Abstract
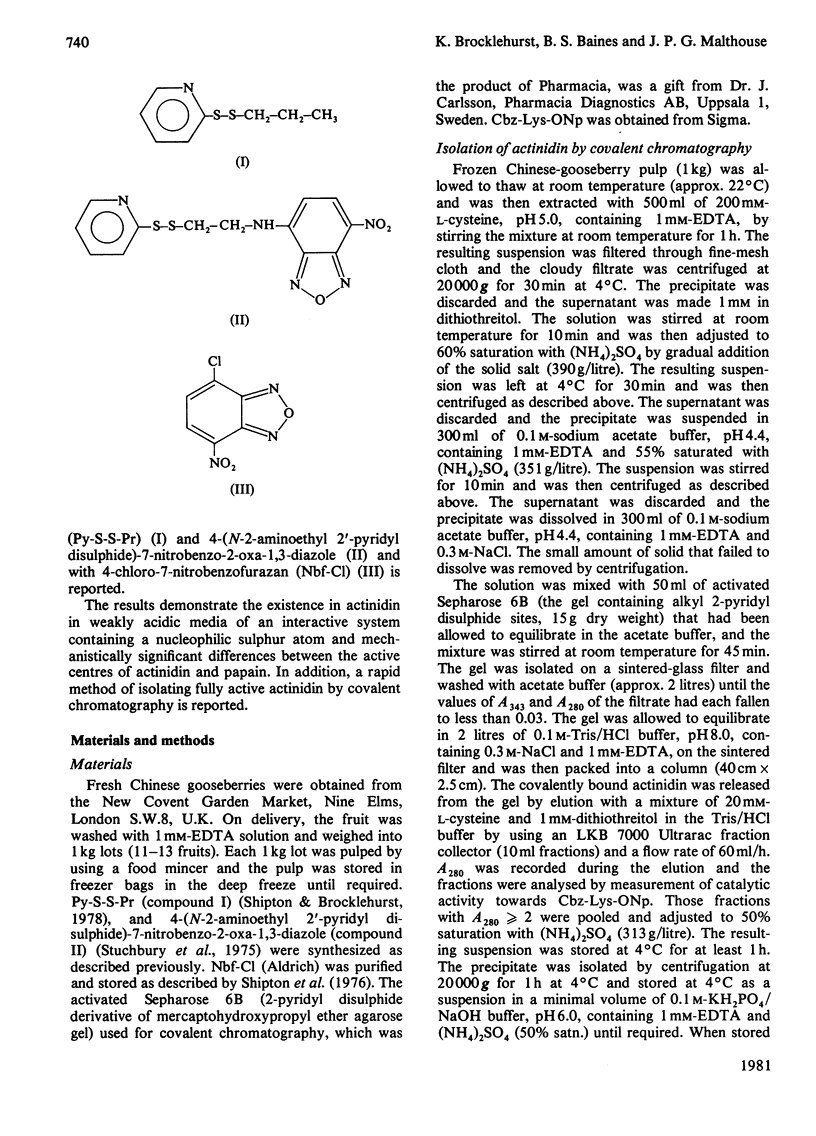
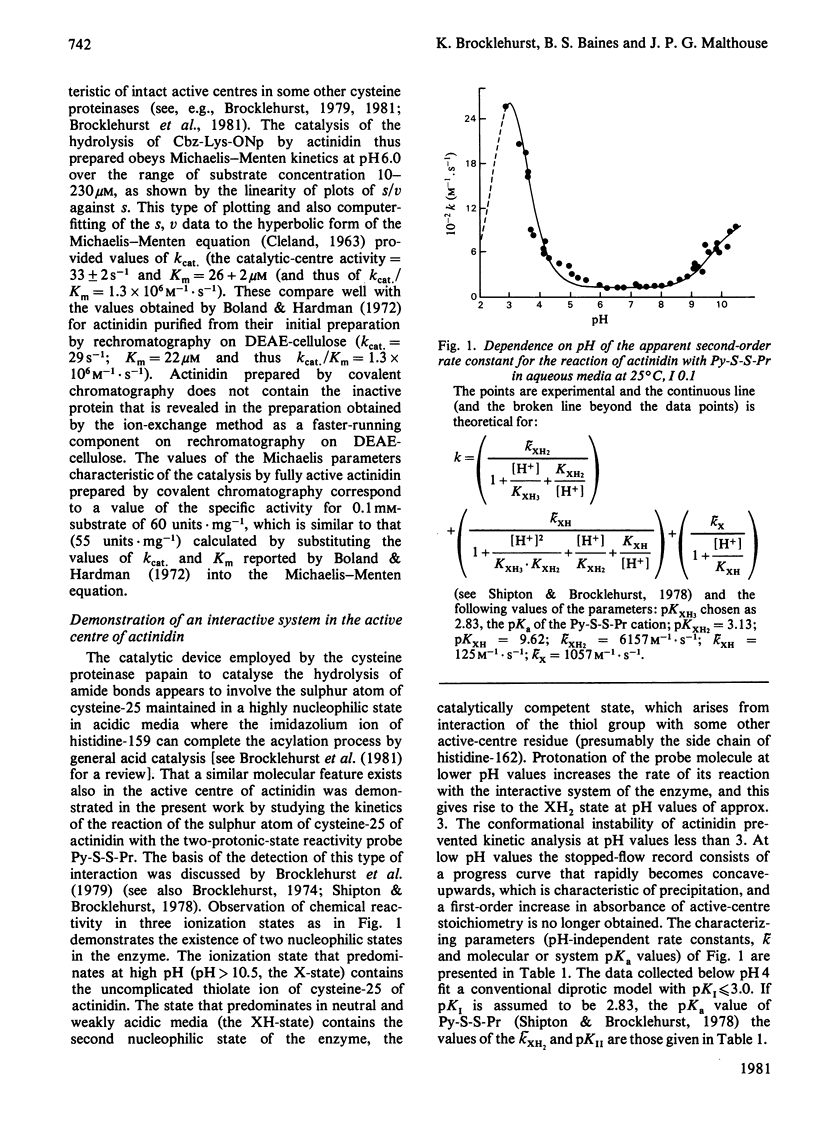
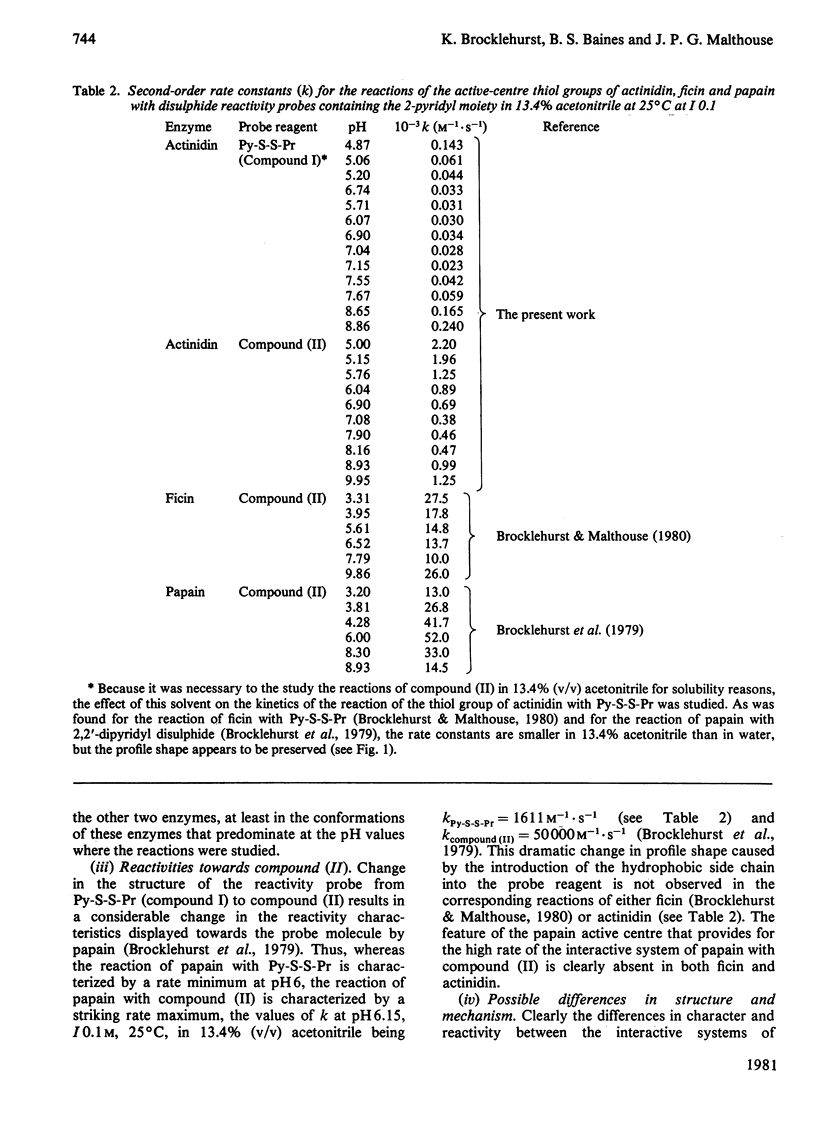
1. A rapid method of isolation of fully active actinidin, the cysteine proteinase from Actinidia chinensis (Chinese gooseberry or kiwifruit), by covalent chromatography, was devised. 2. The active centre of actinidin was investigated by using n-propyl 2-pyridyl disulphide, 4-(N-aminoethyl 2'-pyridyl disulphide)-7-nitrobenzo-2-oxa-1,3-diazole and 4-chloro-7-nitrobenzofurazan as reactivity probes. 3. The presence in actinidin in weakly acidic media of an interactive system containing a nucleophilic sulphur atom was demonstrated. 4. The pKa values (3.1 and 9.6) that characterize this interactive system are more widely separated than those that characterize the interactive active centre systems of ficin (EC 3.4.22.3) and papain (EC 3.4.22.2) (3.8 and 8.6, and 3.9 and 8.8 respectively). 5. Actinidin was shown to resemble ficin rather than papain in (i) the disposition of the active-centre imidazole group with respect to hydrophobic binding areas, and (ii) the inability of the active-centre aspartic acid carboxy group to influence the reactivity of the active-centre thiol group at pH values of about 4. 6. The implications of the results for one-state and two-state mechanisms for cysteine-proteinase catalysis are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelides K. J., Fink A. L. Cryoenzymology of papain: reaction mechanism with an ester substrate. Biochemistry. 1978 Jun 27;17(13):2659–2668. doi: 10.1021/bi00606a032. [DOI] [PubMed] [Google Scholar]
- Angelides K. J., Fink A. L. Mechanism of Action of papain with a specific anilide substrate. Biochemistry. 1979 May 29;18(11):2355–2363. doi: 10.1021/bi00578a034. [DOI] [PubMed] [Google Scholar]
- Baker E. N., Boland M. J., Calder P. C., Hardman M. J. The specificity of actinidin and its relationship to the structure of the enzyme. Biochim Biophys Acta. 1980 Nov 6;616(1):30–34. doi: 10.1016/0005-2744(80)90260-0. [DOI] [PubMed] [Google Scholar]
