Abstract
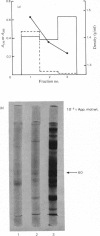
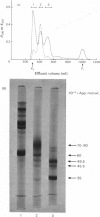
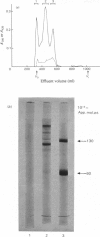
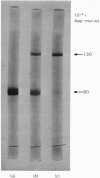
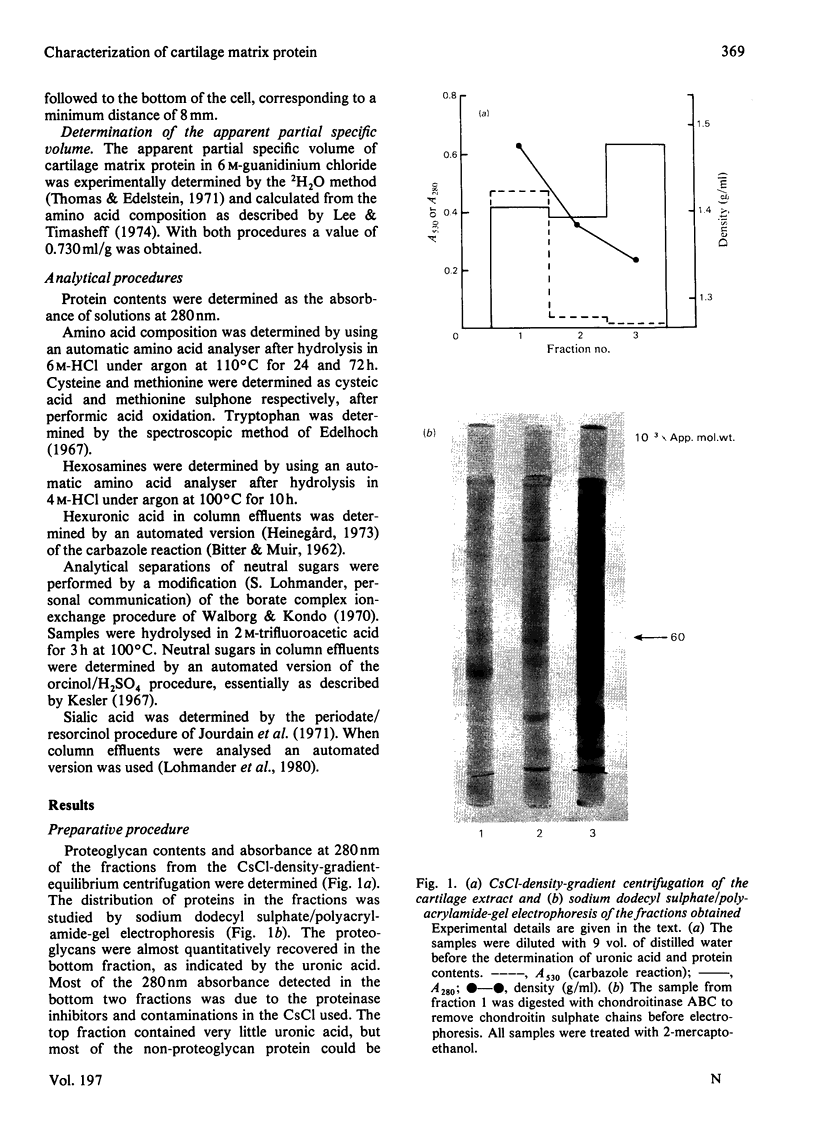
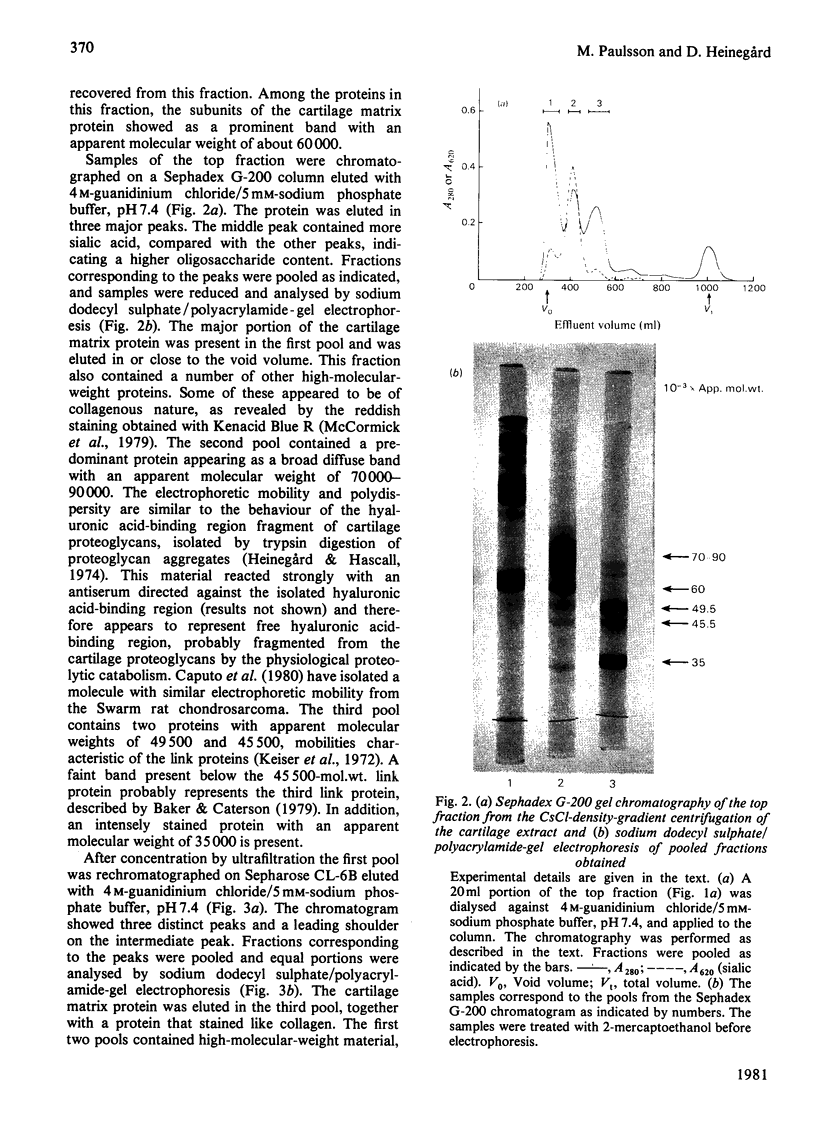
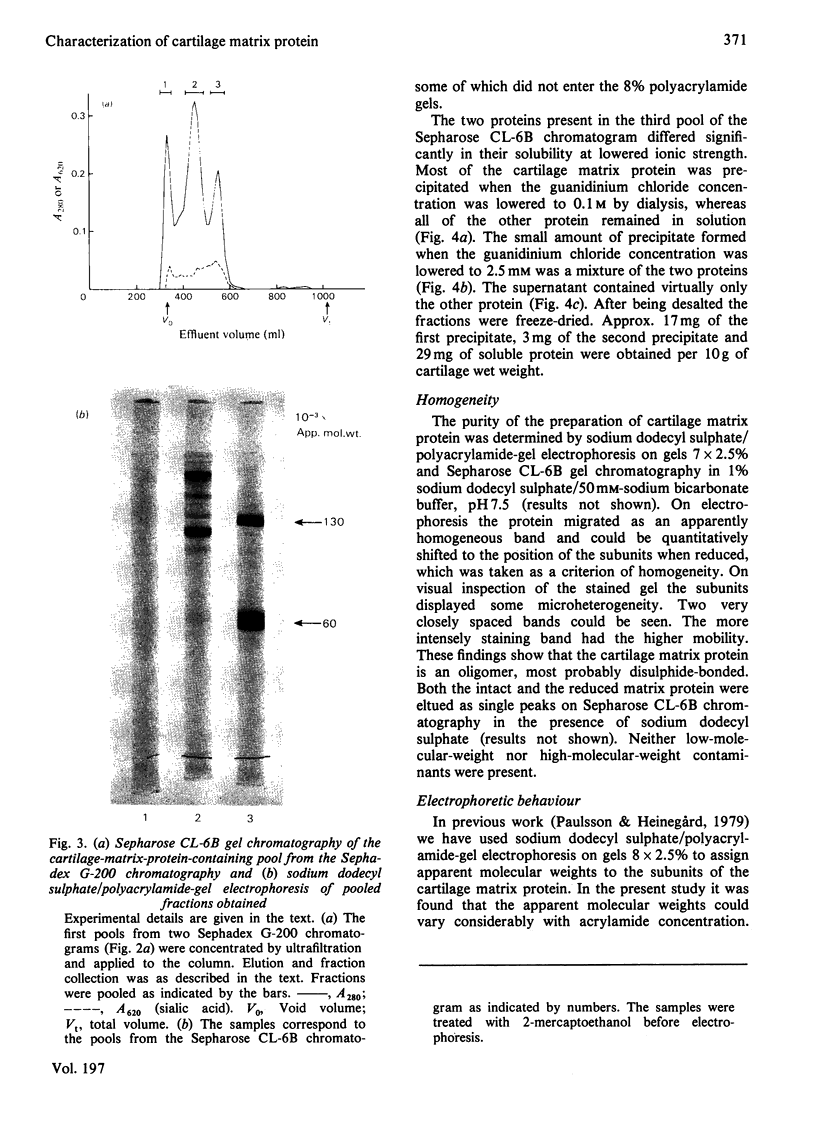
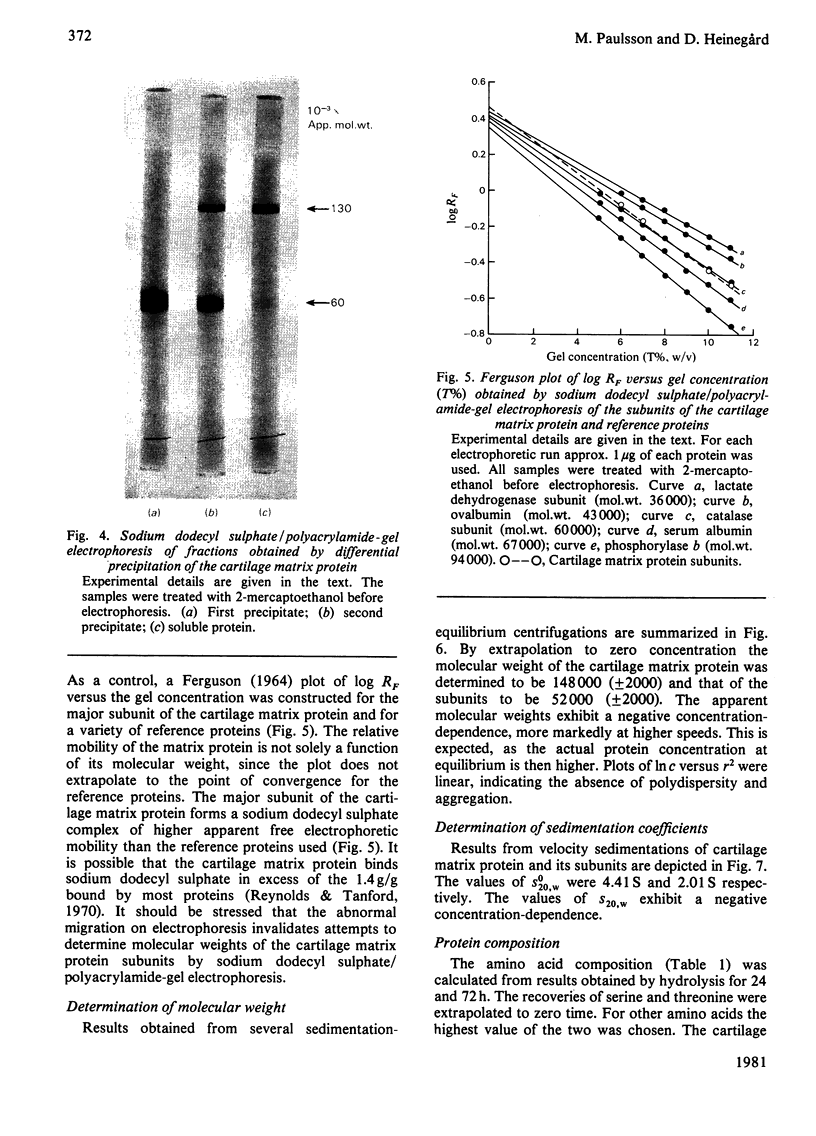
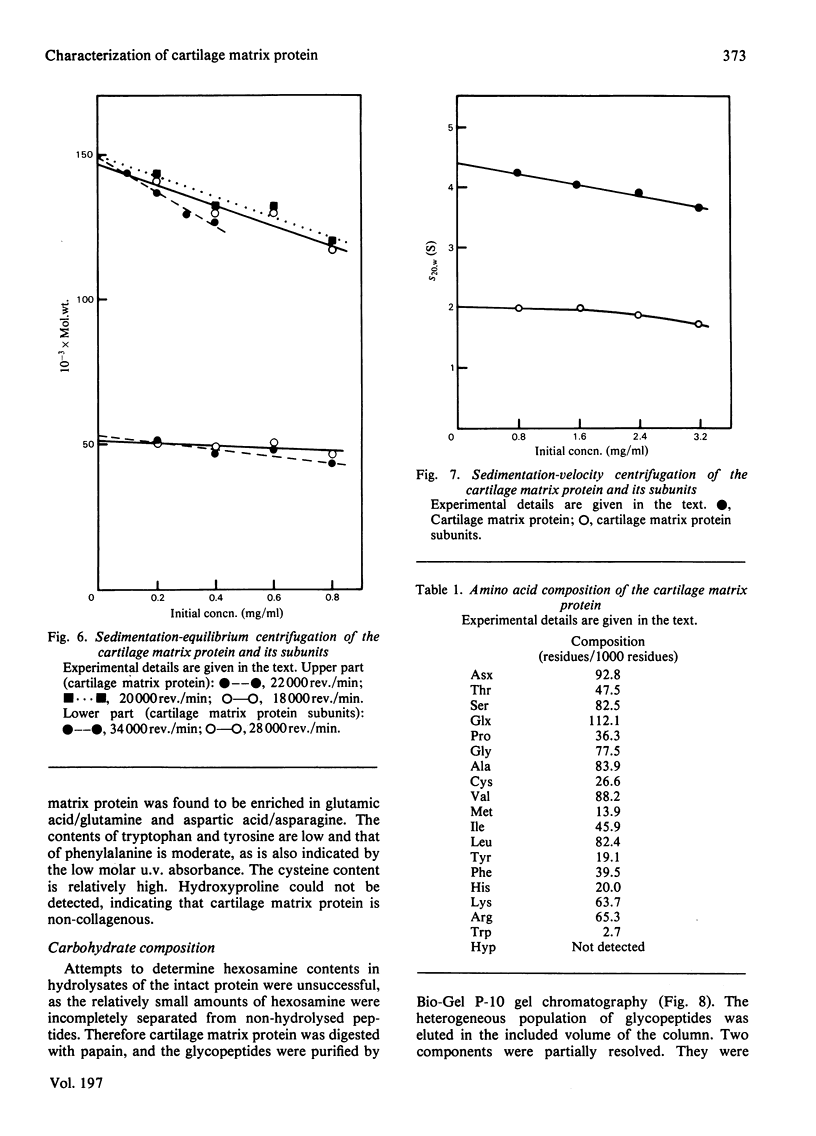
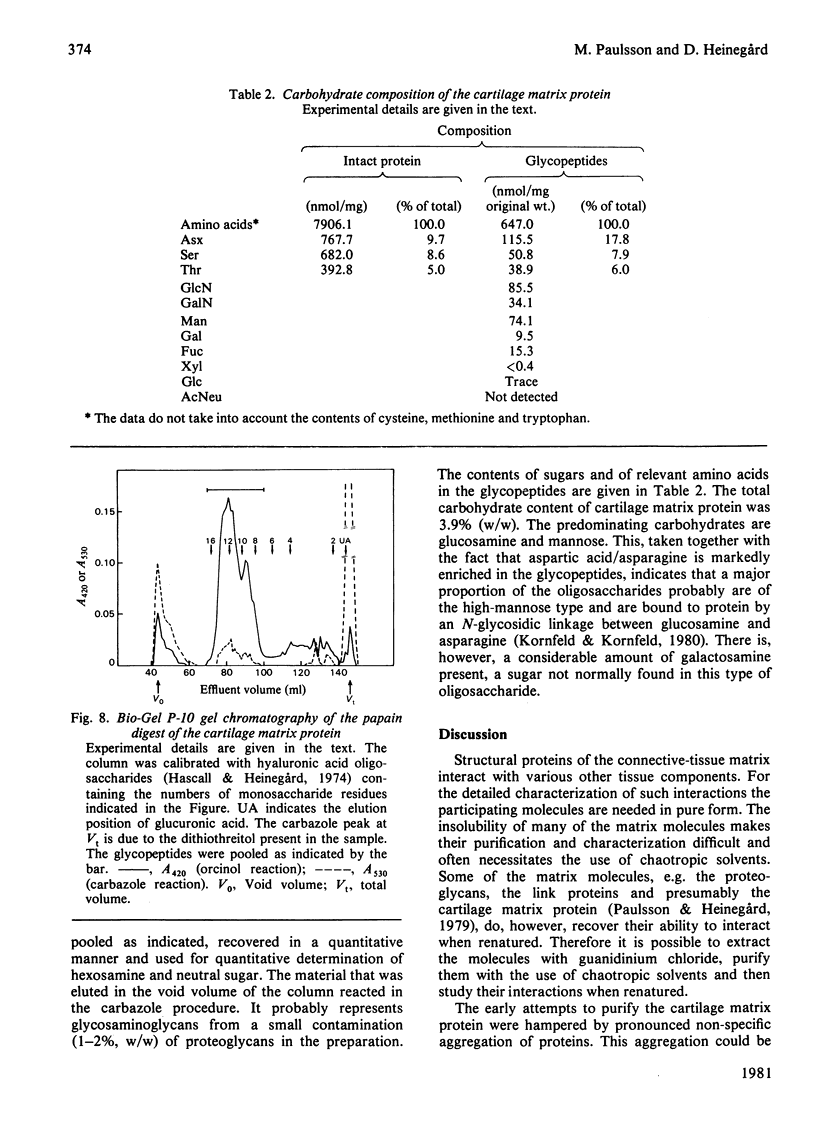
The cartilage matrix protein is a major non-collagenous protein in bovine cartilage. It was purified from a 5 M-guanidinium chloride extract of bovine tracheal cartilage by sequential CsCl-density-gradient centrifugation, gel chromatography in guanidinium chloride and differential precipitation. The molecular weight of the intact protein is 148 000, determined by sedimentation-equilibrium centrifugation. It was dissociated to three subunits of