Abstract
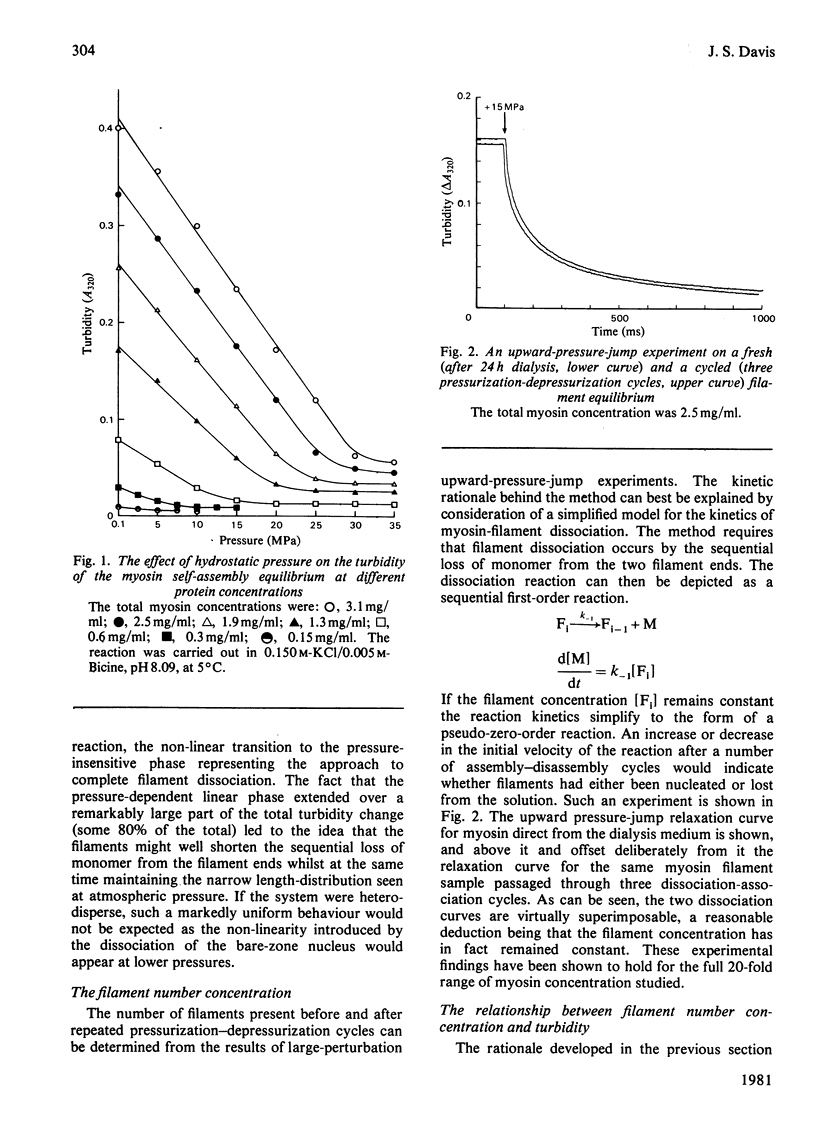
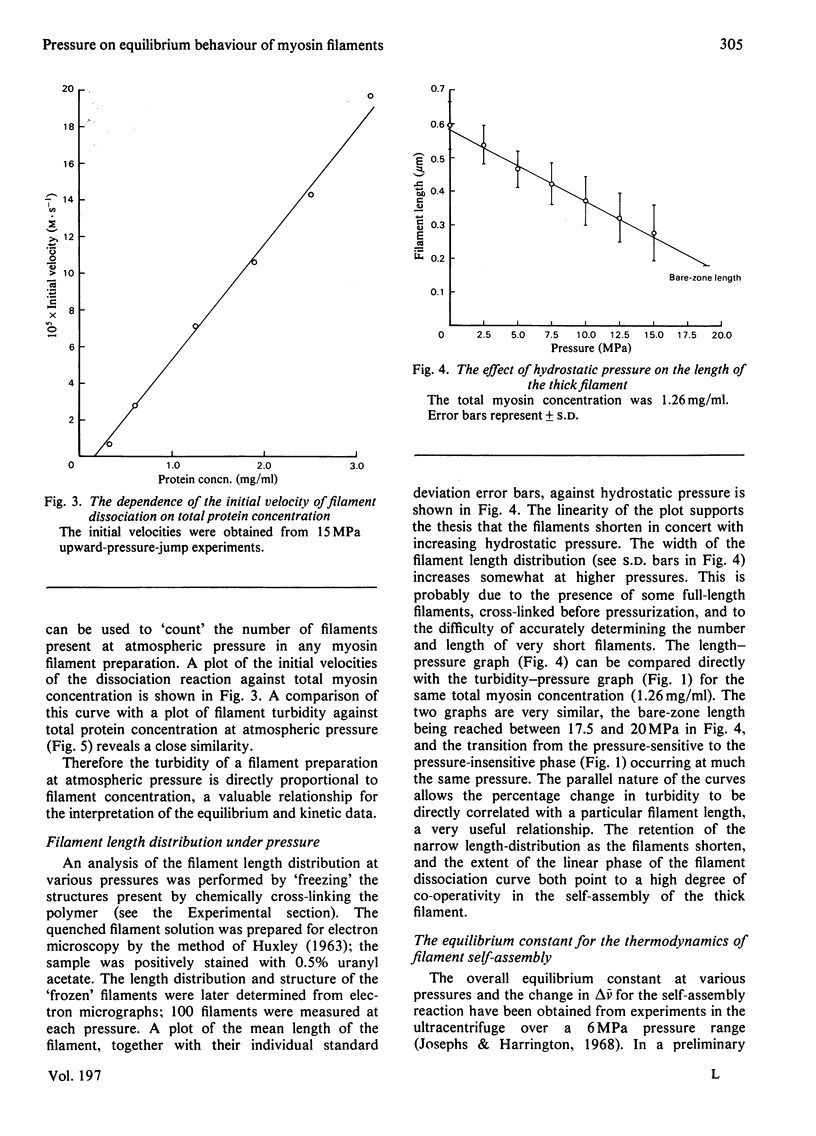
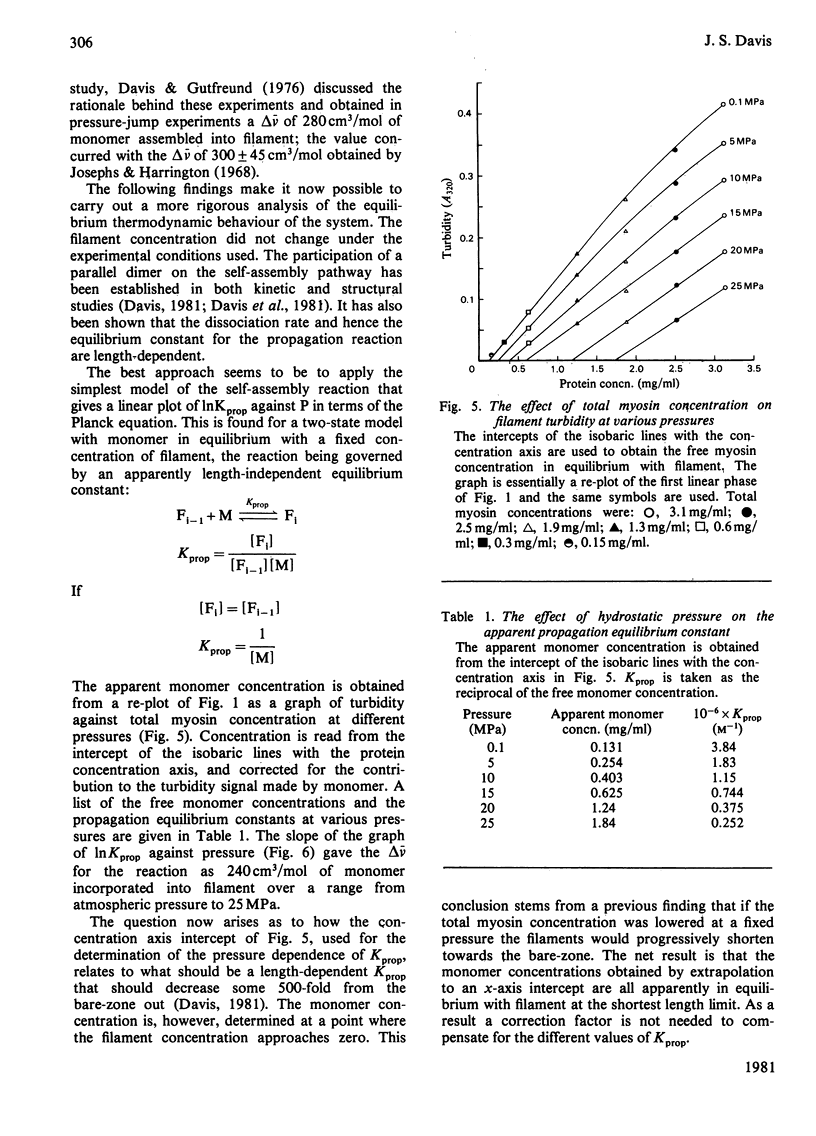
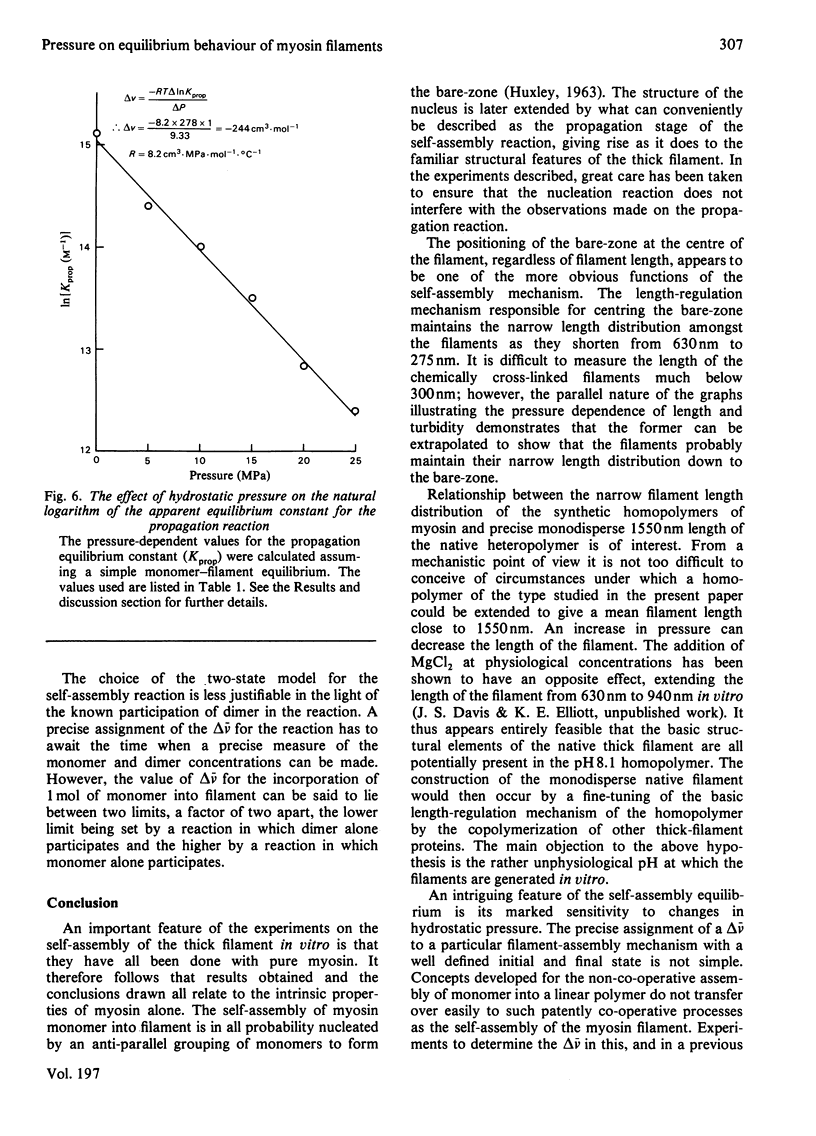
The thick-filament-monomeric-myosin equilibrium was prepared from pure myosin at pH 8.1. The application of hydrostatic pressure to the self-assembly equilibrium resulted in a biphasic dissociation curve in which a linear decrease in turbidity (a measure of weight added to or lost from the filament) was followed by a transition to a second pressure-insensitive phase. The first phase represents the effect of hydrostatic pressure on the growth or propagation phase of filament assembly. Here is was shown that hydrostatic pressure served to shorten the filaments in concert towards the bare zone whilst maintaining the narrow length distribution seen