- Baker E. N. Preliminary crystallographic data for actinidin, a thiol protease from Actinidia chinensis. J Mol Biol. 1973 Mar 5;74(3):411–412. doi: 10.1016/0022-2836(73)90382-3. [DOI] [PubMed] [Google Scholar]
- Baker E. N. Structure of actinidin, after refinement at 1.7 A resolution. J Mol Biol. 1980 Aug 25;141(4):441–484. doi: 10.1016/0022-2836(80)90255-7. [DOI] [PubMed] [Google Scholar]
- Baker E. N. The structure of actinidin at 5-5 A resolution. J Mol Biol. 1976 Feb 25;101(2):185–196. doi: 10.1016/0022-2836(76)90371-5. [DOI] [PubMed] [Google Scholar]
- Berger A., Schechter I. Mapping the active site of papain with the aid of peptide substrates and inhibitors. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):249–264. doi: 10.1098/rstb.1970.0024. [DOI] [PubMed] [Google Scholar]
- Boland M. J., Hardman M. J. Kinetic studies on the thiol protease from Actinidia chinensis. FEBS Lett. 1972 Nov 1;27(2):282–284. doi: 10.1016/0014-5793(72)80641-0. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Baines B. S., Mushiri M. S. Evidence that the active centre of chymopapain A is different from the active centres of some other cysteine proteinases and that the Brønsted coefficient (beta nuc.) for the reactions of thiolate anions with 2,2'-dipyridyl disulphide may be decreased by reagent protonation. Biochem J. 1980 Jul 1;189(1):189–129. doi: 10.1042/bj1890189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Carlsson J., Kierstan M. P., Crook E. M. Covalent chromatography by thiol-disulfide interchange. Methods Enzymol. 1974;34:531–544. doi: 10.1016/s0076-6879(74)34069-4. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Carlsson J., Kierstan M. P., Crook E. M. Covalent chromatography. Preparation of fully active papain from dried papaya latex. Biochem J. 1973 Jul;133(3):573–584. doi: 10.1042/bj1330573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Malthouse J. P. Evidence for a two-state transition in papain that may have no close analogue in ficin. Differences