Abstract
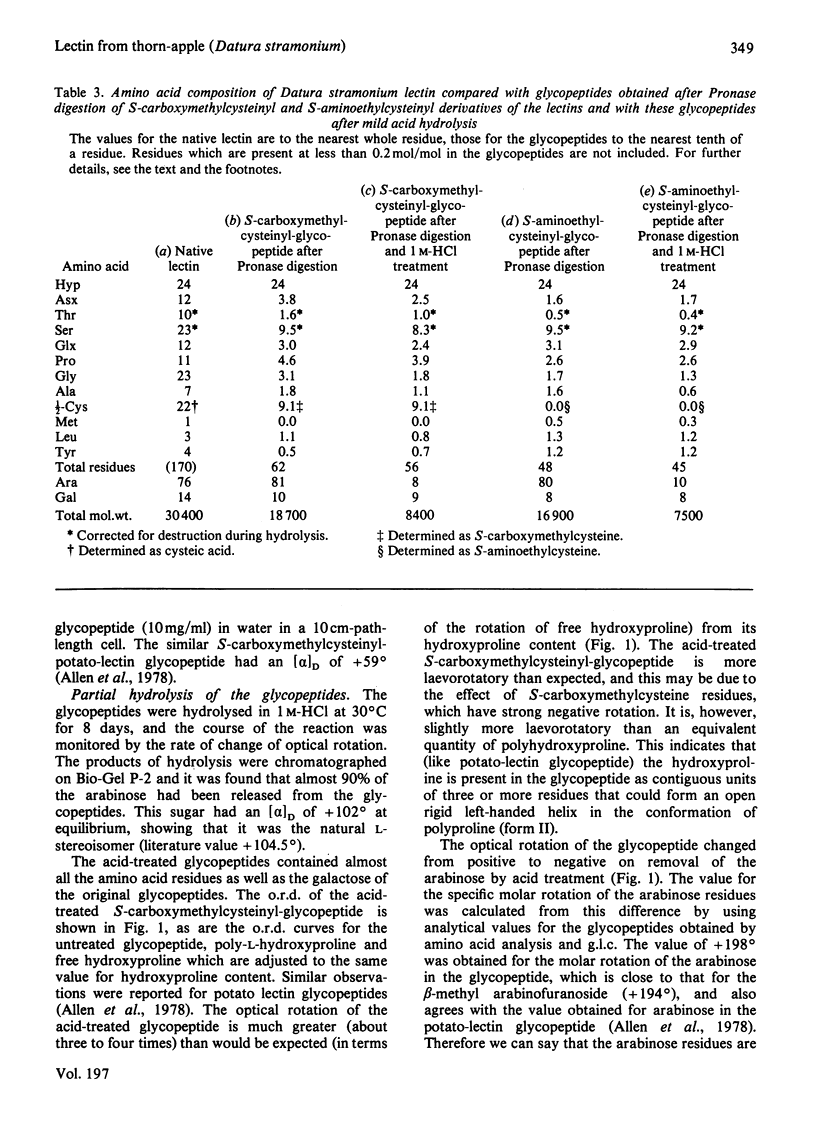
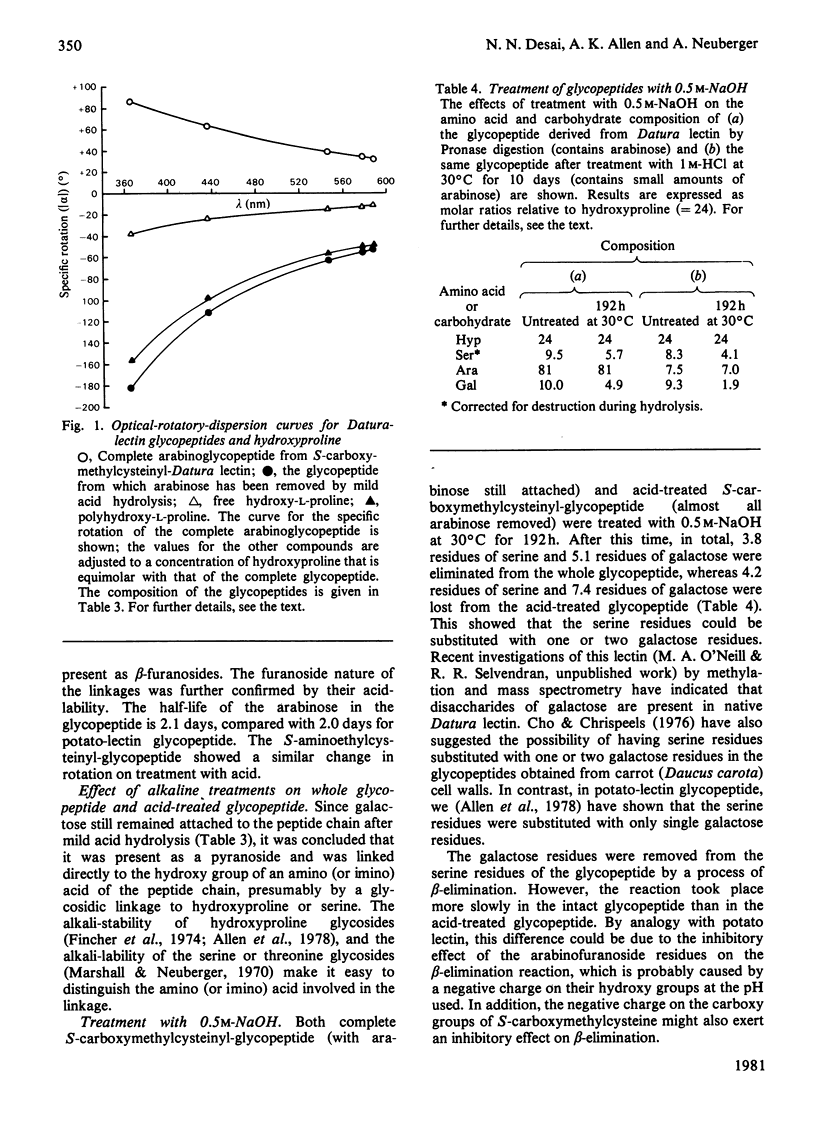
The lectin from Datura stramonium (thorn-apple; Solanaceae) has been purified by affinity chromatography and shown to be a glycoprotein containing about 40% (w/w) of carbohydrate. The most abundant amino acids are hydroxyproline, cystine, glycine and serine. Results obtained by gel filtration in 6m-guanidinium chloride on Sepharose 4B suggest that it has a subunit mol.wt. of about 30000 and that it probably associates into dimers. The lectin is inhibited specifically by chitin oligosaccharides and bacterial-cell-wall oligosaccharides, but only weakly by N-acetylglucosamine. Glycopeptides from soya-bean (Glycine max) lectin and fetuin are also strong inhibitors of Datura lectin, indicating that it interacts with internal N-acetylglucosamine residues. Its specificity is similar to, but not identical with, that of potato (Solanum tuberosum) lectin. After prolonged proteolytic digestion of reduced and S-carboxymethylated or S-aminoethylated derivatives of the lectin, glycopeptides of mol.wt. of about 18000 were isolated. The glycopeptides contained all the carbohydrate and hydroxyproline of the original glycoprotein, and lesser amounts of serine, S-carboxymethylcysteine and other amino acids. The arabinose residues of the glycoprotein are present as β-l-arabinofuranosides linked to the polypeptide chain through the hydroxyproline residues, and can be removed by mild acid treatment; the ratio of arabinose to hydroxyproline is 3.4:1. Some of the serine residues of the polypeptide chain are substituted with one or two α-galactopyranoside residues, most of which can be removed by the action of α-galactosidase. The galactose residues are more easily removed from the acid-treated glycopeptide (from which arabinose has been removed) than from the complete glycopeptide, indicating a steric hindrance of the galactosidase action by the adjacent chains of arabinosides. There is a slow release of galactose residues by a process of β-elimination in 0.5m-NaOH (pH13.7) from the complete glycopeptide, and a fairly rapid release of galactose by this process from the acid-treated glycopeptide, which lacks arabinose. This is probably due to the inhibitory effect of the negative charge on the adjacent arabinofuranoside residues. The similarities and differences between the lectins from Datura and potato are discussed, as are their structural resemblance to glycopeptides that have been isolated from plant cell walls.