Abstract
Racemic 3,4-methylenedioxymethamphetamine (MDMA) acutely increases mood, feelings of empathy, trust, and closeness to others and is investigated to assist psychotherapy. Preclinical research indicates that S-MDMA releases monoamines and oxytocin more potently than R-MDMA, whereas R-MDMA more potently stimulates serotonin 5-hydroxytryptamine-2A receptors. S-MDMA may have more stimulant properties, and R-MDMA may be more psychedelic-like. However, acute effects of S- and R-MDMA have not been examined in a controlled human study. We used a double-blind, randomized, placebo-controlled, crossover design to compare acute effects of MDMA (125 mg), S-MDMA (125 mg), R-MDMA (125 mg and 250 mg), and placebo in 24 healthy participants. Outcome measures included subjective, autonomic, and adverse effects, pharmacokinetics, and plasma oxytocin, prolactin, and cortisol concentrations. S-MDMA (125 mg) induced greater subjective effects (“stimulation,” “drug high,” “happy,” “open”) and higher increases in blood pressure than R-MDMA (both 125 and 250 mg) and MDMA (125 mg). Unexpectedly, R-MDMA did not produce more psychedelic-like effects than S-MDMA. S-MDMA increased plasma prolactin more than MDMA, and S-MDMA increased plasma cortisol and oxytocin more than MDMA and R-MDMA. The plasma elimination half-life of S-MDMA was 4.1 h after administration. The half-life of R-MDMA was 12 and 14 h after the administration of 125 and 250 mg, respectively. Half-lives for S-MDMA and R-MDMA were 5.1 h and 11 h, respectively, after racemic MDMA administration. Concentrations of the CYP2D6-formed MDMA-metabolite 4-hydroxy-3-methoxymethamphetamine were lower after R-MDMA administration compared with S-MDMA administration. The pharmacokinetic findings are consistent with the R-MDMA-mediated inhibition of CYP2D6. Stronger stimulant-like effects of S-MDMA in the present study may reflect the higher potency of S-MDMA rather than qualitative differences between S-MDMA and R-MDMA. Equivalent acute effects of S-MDMA, MDMA, and R-MDMA can be expected at doses of 100, 125, and 300 mg, respectively, and need to be investigated.
Trial registration: ClinicalTrials.gov identifier: NCT05277636
Subject terms: Pharmacology, Translational research
Introduction
3,4-Methylenedioxymethamphetamine (MDMA) releases serotonin (5-hydroxytryptamine [5-HT]), norepinephrine, dopamine, and oxytocin and induces feelings of well-being, empathy, trust, closeness, and connectedness [1, 2]. Acute subjective effects of MDMA are considered helpful to assist psychotherapy for posttraumatic stress disorder [3]. MDMA is a racemic substance that contains equal amounts of the enantiomers S(+)- and R(-)-MDMA. Preclinical research indicates that S-MDMA more potently releases monoamines and oxytocin than R-MDMA, whereas R-MDMA may act more potently on 5-HT2A receptors [4–10]. Behavioral animal studies indicate that S-MDMA is more stimulant-like than R-MDMA, and R-MDMA may be more psychedelic-like while still producing MDMA-typical effects [11–13]. For example, the stimulant d-amphetamine substituted for S-MDMA- but not R-MDMA-trained animals while the psychedelic 2,5-dimethoxy-4-propylthiophenethylamine substituted for R-MDMA- but not S-MDMA-trained animals in drug-discrimination studies in mice [11]. Additionally, preclinical research indicates that R-MDMA induces less hyperthermia and less neurotoxicity [14–16]. Research on abuse-related behavioral effects in Rhesus monkeys showed comparable [17] or little to no drug self-administration of R-MDMA compared with MDMA and S-MDMA [18]. Consistently, priming with MDMA or S-MDMA but not with R-MDMA reinstated extinguished amphetamine self-administration behavior [19]. Because of these preclinical results, R-MDMA has been discussed as a potentially safer tool for substance-assisted therapy than racemic MDMA [12]. However, acute effects of S- and R-MDMA have not been validly compared in a human study. Therefore, the present study compared acute responses to racemic MDMA, S-MDMA, R-MDMA, and placebo in a double-blind, crossover study in healthy participants.
The primary study hypothesis was that S-MDMA would induce greater ratings of subjective stimulation on the Visual Analog Scale (VAS) than R-MDMA, and R-MDMA would induce more psychedelic-like effects on the 5 Dimensions of Altered States of Consciousness (5D-ASC) scale than S-MDMA.
Methods and materials
Study design
The study used a double-blind, placebo-controlled, crossover design with five experimental test sessions to investigate responses to (i) placebo, (ii) 125 mg racemic MDMA, (iii) 125 mg S-MDMA, (iv) 125 mg R-MDMA, and (v) 250 mg R-MDMA. Participants were informed that they would get all treatments. Block randomization was used with counterbalanced treatment order. The washout periods between sessions were at least 10 days. The study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization Guidelines in Good Clinical Practice and approved by the Ethics Committee of Northwest Switzerland (EKNZ) and Swiss Federal Office for Public Health. The study was registered at ClinicalTrials.gov (NCT05277636).
