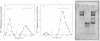Abstract
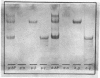
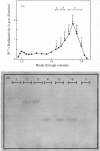
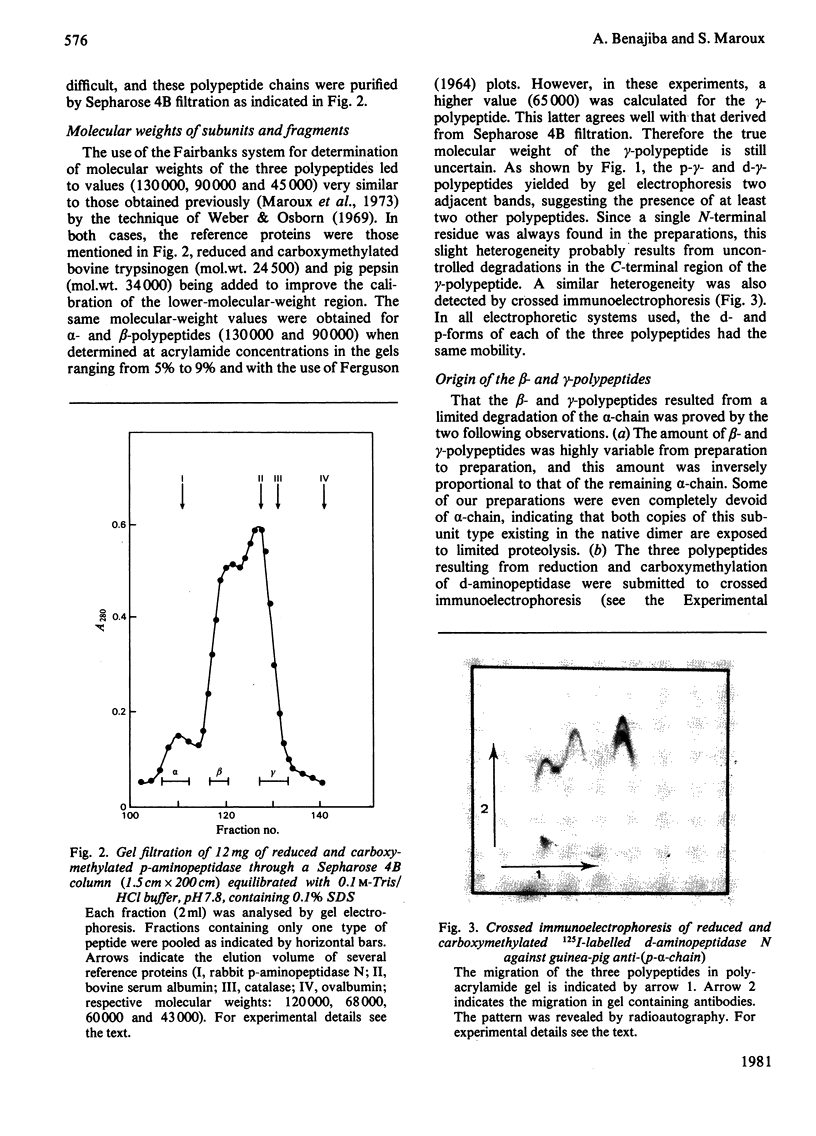
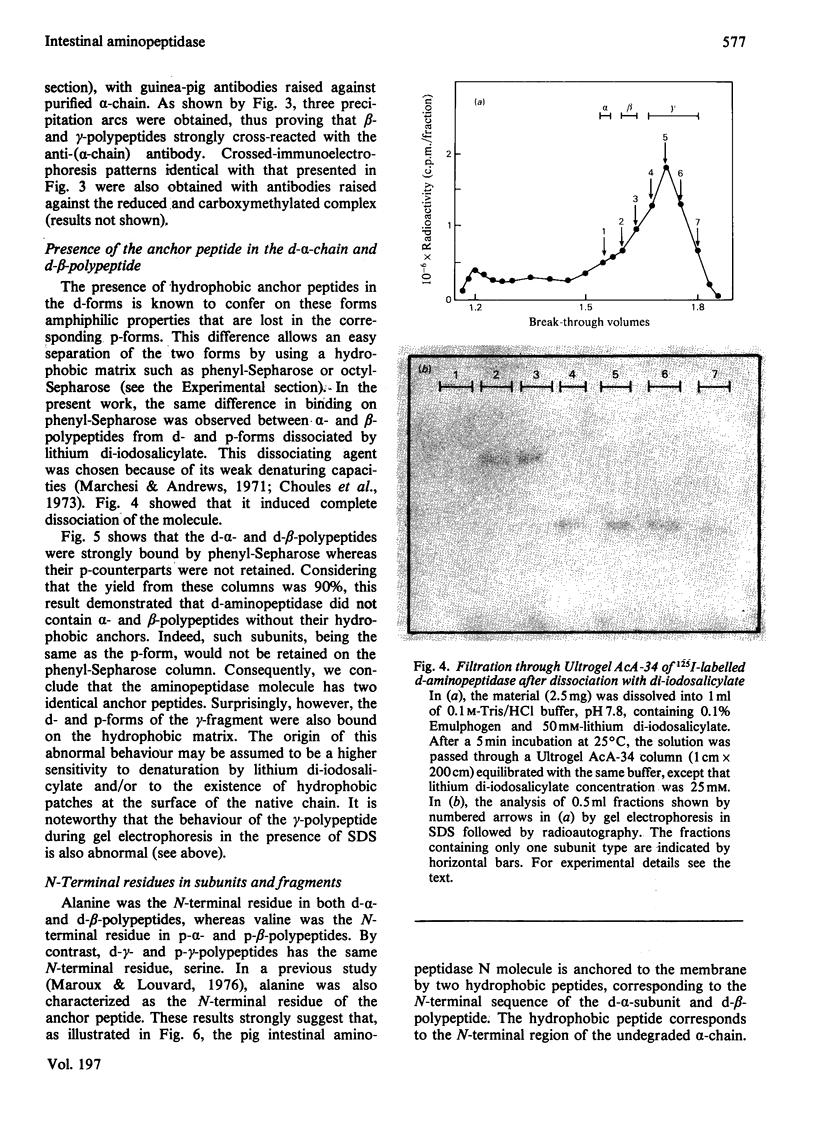
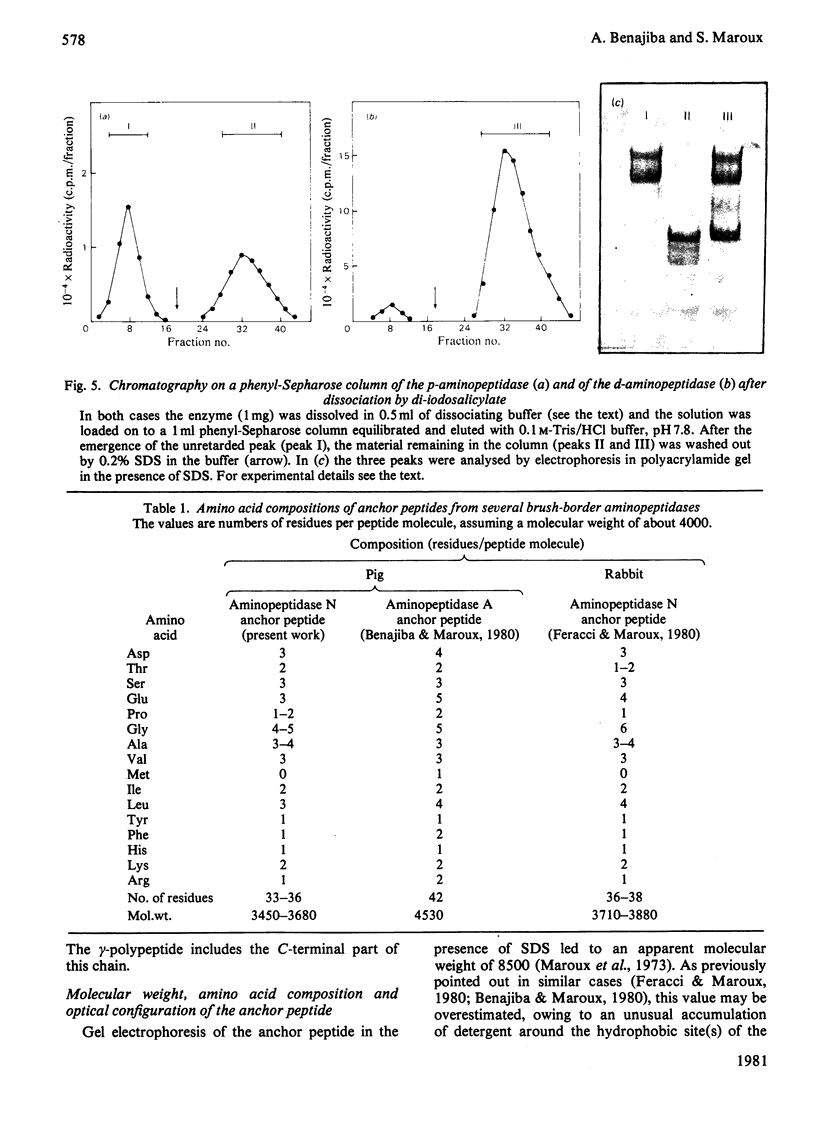
Aminopeptidase N (EC 3.4.11.2), when isolated from pig intestine in either the proteinase- or detergent-released form, frequently appears to contain three polypeptide chains, here termed alpha, beta and gamma. We have established by an immunological technique that the beta- and gamma-polypeptides are derived from the alpha-chain and that the intact enzyme is a dimer, alpha 2. Each alpha-chain of the detergent form was shown to contain a hydrophobic anchor peptide about 35 amino acid residues in length, which included the N-terminal sequences. A peptide bond in the alpha-chain was very sensitive to proteolysis. Its cleavage generated the commonly observed forms: alpha beta gamma and beta 2 gamma 2. The gamma-fragment, which lacked the anchor peptide, was derived from the C-terminal part of the alpha-chain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benajiba A., Maroux S. Purification and characterization of an aminopeptidase A from hog intestinal brush-border membrane. Eur J Biochem. 1980 Jun;107(2):381–388. doi: 10.1111/j.1432-1033.1980.tb06040.x. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Choules G. L., Sandberg R. G., Steggall M., Eyring E. M. Analysis of noncovalent bonding in Mycoplasma membranes. Kinetics of solubilization in sodium dodecyl sulfate and lithium diodosalicylate solutions. Biochemistry. 1973 Oct 23;12(22):4544–4550. doi: 10.1021/bi00746a038. [DOI] [PubMed] [Google Scholar]