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K. A lectin from the exudate of the fruit of the vegetable marrow (Cucurbita pepo) that has a specificity for beta-1,4-linked N-acetylglucosamine oligosaccharides. Biochem J. 1979 Oct 1;183(1):133–137. doi: 10.1042/bj1830133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. K., Desai N. N., Neuberger A., Creeth J. M. Properties of potato lectin and the nature of its glycoprotein linkages. Biochem J. 1978 Jun 1;171(3):665–674. doi: 10.1042/bj1710665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. K., Desai N. N., Neuberger A. Purification of the glycoprotein lectin from the broad bean (Vicia faba) and a comparison of its properties with lectins of similar specificity. Biochem J. 1976 Apr 1;155(1):127–135. doi: 10.1042/bj1550127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. K., Neuberger A., Sharon N. The purification, composition and specificity of wheat-germ agglutinin. Biochem J. 1973 Jan;131(1):155–162. doi: 10.1042/bj1310155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. K., Neuberger A. The purification and properties of the lectin from potato tubers, a hydroxyproline-containing glycoprotein. Biochem J. 1973 Oct;135(2):307–314. doi: 10.1042/bj1350307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. E., Clamp J. R. An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. Biochem J. 1971 Dec;125(4):1009–1018. doi: 10.1042/bj1251009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N. N., Allen A. K. The purification of potato lectin by affinity chromatography on an N,N',N''-triacetylchitotriose-Sepharose matrix. Anal Biochem. 1979 Feb;93(1):88–90. [PubMed] [Google Scholar]
- Fincher G. B., Sawyer W. H., Stone B. A. Chemical and physical properties of an arabinogalactan-peptide from wheat endosperm. Biochem J. 1974 Jun;139(3):535–545. doi: 10.1042/bj1390535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. J., Hammarström S., Sundblad G. Precipitation and carbohydrate-binding specificity studies on wheat germ agglutinin. Biochim Biophys Acta. 1975 Sep 9;405(1):53–61. doi: 10.1016/0005-2795(75)90313-x. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Horejsí V., Kocourek J. Studies on lectins. XXXVII. Isolation and characterization of the lectin from Jimson-weed seeds (Datura stramonium L.). Biochim Biophys Acta. 1978 Jan 25;532(1):92–97. [PubMed] [Google Scholar]
