Abstract
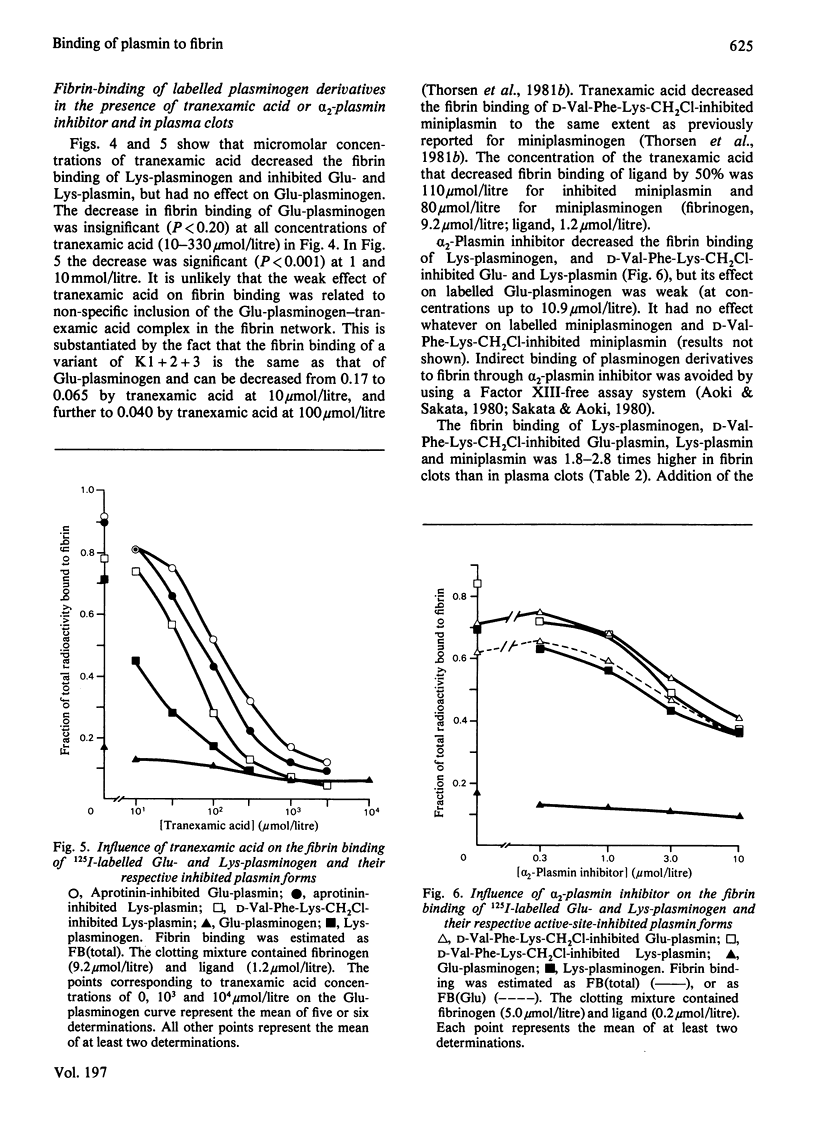
Active-site-inhibited plasmin was prepared by inhibition with d-valyl-l-phenylalanyl-l-lysylchloromethane or by bovine pancreatic trypsin inhibitor (Kunitz inhibitor). Active-site-inhibited Glu-plasmin binds far more strongly to fibrin than Glu-plasminogen [native human plasminogen with N-terminal glutamic acid (residues 1–790)]. This binding is decreased by α2-plasmin inhibitor and tranexamic acid, and is, in the latter case, related to saturation of a strong lysine-binding site. In contrast, α2-plasmin inhibitor and tranexamic acid have only weak effects on the binding of Glu-plasminogen to fibrin. This demonstrates that its strong lysine-binding site is of minor importance to its binding to fibrin. Active-site-inhibited Lys-plasmin and Lys-plasminogen (Glu-plasminogen lacking the N-terminal residues Glu1–Lys76, Glu1–Arg67 or Glu1–Lys77)display binding to fibrin similar to that of active-site inhibited Glu-plasmin. In addition, α2-plasmin inhibitor or tranexamic acid similarly decrease their binding to fibrin. Glu-plasminogen and active-site-inhibited Glu-plasmin have the same gross conformation, and conversion into their respective Lys- forms produces a similar marked change in conformation [Violand, Sodetz & Castellino (1975) Arch. Biochem. Biophys. 170, 300–305]. Our results indicate that this change is not essential to the degree of binding to fibrin or to the effect of α2-plasmin inhibitor and tranexamic acid on this binding. The conversion of miniplasminogen (Glu-plasminogen lacking the N-terminal residues Glu1–Val441) into active-site-inhibited