Participants
Twenty-four healthy participants (12 men and 12 women; mean age ±SD: 29 ± 9 years; range: 18–47 years) were recruited by word of mouth or from a pool of volunteers who had contacted our research group because they were interested in participating in a clinical trial on psychedelics or entactogens. All of the subjects provided written informed consent and were paid for their participation. Exclusion criteria were <18 years or >65 years of age, pregnancy (urine pregnancy test at screening and before each test session), personal or family (first-degree relative) history of major psychiatric disorders (assessed by the Semi-structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 5th edition, Axis I disorders), the use of medications (e.g., antidepressants, antipsychotics, and sedatives) that may interfere with the study medications, chronic or acute physical illness (e.g., abnormal physical exam, electrocardiogram, or hematological and chemical blood analyses), tobacco smoking (>10 cigarettes/day), lifetime prevalence of illicit substances >20 times or use within the last 2 months (except for Δ9-tetrahydrocannabinol; THC), and illicit drug use during the study period (including THC; urine drug test performed randomly prior to one study day). The participants were asked to consume no more than 15 standard alcoholic drinks/week and have no more than one drink on the day before the test sessions. Prior and current substance use is described in the Supplementary Methods and in Supplementary Table S1.
Study drugs
We selected 125 mg racemic MDMA as a common and safe dose [20]. Based on animal data, 125 mg S-MDMA and 125 mg racemic MDMA were expected to be overall equipotent in inducing stimulant-type and adverse effects in humans. R-MDMA was administered at a dose of 125 mg and additionally at a higher dose of 250 mg based on its lower potency and to be able to assess its effect characteristics more fully. Preliminary data indicated that S-MDMA was active at 80–120 mg, and R-MDMA was expected to be active at doses near 300 mg in humans [21]. Fixed rather than weight-based doses were used for practical reasons and because MDMA has not been adjusted to body weight in phase 3 studies and in limited use outside clinical studies. MDMA (ReseaChem, Burgdorf, Switzerland) was administered in opaque capsules that contained 25 mg MDMA hydrochloride and an exact analytically confirmed actual MDMA content of 25.40 ± 0.48 mg (n = 9 samples). S-MDMA (ReseaChem, Burgdorf, Switzerland) was administered in opaque capsules that contained 25 mg S-MDMA hydrochloride and an exact analytically confirmed actual S-MDMA content of 25.56 ± 0.62 mg (n = 10). R-MDMA (ReseaChem, Burgdorf, Switzerland) was administered in opaque capsules that contained 25 mg R-MDMA hydrochloride and an exact analytically confirmed actual R-MDMA content of 25.50 ± 1.30 mg (n = 10). Placebo consisted of identical opaque capsules that were filled with mannitol. All capsules were produced according to Good Manufacturing Practice guidelines (Dr. Hysek AG, Biel, Switzerland). The subjects received 10 capsules in each session: (i) 10 placebo capsules, (ii) five 25 mg (±)-MDMA capsules and five placebo capsules, (iii) five S-MDMA capsules and five placebo capsules, (iv) five 25 mg R-MDMA capsules and five placebo capsules, and (v) ten 25 mg R-MDMA capsules. At the end of each session and at the end of the study, the participants guessed their treatment assignment to evaluate blinding.
Study procedures
The study included a screening visit, five 10-h test sessions with follow-up measurements 24 h after drug intake, and an end-of-study visit that occurred an average of 14 days after the last test session. The sessions were conducted in a calm hospital room. Only one research participant and one investigator were present during each test session. The test sessions began at 8:00 AM. A urine pregnancy test was performed in women with childbearing potential. The participants underwent baseline measurements. A standardized breakfast (two croissants) was served. Substances were administered at 9:00 AM. The outcome measures were repeatedly assessed for 9 h. Standardized lunches were served at 1:30 PM. The participants were sent home at 6:15 PM and returned the next day for follow-up measurements at 9:00 AM.
Subjective drug effects and effect durations
Subjective effects were assessed repeatedly using VASs 0.5 h before and 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7, 8, 9, and 24 h after drug administration. The VAS “simulated” was the primary measure to assess stimulation. The Adjective Mood Rating Scale (AMRS) [22] was used 0.5 h before and 2.5, 5, and 9 h after drug administration. The 5D-ASC scale [23] and the 3D-ASC total score were used as the primary measure to assess psychedelic-like effects. It was administered 9 h after drug administration to retrospectively rate peak drug effects. Mystical experiences were assessed 9 h after drug administration using the Psychedelic Experience Scale (PES) [24], a revalidation of the 100-item States of Consciousness Questionnaire (SOCQ) [25], which includes the 30-item Mystical Experience Questionnaire (MEQ30) [24, 26]. Subjective effect measurements are described in detail in the Supplementary Methods online.