- Colbeau A., Maroux S. Integration of alkaline phosphatase in the intestinal brush border membrane. Biochim Biophys Acta. 1978 Jul 20;511(1):39–51. doi: 10.1016/0005-2736(78)90063-9. [DOI] [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Feracci H., Maroux S. Rabbit intestinal aminopeptidase N. Purification and molecular properties. Biochim Biophys Acta. 1980 Jul;599(2):448–463. doi: 10.1016/0005-2736(80)90190-x. [DOI] [PubMed] [Google Scholar]
- Frank G., Brunner J., Hauser H., Wacker H., Semenza G., Zuber H. The hydrophobic anchor of small-intestinal sucrase--isomaltase: N-terminal sequence of isomaltase subunit. FEBS Lett. 1978 Dec 1;96(1):183–188. doi: 10.1016/0014-5793(78)81090-4. [DOI] [PubMed] [Google Scholar]
- Gorvel J. P., Benajiba A., Maroux S. Purification and characterization of the rabbit intestinal brush-border aminopeptidase A. Biochim Biophys Acta. 1980 Sep 9;615(1):271–274. doi: 10.1016/0005-2744(80)90030-3. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Biogenesis of intestinal plasma membrane: posttranslational route and cleavage of sucrase-isomaltase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5183–5186. doi: 10.1073/pnas.76.10.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lotz B., Colonna-Cesari F., Heitz F., Spach G. A family of double helices of alternating poly(gamma-benzyl-D-L-glutamate), a stereochemical model for gramicidin A. J Mol Biol. 1976 Oct 5;106(4):915–942. doi: 10.1016/0022-2836(76)90343-0. [DOI] [PubMed] [Google Scholar]
