Abstract
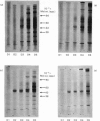
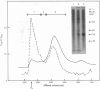
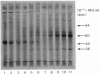
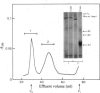
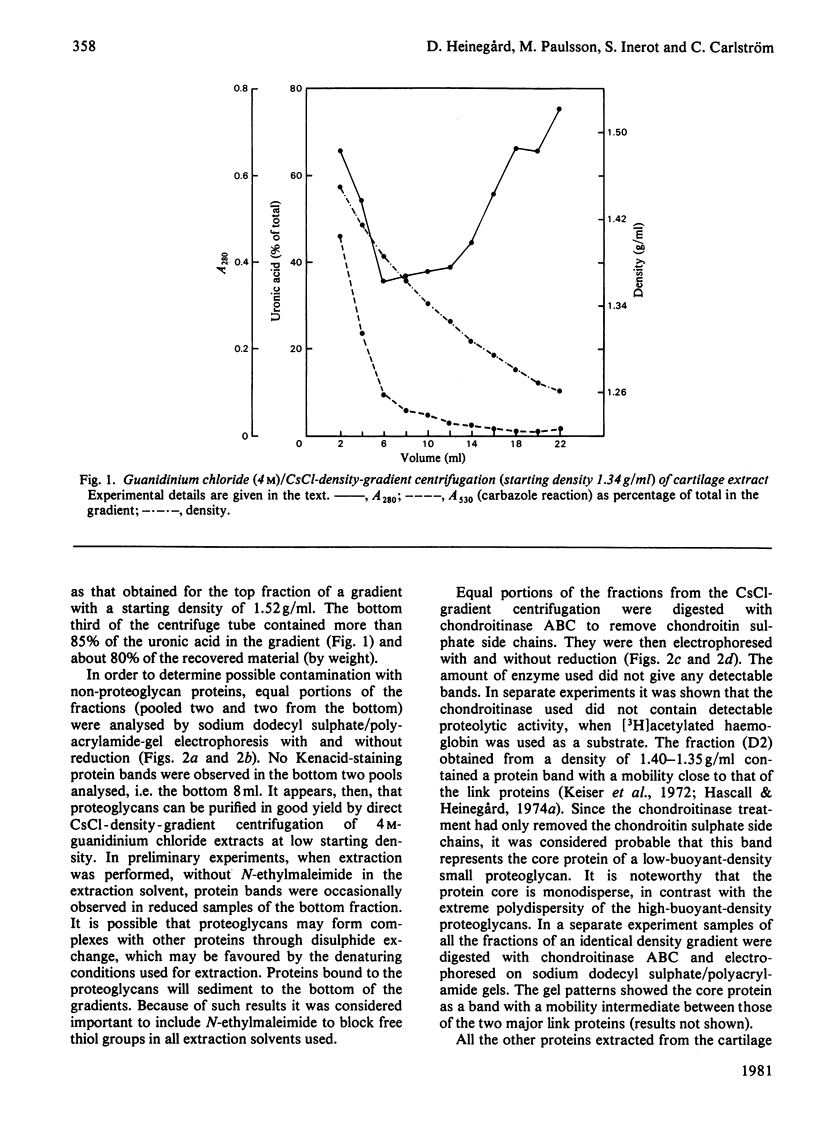
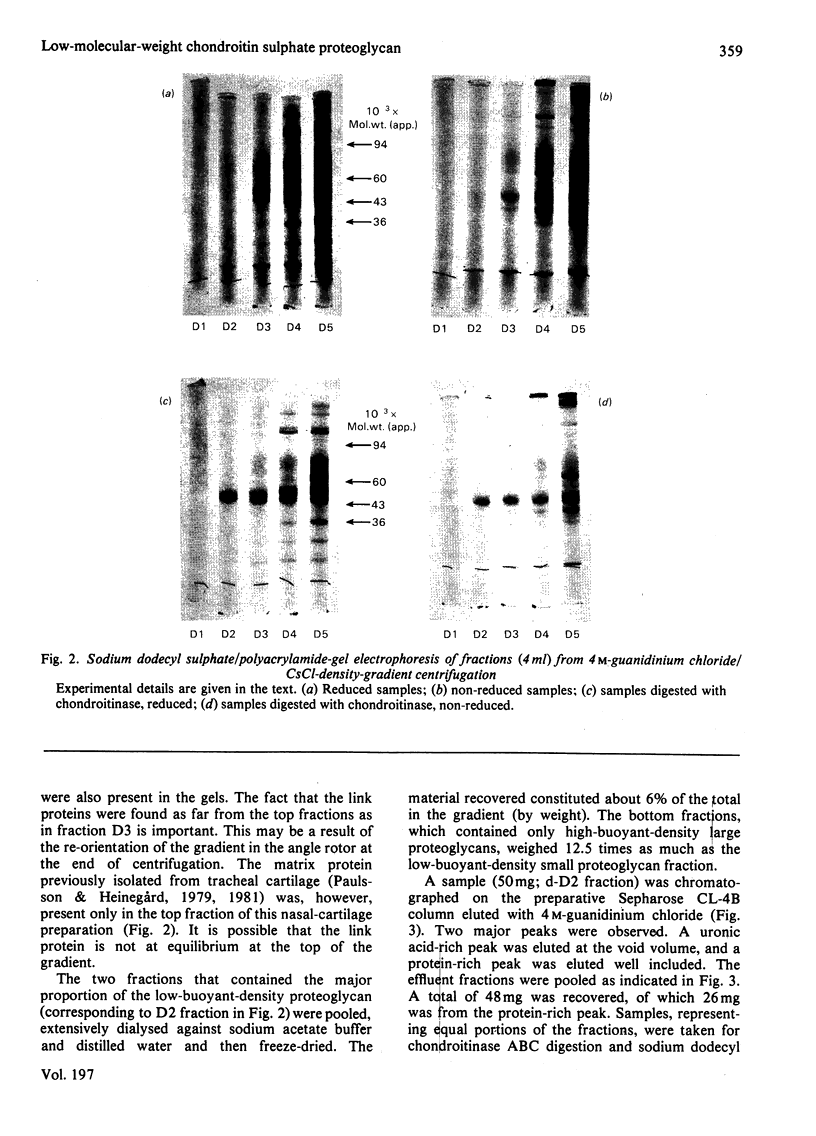
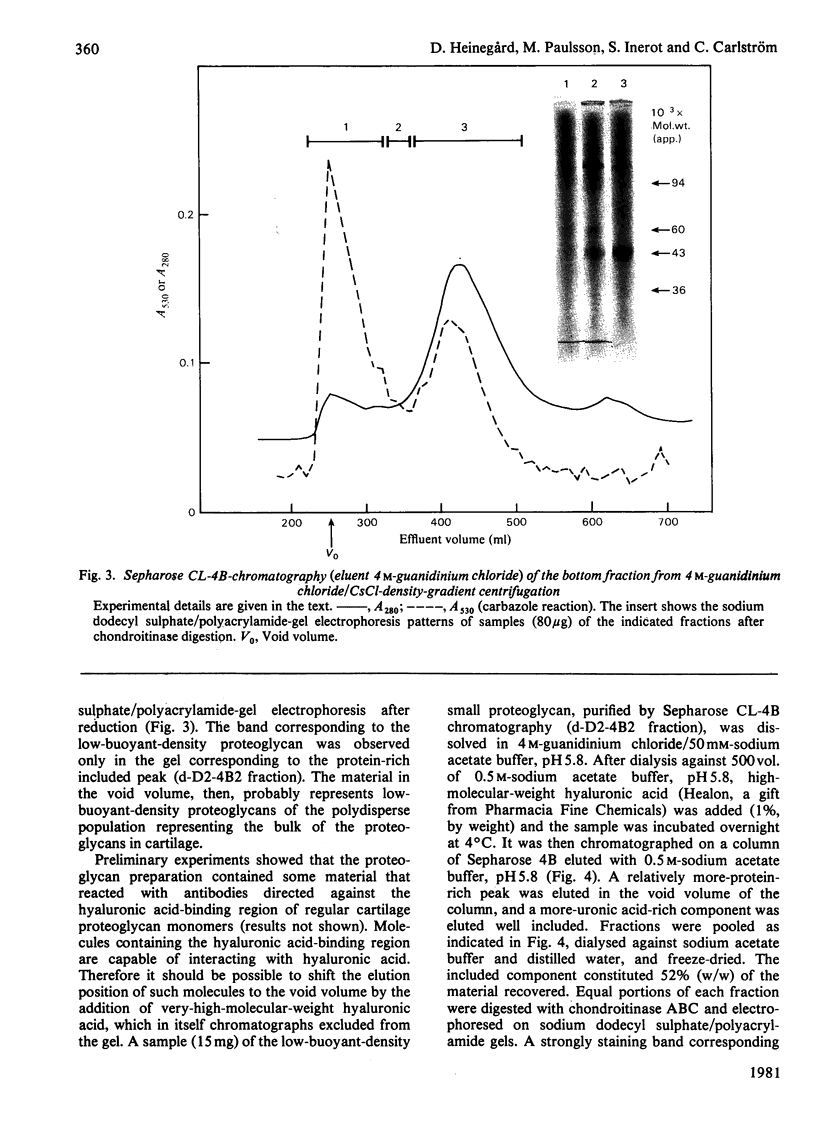
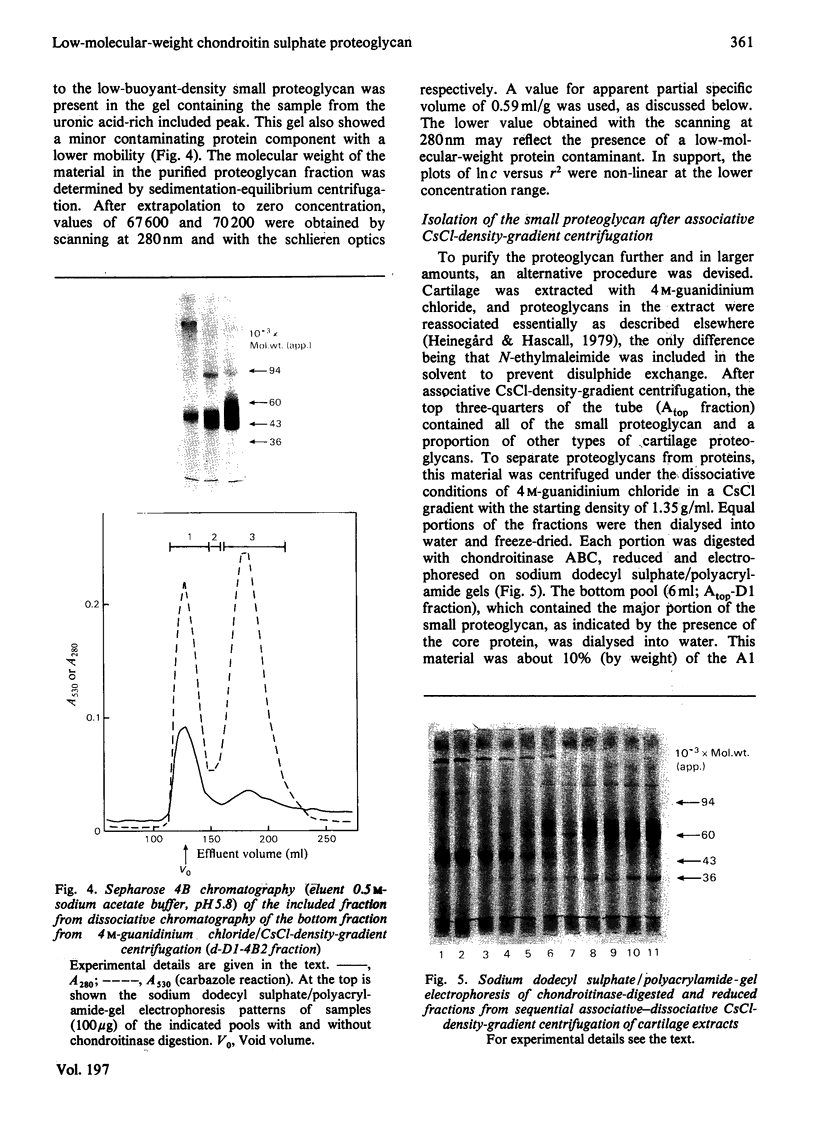
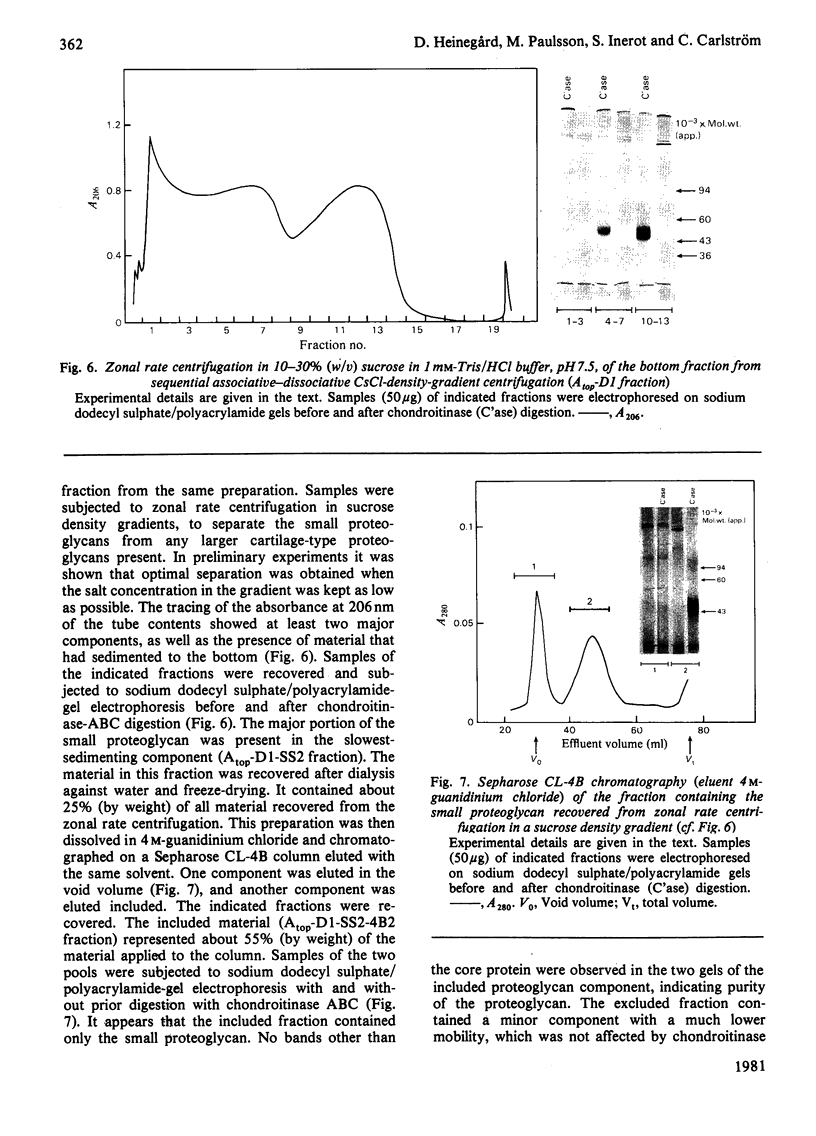
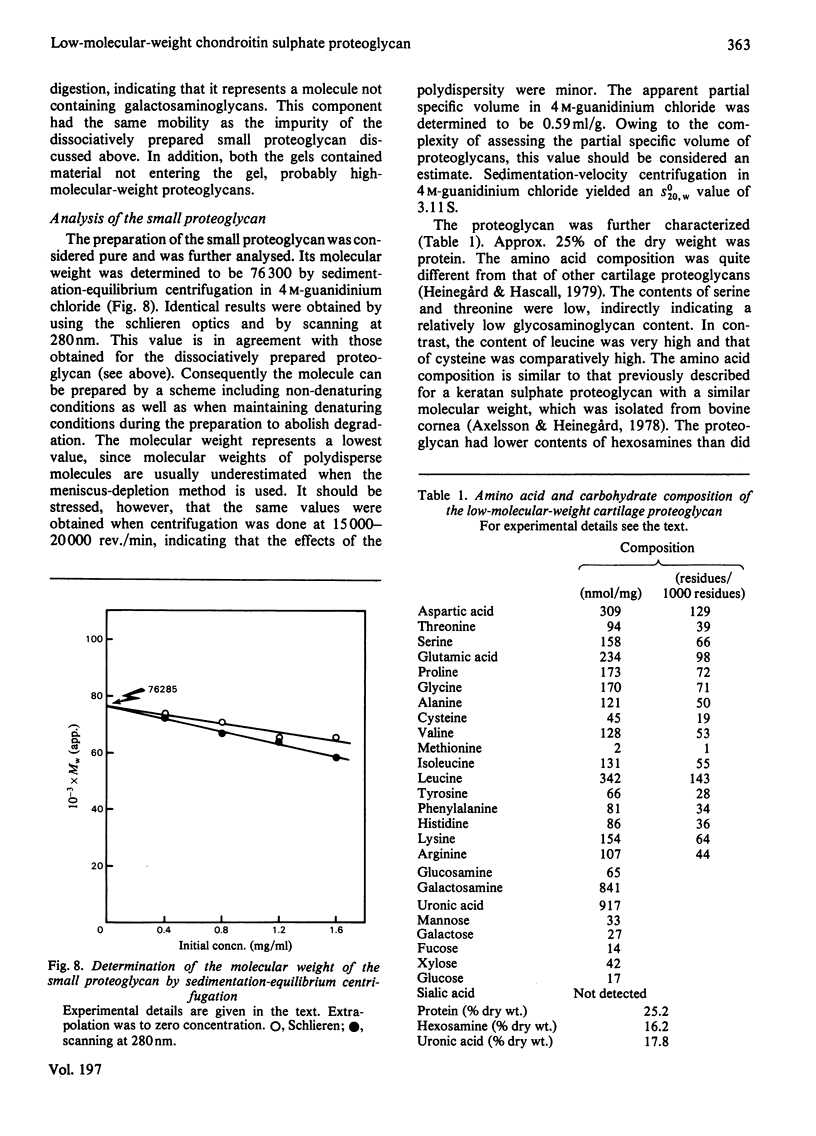
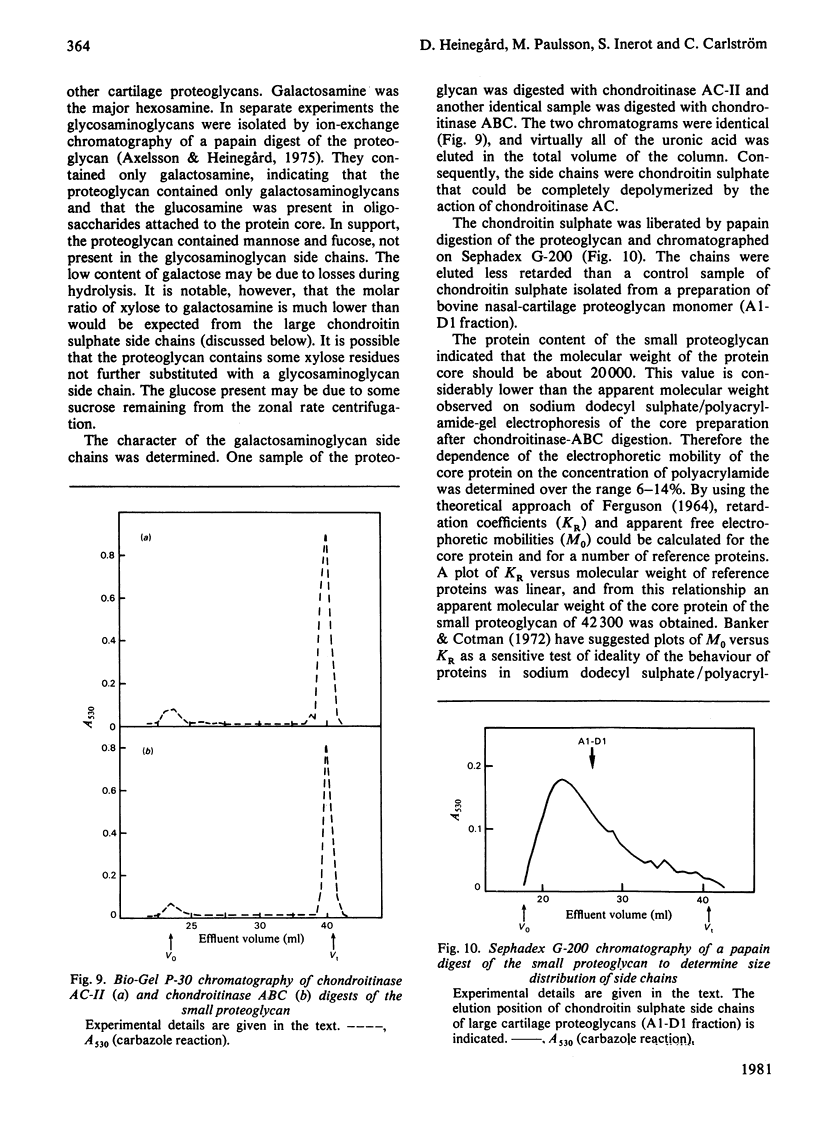
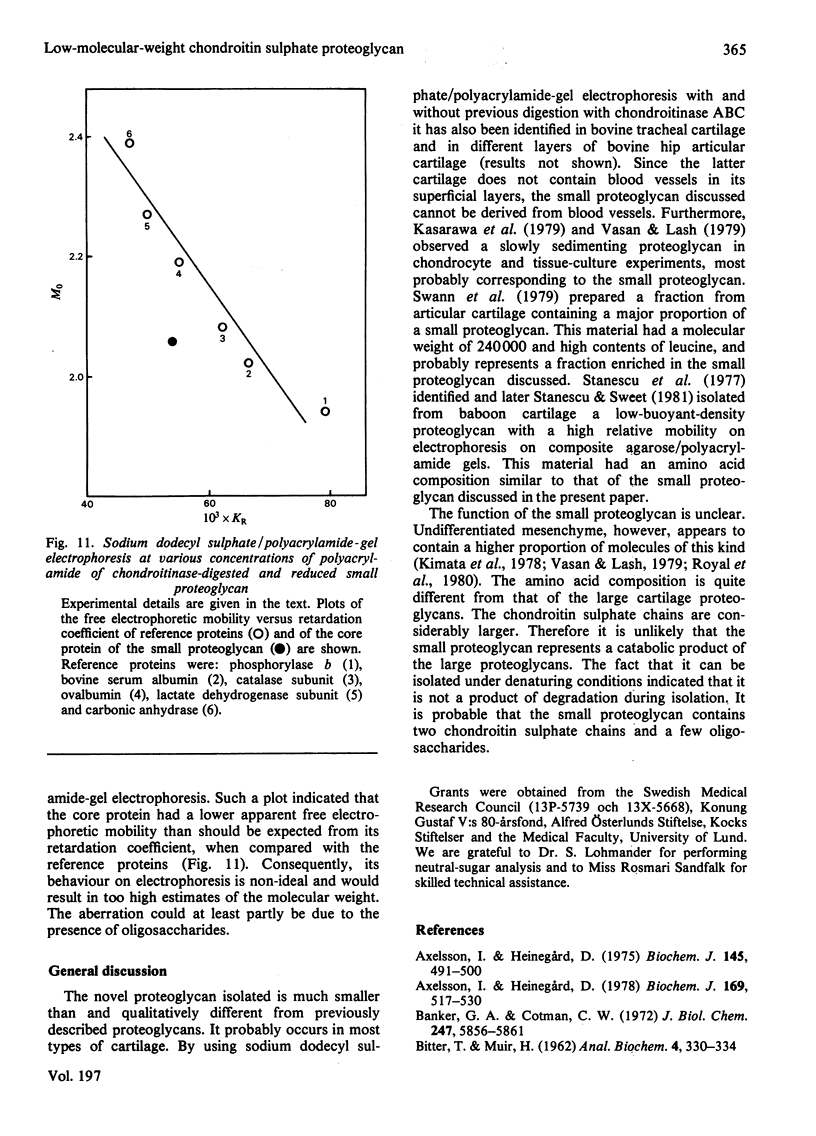
Proteoglycans were isolated from cartilage by extraction with 4M-guanidinium chloride followed by direct centrifugation in 4M-guanidinium chloride/CsCl at a low starting density, 1.34 g/ml. N-Ethylmaleimide was included in the extraction solvent as a precaution against contamination of proteoglycans with unrelated proteins mediated by disulphide exchange. A novel, discrete, low-buoyant-density