miniplasmin makes no difference to the degree of binding to fibrin, which is similarly decreased by the addition of tranexamic acid and unaffected by α2-plasmin inhibitor. Active-site-inhibited Glu-plasmin, Lys-plasmin and miniplasmin have lower fibrin-binding values in a plasma system than in a purified system. Results with miniplasmin(ogen) indicate that plasma proteins other than α2-plasmin inhibitor and histidine-rich glycoprotein decrease the binding of plasmin(ogen) to fibrin.
Full text
PDF


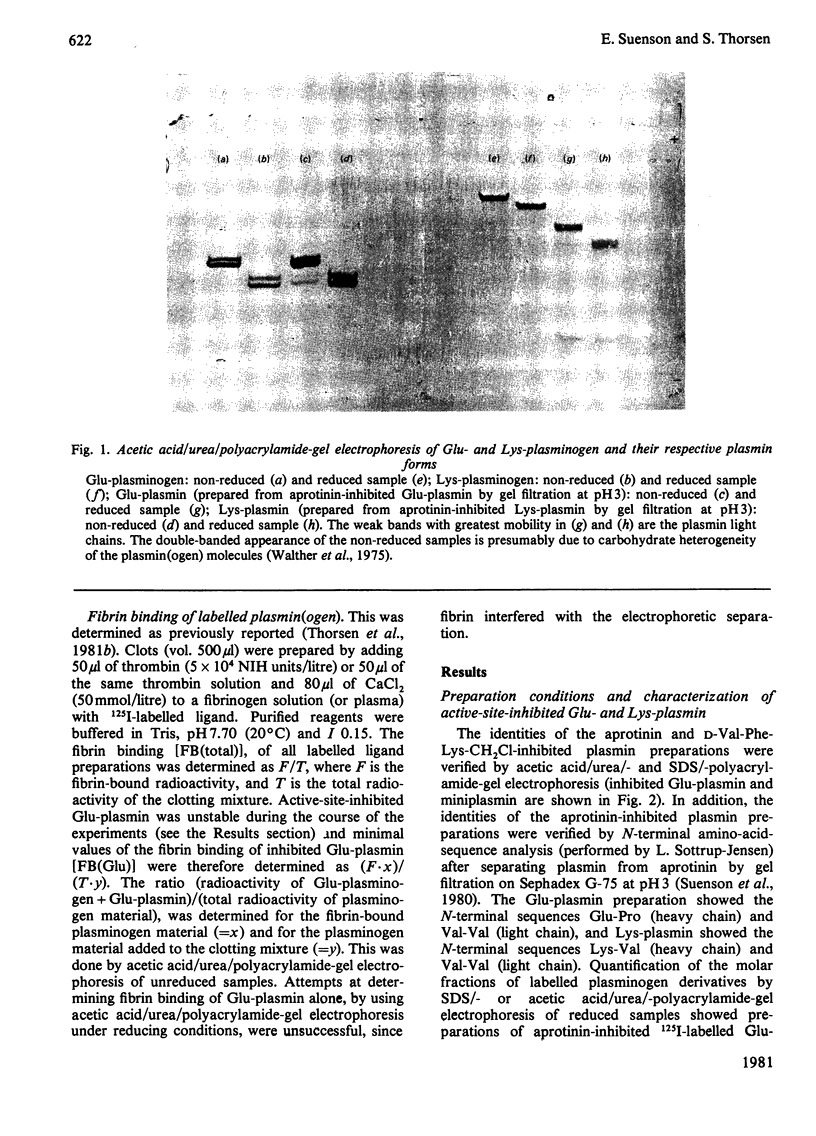
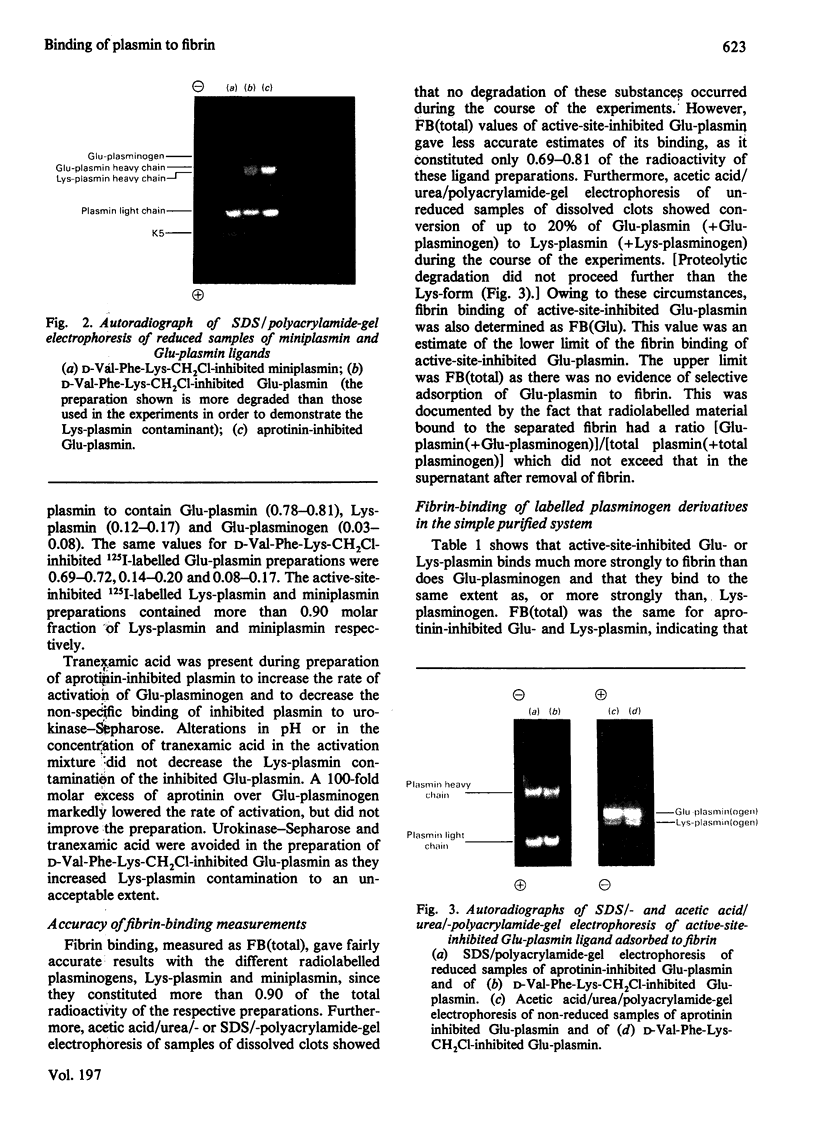
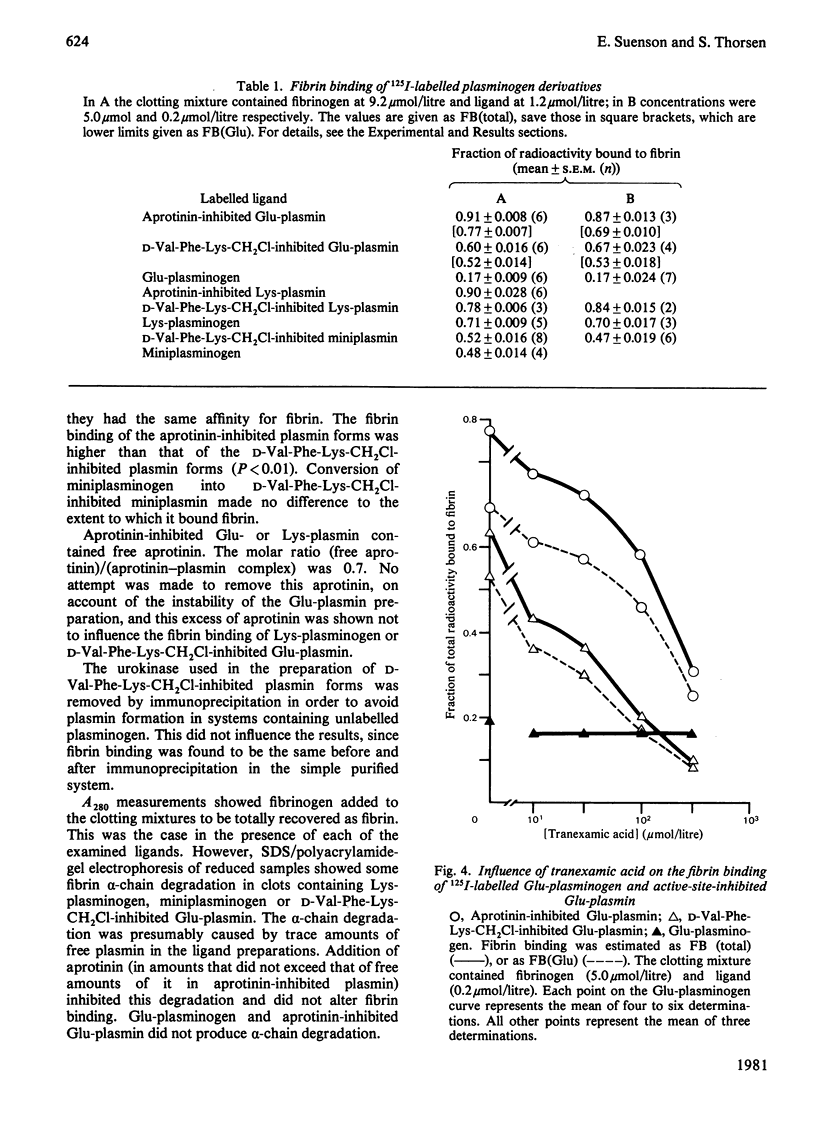



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkjaersig N. The purification and properties of human plasminogen. Biochem J. 1964 Oct;93(1):171–182. doi: 10.1042/bj0930171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki N., Moroi M., Tachiya K. Effects of alpha2-plasmin inhibitor on fibrin clot lysis. Its comparison with alpha2-macroglobulin. Thromb Haemost. 1978 Feb 28;39(1):22–31. [PubMed] [Google Scholar]
- Aoki N., Sakata Y. Influence of alpha 2-plasmin inhibitor on adsorption of plasminogen to fibrin. Thromb Res. 1980 Jul 1;19(1-2):149–155. doi: 10.1016/0049-3848(80)90414-4. [DOI] [PubMed] [Google Scholar]
- Cederholm-Williams S. A., Swain A. The effect of fibrinogen degradation products and some lysine analogues on the dissociation of plasmin(ogen)-fibrin complexes. Thromb Res. 1979;16(5-6):705–713. doi: 10.1016/0049-3848(79)90214-7. [DOI] [PubMed] [Google Scholar]