- Louvard D., Maroux S., Vannier C., Desnuelle P. Topological studies on the hydrolases bound to the intestinal brush border membrane. I. Solubilization by papain and Triton X-100. Biochim Biophys Acta. 1975 Jan 28;375(2):235–248. [PubMed] [Google Scholar]
- Macnair D. C., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic form of dipeptidyl peptidase IV. Biochem J. 1979 May 1;179(2):379–395. doi: 10.1042/bj1790379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Maroux S., Louvard D., Baratti J. The aminopeptidase from hog intestinal brush border. Biochim Biophys Acta. 1973 Sep 15;321(1):282–295. doi: 10.1016/0005-2744(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Maroux S., Louvard D. On the hydrophobic part of aminopeptidase and maltases which bind the enzyme to the intestinal brush border membrane. Biochim Biophys Acta. 1976 Jan 21;419(2):189–195. doi: 10.1016/0005-2736(76)90345-x. [DOI] [PubMed] [Google Scholar]
- Pagès J. M., Louvard D., Lazdunski C. Preparation of active iodinated specific antibodies. FEBS Lett. 1975 Nov 1;59(1):32–35. doi: 10.1016/0014-5793(75)80334-6. [DOI] [PubMed] [Google Scholar]
- Sjöström H., Norén O., Jeppesen L., Staun M., Svensson B., Christiansen L. Purification of different amphiphilic forms of a microvillus aminopeptidase from pig small intestine using immunoadsorbent chromatography. Eur J Biochem. 1978 Aug 1;88(2):503–511. doi: 10.1111/j.1432-1033.1978.tb12476.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]