Abstract
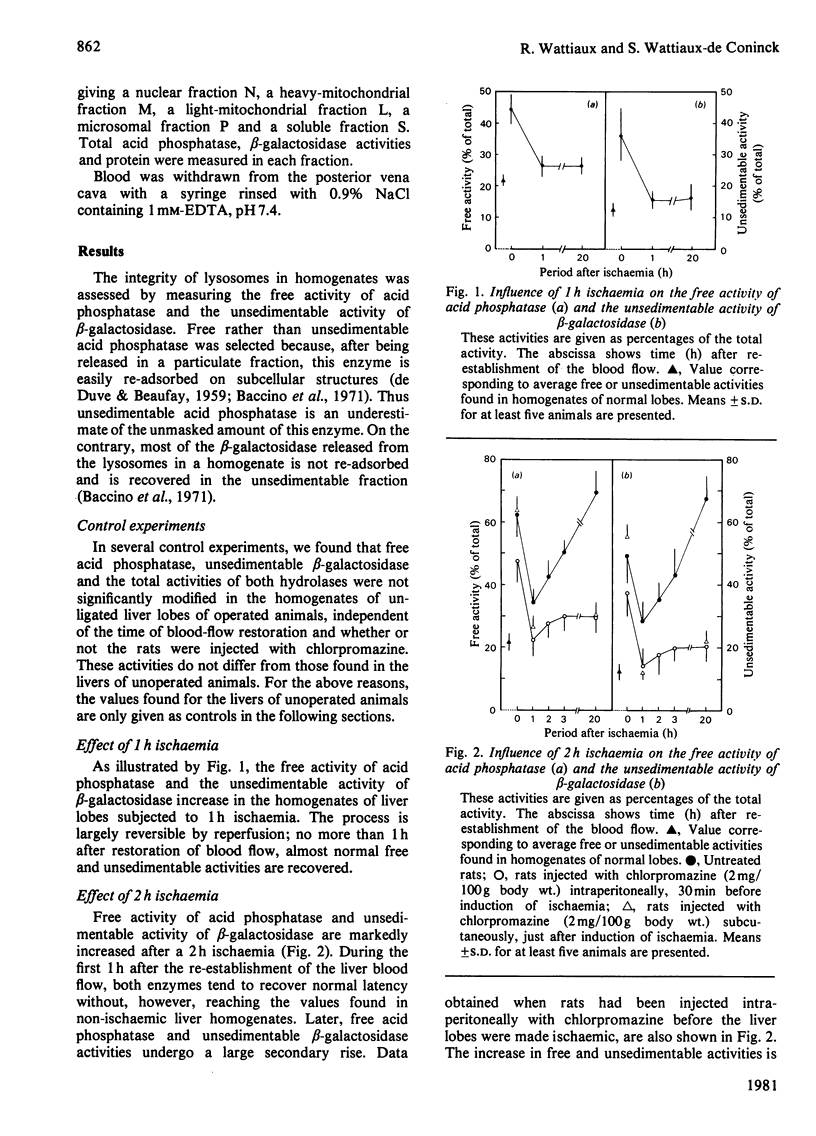
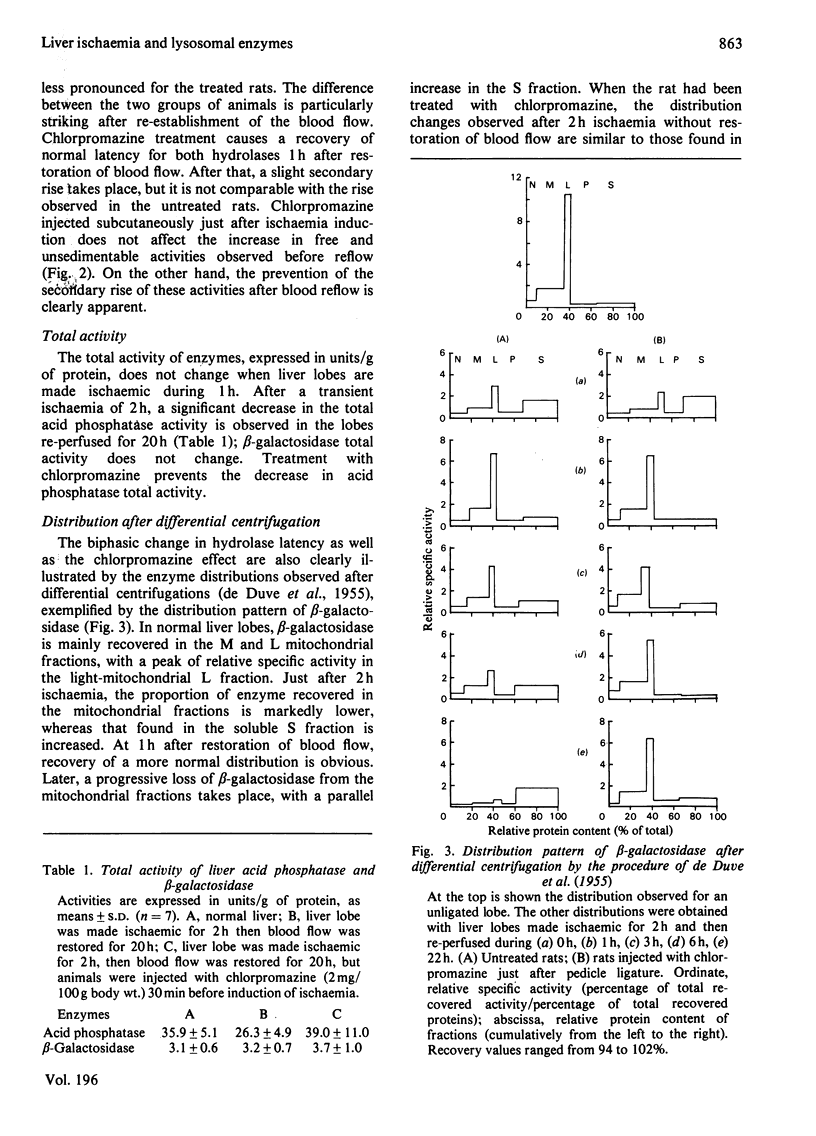
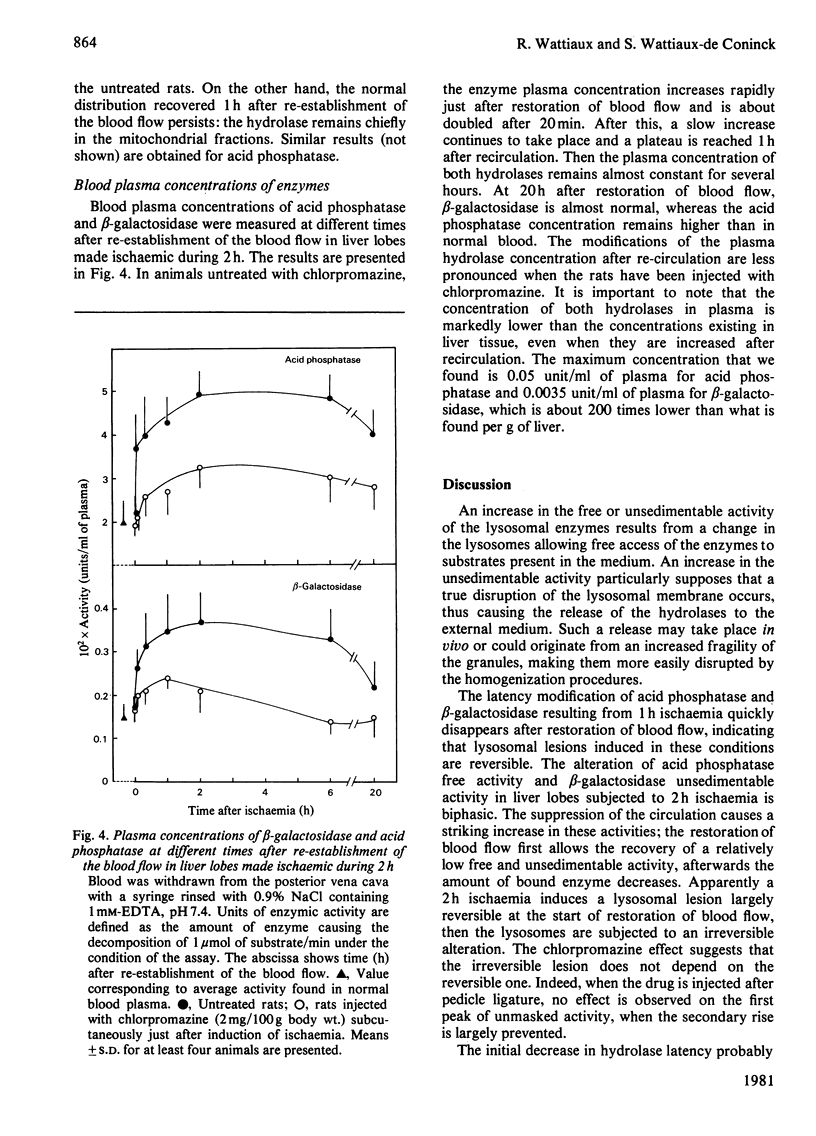
The structure-linked latency of acid phosphatase and beta-galactosidase was studied in rat liver lobes made ischaemic for 1 or 2 h and then recirculated with blood for increasing periods. Free activity of acid phosphatase and unsedimentable activity of beta-galactosidase are increased in homogenates of ischaemic livers. When ischaemia had been maintained for 1 h, the recovery of normal latency for both enzymes was observed 1 h after re-establishment of the blood flow. After a 2 h period of ischaemia, unmasked activity markedly decreases during the first 1 h after restoration of blood flow; after that, a large and irreversible secondary rise takes place. Chlorpromazine, injected 30 min before or just after induction of ischaemia, extensively prevents the latency decrease occurring during restoration of blood flow. Modifications of the hydrolase distribution pattern obtained after differential