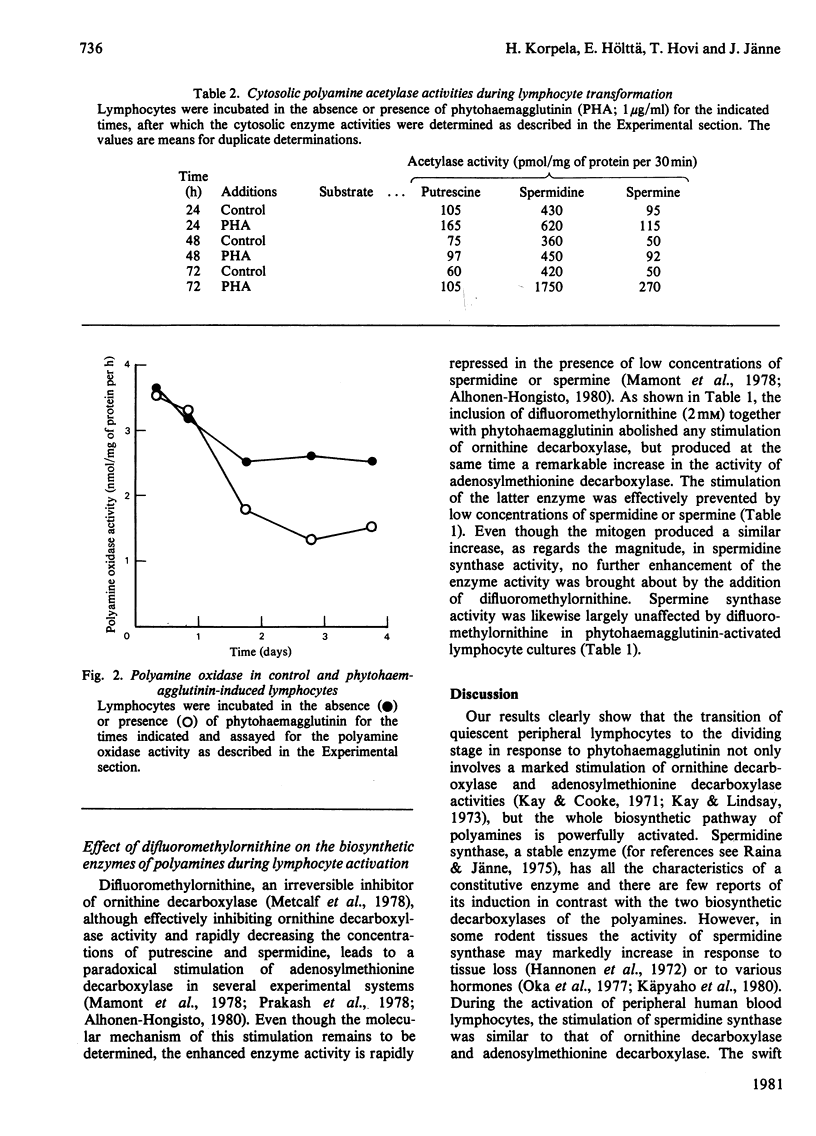
The stimulation of lymphocyte ornithine decarboxylase and adenosylmethionine decarboxylase produced by phytohaemagglutinin was accompanied by an equally marked, but delayed, stimulation of spermidine synthase, which is not commonly considered as an inducible enzyme. In contrast with the marked stimulation of these biosynthetic enzymes, less marked changes were observed in the biodegradative enzymes of polyamines in response to phytohaemagglutinin. Diamine oxidase activity was undetectable during all stages of the transformation. The activity of polyamine oxidase remained either constant or was slightly decreased several days after addition of the mitogen. The activity of polyamine acetylase (employing all the natural polyamines as substrates) distinctly increased both in the cytosolic and crude nuclear preparations of the cells during later stages of mitogen activation. Difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase, although powerfully inhibiting ornithine decarboxylase, produced a gradual enhancement of adenosylmethionine decarboxylase activity during lymphocyte activation, without influencing the activities of the two propylamine transferases (spermidine synthase and spermine synthase).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhonen-Hongisto L. Regulation of S-adenosylmethionine decarboxylase by polyamines in Ehrlich ascites-carcinoma cells grown in culture. Biochem J. 1980 Sep 15;190(3):747–754. doi: 10.1042/bj1900747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylin S. B., Stevens S. A., Shakir K. M. Association of diamine oxidase and ornithine decarboxylase with maturing cells in rapidly proliferating epithelium. Biochim Biophys Acta. 1978 Jul 3;541(3):415–419. doi: 10.1016/0304-4165(78)90200-3. [DOI] [PubMed] [Google Scholar]
- Blankenship J. Metabolic conversion of N1-acetylspermidine to putrescine by a subcellular fraction of rat liver. Proc West Pharmacol Soc. 1979;22:115–118. [PubMed] [Google Scholar]
- Clark J. L., Fuller J. L. Regulation of ornithine decarboxylase in 3T3 cells by putrescine and spermidine: indirect evidence for translational control. Biochemistry. 1975 Oct 7;14(20):4403–4409. doi: 10.1021/bi00691a010. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Dvir R., Harell A., Chayen R. Determination of polyamines in urine. Clin Chim Acta. 1973 Nov 23;49(1):65–72. doi: 10.1016/0009-8981(73)90344-6. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H., Jorstad C. M., Morris D. R. Increased cellular levels of spermidine or spermine are required for optimal DNA synthesis in lymphocytes activated by concanavalin A. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4042–4045. doi: 10.1073/pnas.72.10.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H., Morris D. R. Polyamine accumulation during lymphocyte transformation and its relation to the synthesis, processing, and accumulation of ribonucleic acid. Biochemistry. 1973 Oct 23;12(22):4479–4487. doi: 10.1021/bi00746a028. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H., Morris D. R. S-adenosyl-L-methionine decarboxylase during lymphocyte transformation: decreased degradation in the presence of a specific inhibitor. Biochem Biophys Res Commun. 1973 Jun 8;52(3):1020–1025. doi: 10.1016/0006-291x(73)91039-5. [DOI] [PubMed] [Google Scholar]
