Abstract
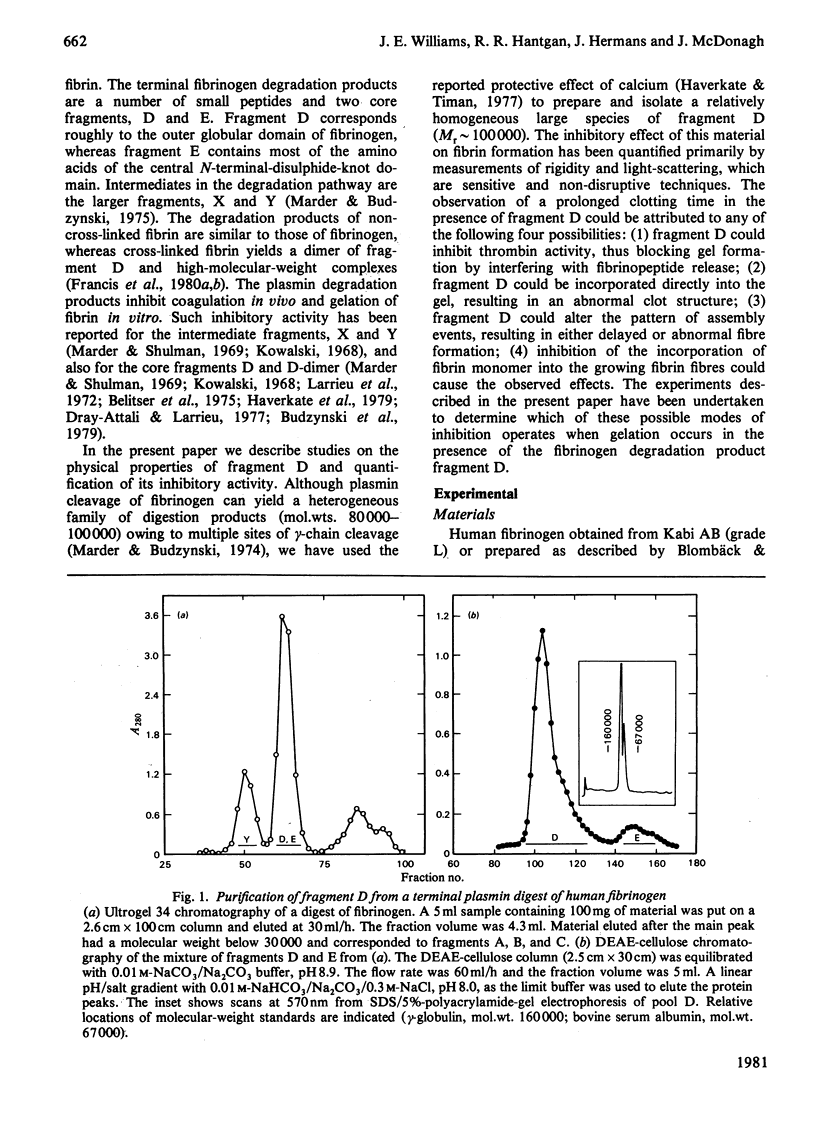
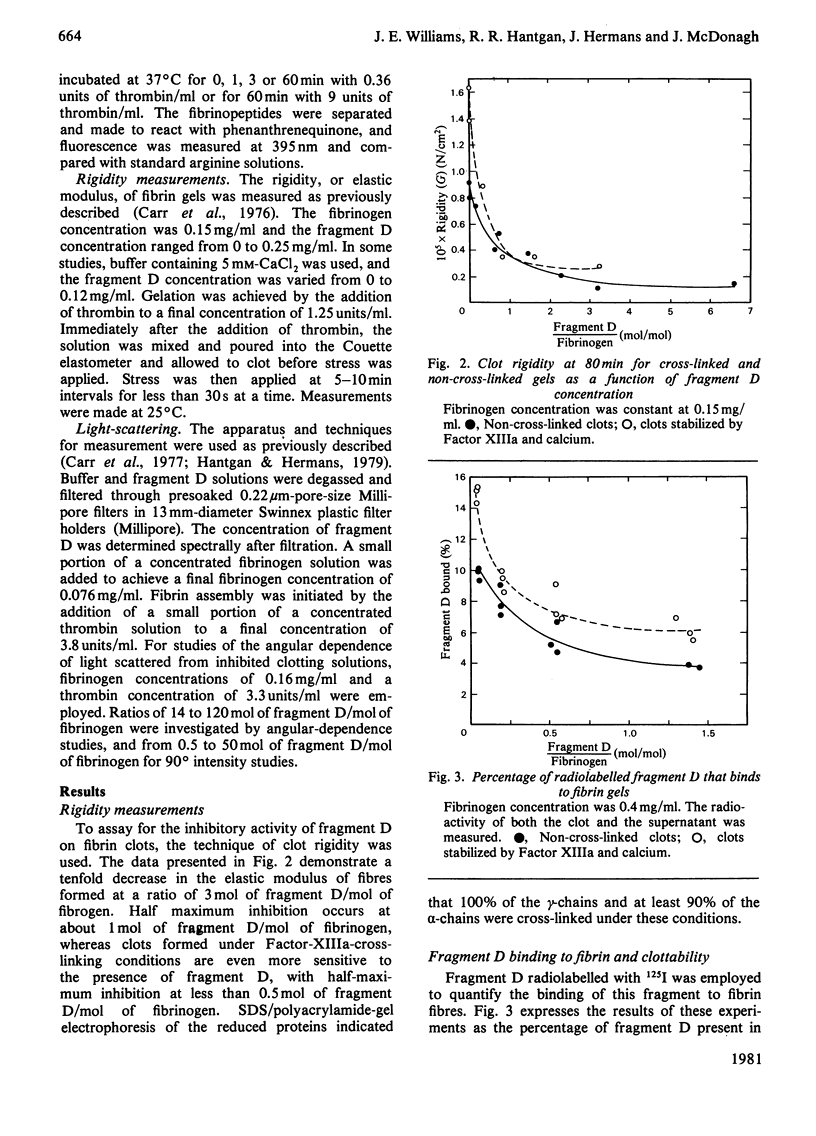
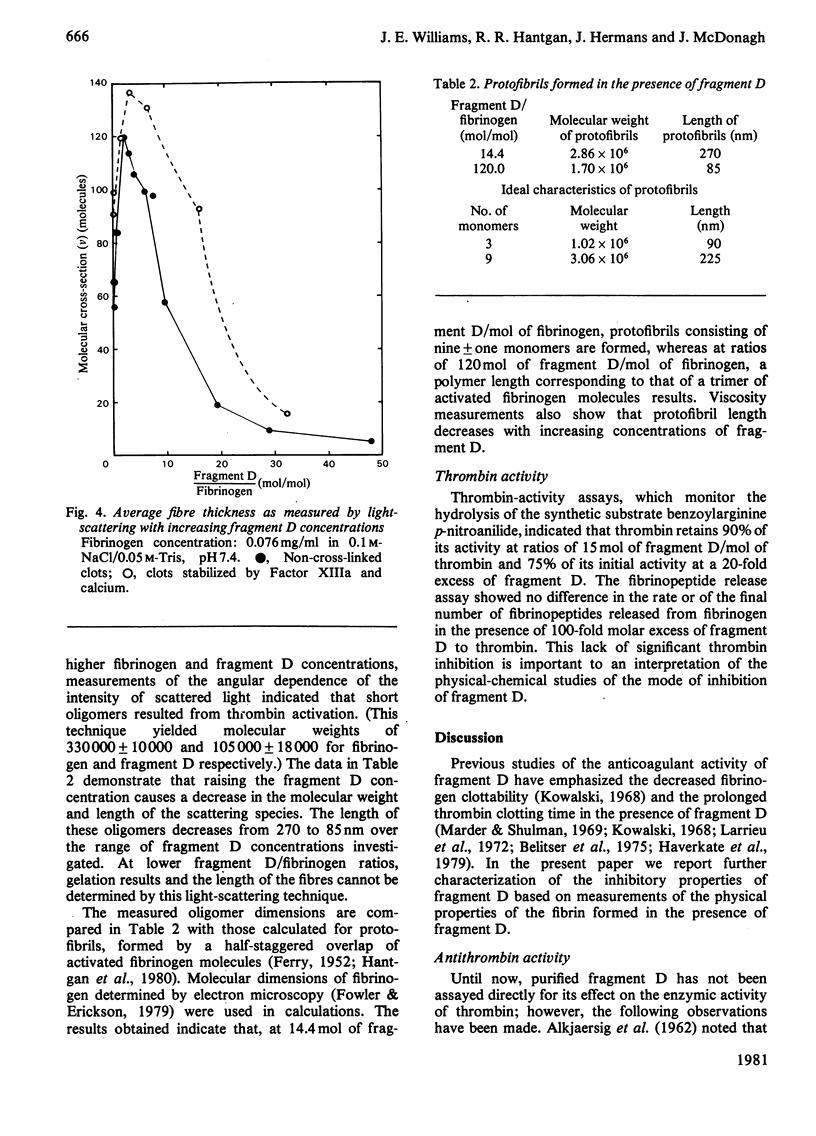
Fragment D (Mr 100 000) prepared from a terminal plasmin digest of fibrinogen was isolated and used to study its effect on fibrin formation. Increasing amounts of fragment D added to a solution of fibrinogen and thrombin decrease the rigidity of the resultant gel (10% of control at 2 mol of fragment D/mol of fibrinogen). Half-maximal inhibition is achieved at 1 mol of fragment D/mol of fibrinogen for non-cross-linked clots and at 1/2 mol of fragment D/mol of fibrinogen for cross-linked clots. "Clottability' decreases concomitantly with the rigidity. Only small amounts of fragment D (less than 10% for non-cross-linked gels) are incorporated into the gel. Light-scattering shows an increase in the final fibre thickness at fragment D concentrations up to 2 mol of fragment D/mol of fibrinogen, from 60 molecules/cross-section for the control to 120 molecules/cross-section. Higher fragment D concentrations lead to a decrease in the final fibre thickness. The limit fibre thickness is 8 nm, with a length of 80 nm, which is equivalent to a fibrin trimer. On the basis of results of synthetic-substrate and fibrinopeptide-release assays, it is clear that thrombin inactivation is not responsible for this effect. These data suggest that fragment D may inhibit fibrin formation by blocking the bimolecular polymerization of activated fibrin monomer molecules to form protofibrils, although additional effects on subsequent assembly steps may also be involved.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALKJAERSIG N., FLETCHER A. P., SHERRY S. Pathogenesis of the coagulation defect developing during pathological plasma proteolytic ("fibrinolytic") states. II. The significance, mechanism and consequences of defective fibrin polymerization. J Clin Invest. 1962 Apr;41:917–934. doi: 10.1172/JCI104547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BETTELHEIM F. R. The clotting of fibrinogen. II. Fractionation of peptide material liberated. Biochim Biophys Acta. 1956 Jan;19(1):121–130. doi: 10.1016/0006-3002(56)90393-6. [DOI] [PubMed] [Google Scholar]