- Christensen U., Sottrup-Jensen L., Magnusson S., Petersen T. E., Clemmensen I. Enzymic properties of the neo-plasmin-Val-422 (miniplasmin). Biochim Biophys Acta. 1979 Apr 12;567(2):472–481. doi: 10.1016/0005-2744(79)90133-5. [DOI] [PubMed] [Google Scholar]
- Clemmensen I., Christensen F. Inhibition of urokinase by complex formation with human alpha1-antitrypsin. Biochim Biophys Acta. 1976 Apr 8;429(2):591–599. doi: 10.1016/0005-2744(76)90307-7. [DOI] [PubMed] [Google Scholar]
- Collen D., Lijnen H. R., De Cock F., Durieux J. P., Loffet A. Kinetic properties of tripeptide lysyl chloromethyl ketone and lysyl p-nitroanilide derivatives towards trypsin-like serine proteinases. Biochim Biophys Acta. 1980 Sep 9;615(1):158–166. doi: 10.1016/0005-2744(80)90019-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gronow M., Violand B. N., Castellino F. J. Purification and some properties of the Glu- and Lys-human plasmin heavy chains. J Biol Chem. 1977 Apr 10;252(7):2175–2177. [PubMed] [Google Scholar]
- Lerch P. G., Rickli E. E., Lergier W., Gillessen D. Localization of individual lysine-binding regions in human plasminogen and investigations on their complex-forming properties. Eur J Biochem. 1980;107(1):7–13. doi: 10.1111/j.1432-1033.1980.tb04617.x. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Hoylaerts M., Collen D. Isolation and characterization of a human plasma protein with affinity for the lysine binding sites in plasminogen. Role in the regulation of fibrinolysis and identification as histidine-rich glycoprotein. J Biol Chem. 1980 Nov 10;255(21):10214–10222. [PubMed] [Google Scholar]
- Markus G., DePasquale J. L., Wissler F. C. Quantitative determination of the binding of epsilon-aminocaproic acid to native plasminogen. J Biol Chem. 1978 Feb 10;253(3):727–732. [PubMed] [Google Scholar]