molecular weight 52 000 by reduction of disulphide bonds. The cartilage matrix protein was insoluble in low-salt solutions and behaved abnormally on sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. The content of cysteine was high, whereas the contents of aromatic amino acids were low. The carbohydrate content was 3.9% (w/w). Glycopeptides obtained after papain digestion were heterogenous on gel chromatography. Asparagine/aspartic acid was enriched in the purified glycopeptides, indicating the presence of N-glycosidic linkages to protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Baker J. R., Caterson B. The isolation and characterization of the link proteins from proteoglycan aggregates of bovine nasal cartilage. J Biol Chem. 1979 Apr 10;254(7):2387–2393. [PubMed] [Google Scholar]
- Caputo C. B., MacCallum D. K., Kimura J. H., Schrode J., Hascall V. C. Characterization of fragments produced by clostripain digestion of proteoglycans from the Swarm rat chondrosarcoma. Arch Biochem Biophys. 1980 Oct 1;204(1):220–233. doi: 10.1016/0003-9861(80)90027-2. [DOI] [PubMed] [Google Scholar]
- Caterson B., Baker J. The interaction of link proteins with proteoglycan monomers in the absence of hyaluronic acid. Biochem Biophys Res Commun. 1978 Feb 14;80(3):496–503. doi: 10.1016/0006-291x(78)91596-6. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974 Jul 10;249(13):4242–4249. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- Keiser H., Shulman H. J., Sandson J. I. Immunochemistry of cartilage proteoglycan. Immunodiffusion and gel-electrophoretic studies. Biochem J. 1972 Jan;126(1):163–169. doi: 10.1042/bj1260163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. The calculation of partial specific volumes of proteins in guanidine hydrochloride. Arch Biochem Biophys. 