centrifugation are in agreement with the latency changes. These results suggest that a 2 h ischaemia causes an alteration of the liver lysosomes that is largely reversible and that restoration of blood flow induces an irreversible alteration of these organelles. Chlorpromazine treatment prevents the irreversible lesion from taking place.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BASSI M., BERNELLI-ZAZZERA A. ULTRASTRUCTURAL CYTOPLASMIC CHANGES OF LIVER CELLS AFTER REVERSIBLE AND IRREVERSIBLE ISCHEMIA. Exp Mol Pathol. 1964 Aug;17:332–350. doi: 10.1016/0014-4800(64)90006-1. [DOI] [PubMed] [Google Scholar]
- Baccino F. M., Rita G. A., Zuretti M. F. Studies on the structure-bound sedimentabolity of some rat liver lysosome hydrolases. Biochem J. 1971 Apr;122(3):363–371. doi: 10.1042/bj1220363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Pfau R. G., Farber J. L. Prevention by chlorpromazine of ischemic liver cell death. Am J Pathol. 1977 Sep;88(3):539–557. [PMC free article] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Serroni A., Martin J. T., Farber J. L. Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem. 1978 Jul 10;253(13):4809–4817. [PubMed] [Google Scholar]
- Chien K. R., Farber J. L. Microsomal membrane dysfunction in ischemic rat liver cells. Arch Biochem Biophys. 1977 Apr 15;180(1):191–198. doi: 10.1016/0003-9861(77)90025-x. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., BEAUFAY H. Tissue fractionation studies. 10. Influence of ischaemia on the state of some bound enzymes in rat liver. Biochem J. 1959 Dec;73:610–616. doi: 10.1042/bj0730610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., WATTIAUX R. Tissue fractionation studies. VII. Release of bound hydrolases by means of triton X-100. Biochem J. 1956 Aug;63(4):606–608. doi: 10.1042/bj0630606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze H., Nahas N., Traynor J. R., Wurl M. Effects of local anaesthetics on phospholipases. Biochim Biophys Acta. 1976 Jul 20;441(1):93–102. doi: 10.1016/0005-2760(76)90284-8. [DOI] [PubMed] [Google Scholar]