- Markus G., Priore R. L., Wissler F. C. The binding of tranexamic acid to native (Glu) and modified (Lys) human plasminogen and its effect on conformation. J Biol Chem. 1979 Feb 25;254(4):1211–1216. [PubMed] [Google Scholar]
- Mcdonagh J., Waggoner W. G., Hamilton E. G., Hindenbach B., Mcdonagh R. P. Affinity chromatography of human plasma and platelet factor XIII on organomercurial agarose. Biochim Biophys Acta. 1976 Oct 28;446(2):345–357. doi: 10.1016/0005-2795(76)90002-7. [DOI] [PubMed] [Google Scholar]
- Norén I., Ramström G., Wallén P. Fibrin plate method with reagents purified by affinity chromatography and its use for determination of fibrinolytic and other proteolytic activity in saliva, bile and plasma. Haemostasis. 1975;4(2):110–124. doi: 10.1159/000214094. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Summaria L., Hsieh B., Shah R. J. The peptide chains of human plasmin. Mechanism of activation of human plasminogen to plasmin. J Biol Chem. 1967 May 25;242(10):2333–2342. [PubMed] [Google Scholar]
- Rákóczi I., Wiman B., Collen D. On the biological significance of the specific interaction between fibrin, plasminogen and antiplasmin. Biochim Biophys Acta. 1978 May 3;540(2):295–300. doi: 10.1016/0304-4165(78)90142-3. [DOI] [PubMed] [Google Scholar]
- Sakata Y., Aoki N. Cross-linking of alpha 2-plasmin inhibitor to fibrin by fibrin-stabilizing factor. J Clin Invest. 1980 Feb;65(2):290–297. doi: 10.1172/JCI109671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodetz J. M., Castellino F. J. The mechanism of activation of rabbit plasminogen by urokinase. J Biol Chem. 1975 Apr 25;250(8):3041–3049. [PubMed] [Google Scholar]