- Hannonen P., Raina A., Jänne J. Polyamine synthesis in the regenerating rat liver: stimulation of S-adenosyl methionine decarboxylase, and spermidine and spermine synthases after partial hepatectomy. Biochim Biophys Acta. 1972 Jun 26;273(1):84–90. doi: 10.1016/0304-4165(72)90194-8. [DOI] [PubMed] [Google Scholar]
- Hovi T., Allison A. C., Williams S. C. Proliferation of human peripheral blood lymphocytes induced by A23187, a streptomyces antibiotic. Exp Cell Res. 1976 Jan;97:92–100. doi: 10.1016/0014-4827(76)90658-3. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Jänne J., Hovi T. Suppression of the formation of polyamines and macromolecules by DL-alpha-difluoromethylornithine and methylglyoxal bis(guanylhydrazone) in phytohaemagglutinin-activated human lymphocytes. Biochem J. 1979 Jan 15;178(1):109–117. doi: 10.1042/bj1780109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E., Sinervirta R., Jänne J. Synthesis and accumulation of polyamines in rat liver regenerating after treatment with carbon tetrachloride. Biochem Biophys Res Commun. 1973 Sep 5;54(1):350–357. doi: 10.1016/0006-291x(73)90929-7. [DOI] [PubMed] [Google Scholar]
- Jänne J., Hölttä E. Putrescine metabolizing enzyme activities in some rat tissues during postnatal development. Acta Chem Scand. 1973;27(7):2399–2404. doi: 10.3891/acta.chem.scand.27-2399. [DOI] [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. Dissociation of putrescine-activated decarboxylation of S-adenosyl-L-methionine from the enzymic synthesis of spermidine and spermine by purified prostatic enzyme preparations. Biochem Biophys Res Commun. 1971 Jan 22;42(2):222–229. [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- Kay J. E., Lindsay V. J. Polyamine synthesis during lymphocyte activation. Induction of ornithine decarboxylase and S-adenosyl methionine decarboxylase. Exp Cell Res. 1973 Mar 15;77(1):428–436. doi: 10.1016/0014-4827(73)90597-1. [DOI] [PubMed] [Google Scholar]
- Kay John E., Cooke Anne. Ornithine decarboxylase and ribosomal RNA synthesis during the stimulation of lymphocytes by phytohaemagglutinin. FEBS Lett. 1971 Jul 15;16(1):9–12. doi: 10.1016/0014-5793(71)80671-3. [DOI] [PubMed] [Google Scholar]
- Käpyaho K., Pösö H., Jänne J. Role of propylamine transferases in hormone-induced stimulation of polyamine biosynthesis. Biochem J. 1980 Oct 15;192(1):59–63. doi: 10.1042/bj1920059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Libby P. R. Calf liver nuclear N-acetyltransferases. Purification and properties of two enzymes with both spermidine acetyltransferase and histone acetyltransferase activities. J Biol Chem. 1978 Jan 10;253(1):233–237. [PubMed] [Google Scholar]