- Belitser V. A., Varetska T. V., Tolstykh V. M., Tsaryuk L. A., Pozdnyakova T. M. Enhanced anticlotting activity of fragments D formed during plasmin hydrolysis of fibrinogen in the presence of calcium chloride. Thromb Res. 1975 Nov;7(5):797–806. doi: 10.1016/0049-3848(75)90204-2. [DOI] [PubMed] [Google Scholar]
- Blombäck B., Hessel B., Hogg D., Therkildsen L. A two-step fibrinogen--fibrin transition in blood coagulation. Nature. 1978 Oct 12;275(5680):501–505. doi: 10.1038/275501a0. [DOI] [PubMed] [Google Scholar]
- Budzynski A. Z., Olexa S. A., Brizuela B. S. The interference of plasmic degradation products of human crosslinked fibrin with clot formation. Biochim Biophys Acta. 1979 May 1;584(2):284–287. doi: 10.1016/0304-4165(79)90273-3. [DOI] [PubMed] [Google Scholar]
- Carr M. E., Jr, Shen L. L., Hermans J. Mass-length ratio of fibrin fibers from gel permeation and light scattering. Biopolymers. 1977 Jan;16(1):1–15. doi: 10.1002/bip.1977.360160102. [DOI] [PubMed] [Google Scholar]
- Carr M. E., Shen L. L., Hermans J. A physical standard of fibrinogen: measurement of the elastic modulus of dilute fibrin gels with a new elastometer. Anal Biochem. 1976 May 7;72:202–211. doi: 10.1016/0003-2697(76)90522-4. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Cassman K. G., Cottrell B. A., Friezner S. J., Takagi T. Amino acid sequence studies on the alpha chain of human fibrinogen. Covalent structure of the alpha-chain portion of fragment D. Biochemistry. 1977 Apr 19;16(8):1710–1715. doi: 10.1021/bi00627a029. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Structure and function of fibrinogen. Horiz Biochem Biophys. 1977;3:164–191. [PubMed] [Google Scholar]
- Dray-Attali L., Larrieu M. J. Fragments d - correlation between structure and biological activity. Thromb Res. 1977 Apr;10(4):575–586. doi: 10.1016/0049-3848(77)90213-4. [DOI] [PubMed] [Google Scholar]