proteoglycan (1.40--1.35 g/ml) was demonstrated by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. Its proteoglycan nature was revealed by the shift in the molecular size observed on gel electrophoresis after treatment with chondroitinase ABC. The core protein was monodisperse. The proteoglycan was further purified by gel chromatography with and without addition of hyaluronate. The proteoglycan constitutes less than 2% (by weight) of the total extracted proteoglycans and is not capable of interacting with hyaluronate. The same proteoglycan was purified in larger quantities by sequential associative and dissociative CsCl-density-gradient centrifugation, zonal rate sedimentation in a sucrose gradient and gel chromatography on Sepharose CL-4B. The pure proteoglycan had a molecular weight of 76 300 determined by sedimentation-equilibrium centrifugation and an apparent partial specific volume of 0.59 ml/g. It contained about 25% protein (of dry weight) and had remarkably high contents of leucine and cysteine as compared with other proteoglycans. The proteoglycan contained two to three large chondroitin sulphate chains and some oligosaccharides.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsson I., Heinegård D. Characterization of the keratan sulphate proteoglycans from bovine corneal stroma. Biochem J. 1978 Mar 1;169(3):517–530. doi: 10.1042/bj1690517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson I., Heinegård D. Fractionation of proteoglycans from bovine corneal stroma. Biochem J. 1975 Mar;145(3):491–500. doi: 10.1042/bj1450491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Banker G. A., Cotman C. W. Measurement of free electrophoretic mobility and retardation coefficient of protein-sodium dodecyl sulfate complexes by gel electrophoresis. A method to