in the disposition of cationic sites and hydrophobic binding areas in the active centres of papain and ficin. Biochem J. 1980 Dec 1;191(3):707–718. doi: 10.1042/bj1910707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Malthouse J. P. Evidence that the lack of high catalytic activity of thiolsubtilisin towards specific substrates may be due to an inappropriately located proton-distribution system. Demonstration of highly nucleophilic character of the thiol group of thiolsubtilisin in the catalytically relevant ionization state of the active centre by use of a two-protonic-state reactivity probe. Biochem J. 1981 Mar 1;193(3):819–823. doi: 10.1042/bj1930819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Malthouse J. P., Shipton M. Evidence that binding to the s2-subsite of papain may be coupled with catalytically relevant structural change involving the cysteine-25-histidine-159 diad. Kinetics of the reaction of papain with a two-protonic-state reactivity probe containing a hydrophobic side chain. Biochem J. 1979 Nov 1;183(2):223–231. doi: 10.1042/bj1830223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K. Specific covalent modification of thiols: applications in the study of enzymes and other biomolecules. Int J Biochem. 1979;10(4):259–274. doi: 10.1016/0020-711x(79)90088-0. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. Computer programmes for processing enzyme kinetic data. Nature. 1963 May 4;198:463–465. doi: 10.1038/198463a0. [DOI] [PubMed] [Google Scholar]
- Carne A., Moore C. H. The amino acid sequence of the tryptic peptides from actinidin, a proteolytic enzyme from the fruit of Actinidia chinensis. Biochem J. 1978 Jul 1;173(1):73–83. doi: 10.1042/bj1730073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth J., Kalk K. H., Swen H. M. Binding of chloromethyl ketone substrate analogues to crystalline papain. Biochemistry. 1976 Aug 24;15(17):3731–3738. doi: 10.1021/bi00662a014. [DOI] [PubMed] [Google Scholar]