- Ferry J. D. The Mechanism of Polymerization of Fibrinogen. Proc Natl Acad Sci U S A. 1952 Jul;38(7):566–569. doi: 10.1073/pnas.38.7.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler W. E., Erickson H. P. Trinodular structure of fibrinogen. Confirmation by both shadowing and negative stain electron microscopy. J Mol Biol. 1979 Oct 25;134(2):241–249. doi: 10.1016/0022-2836(79)90034-2. [DOI] [PubMed] [Google Scholar]
- Francis C. W., Marder V. J., Barlow G. H. Plasmic degradation of crosslinked fibrin. Characterization of new macromolecular soluble complexes and a model of their structure. J Clin Invest. 1980 Nov;66(5):1033–1043. doi: 10.1172/JCI109931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C. W., Marder V. J., Martin S. E. Plasmic degradation of crosslinked fibrin. I. Structural analysis of the particulate clot and identification of new macromolecular-soluble complexes. Blood. 1980 Sep;56(3):456–464. [PubMed] [Google Scholar]
- HALL C. E., SLAYTER H. S. The fibrinogen molecule: its size, shape, and mode of polymerization. J Biophys Biochem Cytol. 1959 Jan 25;5(1):11–16. doi: 10.1083/jcb.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantgan R. R., Hermans J. Assembly of fibrin. A light scattering study. J Biol Chem. 1979 Nov 25;254(22):11272–11281. [PubMed] [Google Scholar]
- Hantgan R., Fowler W., Erickson H., Hermans J. Fibrin assembly: a comparison of electron microscopic and light scattering results. Thromb Haemost. 1980 Dec 19;44(3):119–124. [PubMed] [Google Scholar]
- Haverkate F., Timan G., Nieuwenhuizen W. Anticlotting properties of fragments D from human fibrinogen and fibrin. Eur J Clin Invest. 1979 Aug;9(4):253–255. doi: 10.1111/j.1365-2362.1979.tb00881.x. [DOI] [PubMed] [Google Scholar]
- Haverkate F., Timan G. Protective effect of calcium in the plasmin degradation of fibrinogen and fibrin fragments D. Thromb Res. 1977 Jun;10(6):803–812. doi: 10.1016/0049-3848(77)90137-2. [DOI] [PubMed] [Google Scholar]
- Heene D. L., Matthias F. R., Wegrzynowicz Z., Hocke G. Separation of fibrinogen degradation products (FDP) by means of adsorption to insolubilized fibrinmonomer (FM-ag). Thromb Haemost. 1979 Jun 30;41(4):677–686. [PubMed] [Google Scholar]