- Thorsen S., Clemmensen I., Sottrup-Jensen L., Magnusson S. Adsorption to fibrin of native fragments of known primary structure from human plasminogen. Biochim Biophys Acta. 1981 May 29;668(3):377–387. doi: 10.1016/0005-2795(81)90171-9. [DOI] [PubMed] [Google Scholar]
- Thorsen S. Differences in the binding to fibrin of native plasminogen and plasminogen modified by proteolytic degradation. Influence of omega-aminocarboxylic acids. Biochim Biophys Acta. 1975 May 30;393(1):55–65. doi: 10.1016/0005-2795(75)90216-0. [DOI] [PubMed] [Google Scholar]
- Thorsen S. Human urokinase and porcine tissue plasminogen activator. A comparative study of the mechanism of fibrinolysis, and the effect of natural proteinase inhibitors and omega-aminocarboxylic acids. Dan Med Bull. 1977 Oct;24(5):189–206. [PubMed] [Google Scholar]
- Thorsen S., Müllertz S. Rate of activation and electrophoretic mobility of unmodified and partially degraded plasminogen. Effects of 6-aminohexanoic acid and related compounds. Scand J Clin Lab Invest. 1974 Oct;34(2):167–176. [PubMed] [Google Scholar]
- Violand B. N., Castellino F. J. Mechanism of the urokinase-catalyzed activation of human plasminogen. J Biol Chem. 1976 Jul 10;251(13):3906–3912. [PubMed] [Google Scholar]
- Violand B. N., Sodetz J. M., Castellino F. J. The effect of epsilon-amino caproic acid on the gross conformation of plasminogen and plasmin. Arch Biochem Biophys. 1975 Sep;170(1):300–305. doi: 10.1016/0003-9861(75)90121-6. [DOI] [PubMed] [Google Scholar]