- Hillson D. A. Resolution of thiol-containing proteins by sequential-elution covalent chromatography. J Biochem Biophys Methods. 1981 Feb;4(2):101–111. doi: 10.1016/0165-022x(81)90023-3. [DOI] [PubMed] [Google Scholar]
- Kraut J. Serine proteases: structure and mechanism of catalysis. Annu Rev Biochem. 1977;46:331–358. doi: 10.1146/annurev.bi.46.070177.001555. [DOI] [PubMed] [Google Scholar]
- Malthouse J. P., Brocklehurst K. Preparation of fully active ficin from Ficus glabrata by covalent chromatography and characterization of its active centre by using 2,2'-depyridyl disulphide as a reactivity probe. Biochem J. 1976 Nov;159(2):221–234. doi: 10.1042/bj1590221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowall M. A. Anionic proteinase from Actinidia chinensis. Preparation and properties of the crystalline enzyme. Eur J Biochem. 1970 Jun;14(2):214–221. doi: 10.1111/j.1432-1033.1970.tb00280.x. [DOI] [PubMed] [Google Scholar]
- Shipton M., Brochlehurst K. Characterization of the papain active centre by using two-protonic-state electrophiles as reactivity probes. Evidence for nucleophilic reactivity in the un-interrupted cysteine-25-histidine-159 interactive system. Biochem J. 1978 May 1;171(2):385–401. doi: 10.1042/bj1710385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton M., Stuchbury T., Brocklehurst K. 4-Chloro-7-nitrobenzo-2-oxa-1,3-diazole as a reactivity probe for the investigation of the thiol proteinases. evidence that ficin and bromelain may lack carboxyl groups conformationally equivalent to that of aspartic acid-158 of papain. Biochem J. 1976 Nov;159(2):235–244. doi: 10.1042/bj1590235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuchbury T., Shipton M., Norris R., Malthouse J. P., Brocklehurst K., Herbert J. A., Suschitzky H. A reporter group delivery system with both absolute and selective specificity for thiol groups and an improved fluorescent probe containing the 7-nitrobenzo-2-oxa-1,3-diazole moiety. Biochem J. 1975 Nov;151(2):417–432. doi: 10.1042/bj1510417. [DOI] [PMC free article] [PubMed] [Google Scholar]