- Matsui I., Pegg A. E. Increase in acetylation of spermidine in rat liver extracts brought about by treatment with carbon tetrachloride. Biochem Biophys Res Commun. 1980 Feb 12;92(3):1009–1015. doi: 10.1016/0006-291x(80)90802-5. [DOI] [PubMed] [Google Scholar]
- Menashe M., Faber J., Bachrach U. Formulation of N-acetylputrescine and N1-acetylspermidine in cultured human lymphocytes. Biochem J. 1980 Apr 15;188(1):263–267. doi: 10.1042/bj1880263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUYAMA T., KOBAYASHI Y. Determination of diamine oxidase activity by liquid scintillation counting. Arch Biochem Biophys. 1961 Nov;95:242–250. doi: 10.1016/0003-9861(61)90141-2. [DOI] [PubMed] [Google Scholar]
- Oka T., Perry J. W., Kano K. Hormonal regulation of spermidine synthase during the development of mouse mammary epithelium in vitro. Biochem Biophys Res Commun. 1977 Dec 7;79(3):979–986. doi: 10.1016/0006-291x(77)91206-2. [DOI] [PubMed] [Google Scholar]
- Otani S., Mizoguchi Y., Matsui I., Morisawa S. Inhibition of DNA synthesis by methylglyoxal bis(guanylhydrazone) during lymphocyte transformation. Mol Biol Rep. 1974 Dec;1(8):431–436. doi: 10.1007/BF00360667. [DOI] [PubMed] [Google Scholar]
- Pajula R. L., Raina A., Eloranta T. Polyamine synthesis in mammalian tissues. Isolation and characterization of spermine synthase from bovine brain. Eur J Biochem. 1979 Nov;101(2):619–626. doi: 10.1111/j.1432-1033.1979.tb19756.x. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Prakash N. J., Schechter P. J., Grove J., Koch-Weser J. Effect of alpha-difluoromethylornithine, an enzyme-activated irreversible inhibitor of ornithine decarboxylase, on L1210 leukemia in mice. Cancer Res. 1978 Sep;38(9):3059–3062. [PubMed] [Google Scholar]
- Raina A., Jänne J. Physiology of the natural polyamines putrescine, spermidine and spermine. Med Biol. 1975 Jun;53(3):121–147. [PubMed] [Google Scholar]
- Raina A., Pajula R. L., Eloranta T. A rapid assay method for spermidine and spermine synthases. Distribution of polyamine-synthesizing enzymes and methionine adenosyltransferase in rat tissues. FEBS Lett. 1976 Sep 1;67(3):252–255. doi: 10.1016/0014-5793(76)80540-6. [DOI] [PubMed] [Google Scholar]
- Sakai T., Perry J. W., Hori C., Oka T. Putrescine and the regulation of S-adenosyl-L-methionine decarboxylase in cultured mouse mammary gland. Biochim Biophys Acta. 1980 Aug 7;614(2):577–582. doi: 10.1016/0005-2744(80)90246-6. [DOI] [PubMed] [Google Scholar]
- Seiler N., Al-Therib M. J. Putrescine catabolism in mammalian brain. Biochem J. 1974 Oct;144(1):29–35. doi: 10.1042/bj1440029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N. Use of the dansyl reaction in biochemical analysis. Methods Biochem Anal. 1970;18:259–337. doi: 10.1002/9780470110362.ch5. [DOI] [PubMed] [Google Scholar]
- Tryding N., Willert B. Determination of plasma diamine oxidase (histaminase) in clinical practice. A comparison between a biological method and a radiochemical micromethod. Scand J Clin Lab Invest. 1968;22(1):29–32. doi: 10.3109/00365516809160732. [DOI] [PubMed] [Google Scholar]