- Kilpatrick D. C., Jeffree C. E., Lockhart C. M., Yeoman M. M. Immunological evidence for structural similarity among lectins from species of the Solanaceae. FEBS Lett. 1980 Apr 21;113(1):129–133. doi: 10.1016/0014-5793(80)80511-4. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. C., Yeoman M. M., Gould A. R. Tissue and subcellular distribution of the lectin from Datura stramonium (thorn apple). Biochem J. 1979 Nov 15;184(2):215–219. doi: 10.1042/bj1840215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. C., Yeoman M. M. Purification of the lectin from Datura stramonium. Biochem J. 1978 Dec 1;175(3):1151–1153. doi: 10.1042/bj1751151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDLEY H. A new synthetic substrate for trypsin and its application to the determination of the amino-acid sequence of proteins. Nature. 1956 Sep 22;178(4534):647–648. doi: 10.1038/178647a0. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. Soybean agglutinin--a plant glycoprotein. Structure of the carboxydrate unit. J Biol Chem. 1978 May 25;253(10):3468–3476. [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Fish W. W. Protein polypeptide chain molecular weights by gel chromatography in guanidinium chloride. Methods Enzymol. 1972;26:28–42. doi: 10.1016/s0076-6879(72)26004-9. [DOI] [PubMed] [Google Scholar]
- O'Neill M. A., Selvendran R. R. Glycoproteins from the cell wall of Phaseolus coccineus. Biochem J. 1980 Apr 1;187(1):53–63. doi: 10.1042/bj1870053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoe G. I., Bird G. W., Uhlenbruck G., Sprenger I., Heggen M. Heterophile agglutinins with a broad-spectrum specificity. VI. The nature of cell surface receptors for the agglutinins present in seeds of Amaranthus caudatus, Maclura aurantica, Datura stramonium, Viscum album, Phaseolus vulgaris and Moluccella laevis. Z Immunitatsforsch Allerg Klin Immunol. 1970 Oct;140(4):374–394. [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974 Sep 25;249(18):5704–5717. [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Adv Protein Chem. 1973;27:349–467. doi: 10.1016/s0065-3233(08)60451-9. [DOI] [PubMed] [Google Scholar]
- Tucker S. M., Boyd P. J., Thompson A. E., Price R. G. Automated assay of N-acetyl-beta-glucosaminidase in normal and pathological human urine. Clin Chim Acta. 1975 Jul 23;62(2):333–339. doi: 10.1016/0009-8981(75)90245-4. [DOI] [PubMed] [Google Scholar]
- Uy R., Wold F. 1,4-Butanediol diglycidyl ether coupling of carbohydrates to Sepharose: affinity adsorbents for lectins and glycosidases. Anal Biochem. 1977 Jul;81(1):98–107. doi: 10.1016/0003-2697(77)90602-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]