validate molecular weight estimates. J Biol Chem. 1972 Sep 25;247(18):5856–5861. [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem J. 1976 Jul 1;157(1):127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Binding of oligosaccharides of hyaluronic acid to proteoglycans. Biochem J. 1973 Dec;135(4):905–908. doi: 10.1042/bj1350905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974 Jul 10;249(13):4232–4241. [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974 Jul 10;249(13):4242–4249. [PubMed] [Google Scholar]
- Hascall V. C., Riolo R. L. Characteristics of the protein-keratan sulfate core and of keratan sulfate prepared from bovine nasal cartilage proteoglycan. J Biol Chem. 1972 Jul 25;247(14):4529–4538. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Physical properties and polydispersity of proteoglycan from bovine nasal cartilage. J Biol Chem. 1970 Oct 10;245(19):4920–4930. [PubMed] [Google Scholar]
- Heinegård D. K., Hascall V. C. Characteristics of the nonaggregating proteoglycans isolated from bovine nasal cartilage. J Biol Chem. 1979 Feb 10;254(3):927–934. [PubMed] [Google Scholar]
- Heinegård D., Axelsson I. Distribution of keratan sulfate in cartilage proteoglycans. J Biol Chem. 1977 Mar 25;252(6):1971–1979. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- Karasawa K., Kimata K., Ito K., Kato Y., Suzuki S. Morphological and biochemical differentiation of limb bud cells cultured in chemically defined medium. Dev Biol. 1979 Jun;70(2):287–305. doi: 10.1016/0012-1606(79)90029-0. [DOI] [PubMed] [Google Scholar]
- Keiser H., Shulman H. J., Sandson J. I. Immunochemistry of cartilage proteoglycan. Immunodiffusion and gel-electrophoretic studies. Biochem J. 1972 Jan;126(1):163–169. doi: 10.1042/bj1260163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata K., Oike Y., Ito K., Karasawa K., Suzuki S. The occurrence of low buoyant density proteoglycans in embryonic chick cartilage. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1431–1439. doi: 10.1016/0006-291x(78)91163-4. [DOI] [PubMed] [Google Scholar]
- Mason R. M., Mayes R. W. Extraction of cartilage protein-polysaccharides with inorganic salt solutions. Biochem J. 1973 Mar;131(3):535–540. doi: 10.1042/bj1310535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Paulsson M., Heinegård D. Matrix proteins bound to associatively prepared proteoglycans from bovine cartilage. Biochem J. 1979 Dec 1;183(3):539–545. doi: 10.1042/bj1830539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Heinegård D. Purification and structural characterization of a cartilage matrix protein. Biochem J. 1981 Aug 1;197(2):367–375. doi: 10.1042/bj1970367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. P., Mason R. M. The stability of bovine nasal cartilage proteoglycans during isolation and storage. Biochim Biophys Acta. 1977 Jun 23;498(1):176–188. doi: 10.1016/0304-4165(77)90098-8. [DOI] [PubMed] [Google Scholar]
- Royal P. D., Sparks K. J., Goetinck P. F. Physical and immunochemical characterization of proteoglycans synthesized during chondrogenesis in the chick embryo. J Biol Chem. 1980 Oct 25;255(20):9870–9878. [PubMed] [Google Scholar]
- Stanescu V., Maroteaux P., Sobczak E. Proteoglycan populations of baboon (Papio papio) articular cartilage. Biochem J. 1977 Apr 1;163(1):103–109. doi: 10.1042/bj1630103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanescu V., Sweet M. B. Characterization of a proteoglycan of high electrophoretic mobility. Biochim Biophys Acta. 1981 Feb 18;673(1):101–113. [PubMed] [Google Scholar]
- Swann D. A., Powell S., Sotman S. The heterogeneity of cartilage proteoglycans. Isolation of different types of proteoglycans from bovine articular cartilage. J Biol Chem. 1979 Feb 10;254(3):945–954. [PubMed] [Google Scholar]
- Thomas J. O., Edelstein S. J. Molecular weights and volumes from density perturbation ultracentrifugation. Application to aldolase and deoxyribonucleic acid polymerase in solutions of guanidine hydrochloride. Biochemistry. 1971 Feb 2;10(3):477–482. doi: 10.1021/bi00779a020. [DOI] [PubMed] [Google Scholar]
- Vasan N. S., Lash J. W. Monomeric and aggregate proteoglycans in the chondrogenic differentiation of embryonic chick limb buds. J Embryol Exp Morphol. 1979 Jan;49:47–59. [PubMed] [Google Scholar]
- Walborg E. F., Jr, Kondo L. E. Automated system for ion-exchange chromatography of saccharides. Anal Biochem. 1970 Oct;37(2):320–329. doi: 10.1016/0003-2697(70)90054-0. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]