- Hermans J. Models of fibrin. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1189–1193. doi: 10.1073/pnas.76.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryk B. J., Collen D., Woods K. R., Blombäck B. Evidence for localization of polymerization sites in fibrinogen. J Biol Chem. 1974 May 25;249(10):3322–3325. [PubMed] [Google Scholar]
- LATALLO Z. S., BUDZYNSKI A. Z., LIPINSKI B., KOWALSKI E. INHIBITION OF THROMBIN AND OF FIBRIN POLYMERIZATION, TWO ACTIVITIES DERIVED FROM PLASMIN-DIGESTED FIBRINOGEN. Nature. 1964 Sep 12;203:1184–1185. doi: 10.1038/2031184a0. [DOI] [PubMed] [Google Scholar]
- Larrieu M. J., Rigollot C., Marder V. J. Comparative effects of fibrinogen degradation fragments D and E on coagulation. Br J Haematol. 1972 Jun;22(6):719–733. doi: 10.1111/j.1365-2141.1972.tb05717.x. [DOI] [PubMed] [Google Scholar]
- Marder V. J., Budzynski A. Z. Data for defining fibrinogen and its plasmic degradation products. Thromb Diath Haemorrh. 1975 Apr 30;33(2):199–207. [PubMed] [Google Scholar]
- Marder V. J., Budzynski A. Z. The structure of the fibrinogen degradation products. Prog Hemost Thromb. 1974;2(0):141–174. [PubMed] [Google Scholar]
- Marder V. J., Budzyński A. Z., James H. L. High molecular weight derivatives of human fibrinogen produced by plasmin. 3. Their NH2-terminal amino acids and comparison with the "NH2-terminal disulfide knot". J Biol Chem. 1972 Aug 10;247(15):4775–4781. [PubMed] [Google Scholar]
- Marder V. J., Shulman N. R. High molecular weight derivatives of human fibrinogen produced by plasmin. II. Mechanism of their anticoagulant activity. J Biol Chem. 1969 Apr 25;244(8):2120–2124. [PubMed] [Google Scholar]
- McDonagh J., Messel H., McDonagh R. P., Jr, Murano G., Blombäck B. Molecular weight analysis of fibrinogen and fibrin chains by an improved sodium dodecyl sulfate gel electrophoresis method. Biochim Biophys Acta. 1972 Jan 26;257(1):135–142. doi: 10.1016/0005-2795(72)90262-0. [DOI] [PubMed] [Google Scholar]