at atmospheric pressure; the filament concentration remained constant during the experiment. A more precise definition of the delta-v for the assembly of monomer into filament was obtained than had hitherto been possible. The positioning of the bare zone at the centre of the filament seems to be one of the more obvious functions of the length-regulation mechanism. It also appears that all the basic structural elements of the native thick filament are potentially present in the pH 8.1 homopolymer; its length can be increased by physiological concentrations of MgCl2 and decreased by pressure. The monodisperse native filament could then be formed by a fine tuning of the basic length-regulation mechanism of the homopolymer by the co-polymerization of the other thick-filament proteins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berne B. J. Interpretation of the light scattering from long rods. J Mol Biol. 1974 Nov 15;89(4):755–758. doi: 10.1016/0022-2836(74)90049-7. [DOI] [PubMed] [Google Scholar]
- Craig R., Offer G. Axial arrangement of crossbridges in thick filaments of vertebrate skeletal muscle. J Mol Biol. 1976 Apr 5;102(2):325–332. doi: 10.1016/s0022-2836(76)80057-5. [DOI] [PubMed] [Google Scholar]
- Davis J. S., Gutfreund H. The scope of moderate pressure changes for kinetic and equilibrium studies of biochemical systems. FEBS Lett. 1976 Dec 31;72(2):199–207. doi: 10.1016/0014-5793(76)80971-4. [DOI] [PubMed] [Google Scholar]
- Josephs R., Harrington W. F. On the stability of myosin filaments. Biochemistry. 1968 Aug;7(8):2834–2847. doi: 10.1021/bi00848a020. [DOI] [PubMed] [Google Scholar]
- Josephs R., Harrington W. F. Studies on the formation and physical chemical properties of synthetic myosin filaments. Biochemistry. 1966 Nov;5(11):3474–3487. doi: 10.1021/bi00875a013. [DOI] [PubMed] [Google Scholar]
- Morimoto K., Harrington W. F. Isolation and composition of thick filaments from rabbit skeletal muscle. J Mol Biol. 1973 Jun 15;77(1):165–175. doi: 10.1016/0022-2836(73)90370-7. [DOI] [PubMed] [Google Scholar]
- Richards E. G., Chung C. S., Menzel D. B., Olcott H. S. Chromatography of myosin on diethylaminoethyl-Sephadex A-50. Biochemistry. 1967 Feb;6(2):528–540. doi: 10.1021/bi00854a022. [DOI] [PubMed] [Google Scholar]
- Starr R., Offer G. Polypeptide chains of intermediate molecular weight in myosin preparations. FEBS Lett. 1971 Jun 2;15(1):40–44. doi: 10.1016/0014-5793(71)80075-3. [DOI] [PubMed] [Google Scholar]