1974 Nov;165(1):268–273. doi: 10.1016/0003-9861(74)90164-7. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., De Luca S., Nilsson B., Hascall V. C., Caputo C. B., Kimura J. H., Heinegard D. Oligosaccharides on proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1980 Jul 10;255(13):6084–6091. [PubMed] [Google Scholar]
- McCormick P. J., Chandrasekhar S., Millis A. J. Direct visualization of collagens and procollagens in polyacrylamide gels. Anal Biochem. 1979 Sep 1;97(2):359–366. doi: 10.1016/0003-2697(79)90086-1. [DOI] [PubMed] [Google Scholar]
- Paulsson M., Heinegård D. Matrix proteins bound to associatively prepared proteoglycans from bovine cartilage. Biochem J. 1979 Dec 1;183(3):539–545. doi: 10.1042/bj1830539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périn J. P., Bonnet F., Pizon V., Jollès J., Jollès P. Structural data concerning the link proteins from bovine nasal cartilage proteolycan complex. FEBS Lett. 1980 Oct 6;119(2):333–336. doi: 10.1016/0014-5793(80)80283-3. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1002–1007. doi: 10.1073/pnas.66.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. O., Edelstein S. J. Molecular weights and volumes from density perturbation ultracentrifugation. Application to aldolase and deoxyribonucleic acid polymerase in solutions of guanidine hydrochloride. Biochemistry. 1971 Feb 2;10(3):477–482. doi: 10.1021/bi00779a020. [DOI] [PubMed] [Google Scholar]
- Walborg E. F., Jr, Kondo L. E. Automated system for ion-exchange chromatography of saccharides. Anal Biochem. 1970 Oct;37(2):320–329. doi: 10.1016/0003-2697(70)90054-0. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]