- Mihalyi E. Physicochemical studies of bovine fibrinogen. IV. Ultraviolet absorption and its relation to the structure of the molecule. Biochemistry. 1968 Jan;7(1):208–223. doi: 10.1021/bi00841a026. [DOI] [PubMed] [Google Scholar]
- Nelb G. W., Gerth C., Ferry J. D. Rheology of fibrin clots. III. Shear creep and creep recovery of fine ligated and coarse unligated closts. Biophys Chem. 1976 Sep;5(3):377–387. doi: 10.1016/0301-4622(76)80050-6. [DOI] [PubMed] [Google Scholar]
- Olexa S. A., Budzynski A. Z. Evidence for four different polymerization sites involved in human fibrin formation. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1374–1378. doi: 10.1073/pnas.77.3.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo S. V., Schwartz M. L., Hill R. L., McKee P. A. The effect of plasmin on the subunit structure of human fibrinogen. J Biol Chem. 1972 Feb 10;247(3):636–645. [PubMed] [Google Scholar]
- Shen L. L., Hermans J., McDonagh J., McDonagh R. P., Carr M. Effects of calcium ion and covalent crosslinking on formation and elasticity of fibrin cells. Thromb Res. 1975 Mar;6(3):255–265. doi: 10.1016/0049-3848(75)90073-0. [DOI] [PubMed] [Google Scholar]
- Shen L. L., McDonagh R. P., McDonagh J., Hermans J. Early events in the plasmin digestion of fibrinogen and fibrin. Effects of plasmin on fibrin polymerization. J Biol Chem. 1977 Sep 10;252(17):6184–6189. [PubMed] [Google Scholar]
- Weisel J. W., Phillips G. N., Jr, Cohen C. A model from electron microscopy for the molecular structure of fibrinogen and fibrin. Nature. 1981 Jan 22;289(5795):263–267. doi: 10.1038/289263a0. [DOI] [PubMed] [Google Scholar]
- Yamada S., Itano H. Phenanthrenequinone as an analytical reagent for arginine and other monosubstituted guanidines. Biochim Biophys Acta. 1966 Dec 28;130(2):538–540. doi: 10.1016/0304-4165(66)90256-x. [DOI] [PubMed] [Google Scholar]
- York L. L., Blombäck B. Interaction of fragments of fibrinogen with insolubilized fibrin monomers (activated fibrinogen). Thromb Res. 1976 May;8(5):607–618. doi: 10.1016/0049-3848(76)90242-5. [DOI] [PubMed] [Google Scholar]