- Walther P. J., Hill R. L., McKee P. A. The importance of the preactivation peptide in the two-stage mechanism of human plasminogen activation. J Biol Chem. 1975 Aug 10;250(15):5926–5933. [PubMed] [Google Scholar]
- Wiman B., Boman L., Collen D. On the kinetics of the reaction between human antiplasmin and a low-molecular-weight form of plasmin. Eur J Biochem. 1978 Jun 1;87(1):143–146. doi: 10.1111/j.1432-1033.1978.tb12360.x. [DOI] [PubMed] [Google Scholar]
- Wiman B., Collen D. On the kinetics of the reaction between human antiplasmin and plasmin. Eur J Biochem. 1978 Mar 15;84(2):573–578. doi: 10.1111/j.1432-1033.1978.tb12200.x. [DOI] [PubMed] [Google Scholar]
- Wiman B., Lijnen H. R., Collen D. On the specific interaction between the lysine-binding sites in plasmin and complementary sites in alpha2-antiplasmin and in fibrinogen. Biochim Biophys Acta. 1979 Jul 25;579(1):142–154. doi: 10.1016/0005-2795(79)90094-1. [DOI] [PubMed] [Google Scholar]
- Wiman B. Primary structure of the B-chain of human plasmin. Eur J Biochem. 1977 Jun 1;76(1):129–137. doi: 10.1111/j.1432-1033.1977.tb11578.x. [DOI] [PubMed] [Google Scholar]
- Wiman B., Wallén P. On the primary structure of human plasminogen and plasmin. Purification and characterization of cyanogen-bromide fragments. Eur J Biochem. 1975 Sep 15;57(2):387–394. doi: 10.1111/j.1432-1033.1975.tb02312.x. [DOI] [PubMed] [Google Scholar]
- Wiman B., Wallén P. The specific interaction between plasminogen and fibrin. A physiological role of the lysine binding site in plasminogen. Thromb Res. 1977 Feb;10(2):213–222. doi: 10.1016/0049-3848(77)90003-2. [DOI] [PubMed] [Google Scholar]