The time to onset, time to maximal effect, time to offset, effect duration, and area under the effect curve were assessed using Phoenix WinNonlin 8.3 (Certara, Princeton, NJ, USA) and “any drug effect” VAS effect-time plots and an onset/offset threshold of 10% of the maximum possible response. Participants with responses <10% on this scale were not used to determine the time to onset, time to offset, or effect duration.
Autonomic and adverse effects
Blood pressure, heart rate, and tympanic body temperature were repeatedly measured at baseline and 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7, 8, 9, and 24 h after drug administration. Adverse effects were assessed 0.5 h before and 9, 24 and 72 h after drug administration using the List of Complaints [27]. To assess adverse effects on mood 1–3 days after substance administration, the Beck Depression Inventory (BDI) [28] and Symptom-Check-List-90-R (SCL-90-R) [29] were used 72 h after administration.
Endocrine effects
Plasma concentrations of oxytocin were measured before and 2, 3, and 6 h after drug administration and determined as previously described [30]. Plasma concentrations of cortisol and prolactin were measured at baseline and 2 and 3 h after drug administration using an electrochemiluminescence immunoassay as previously described [31].
Plasma MDMA concentrations
Plasma concentrations of MDMA, S-MDMA, R-MDMA, and their metabolites were measured before and 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7, 8, 9, and 24 h after drug administration. Blood was collected into lithium heparin tubes. The blood samples were immediately centrifuged, and the plasma was subsequently stored at −80 °C until analysis.
MDMA, S-MDMA, R-MDMA, and their metabolites 3,4-methylenedioxyamphetamine (MDA) and 4-hydroxy-3-methoxymethamphetamine (HMMA) were analyzed in human plasma using an achiral high-performance liquid chromatography tandem mass spectrometry method and additionally an enantioselective method for racemic MDMA as previously described [32]. HMMA concentrations were determined after enzymatic deglucuronidation.
Pharmacokinetic analyses
Pharmacokinetic parameters were estimated using non-compartmental methods. Analyses were conducted using Phoenix WinNonlin 8.3 (Certara, Princeton, NJ, USA).
Data analysis
Peak (Emax and/or Emin) or peak change from baseline (ΔEmax) values were determined for repeated measures. The values were then analyzed using repeated-measures analysis of variance (ANOVA), with drug as the within-subjects factor, followed by the Tukey post hoc tests using R 4.2.1 software (RStudio, PBC, Boston, MA, USA). The criterion for significance was p < 0.05.
Results
Subjective drug effects
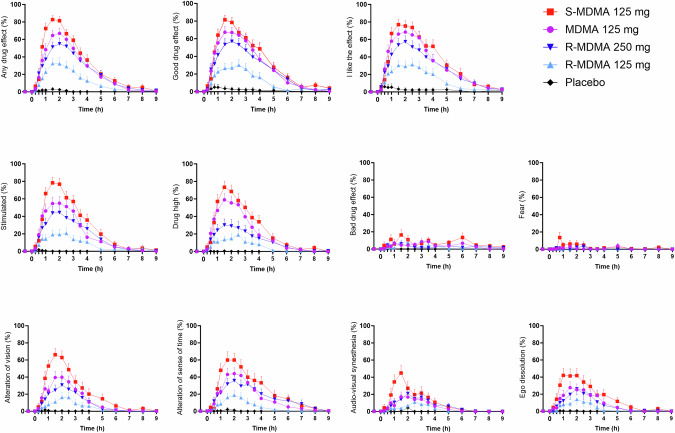
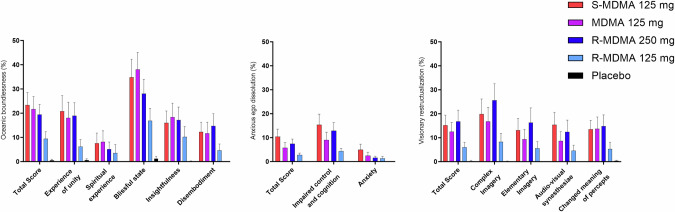
Subjective effects over time on the VAS are shown in Fig. 1 and Supplementary Fig. S1. Subjective peak responses and statistics are shown in Table 1. S-MDMA produced overall greater subjective effects than MDMA and R-MDMA at the doses used. Specifically, S-MDMA induced significantly stronger “bad drug effects,” “alteration of vision,” and “audio-visual synesthesia” than MDMA and significantly stronger effects than 250 mg R-MDMA on most VASs including “stimulation”. Both R-MDMA doses induced lower effects on “drug high,” “happy,” “content,” “talkative,” “open,” “trust,” and “I feel close to others” than MDMA and S-MDMA (Fig. 1, Supplementary Fig. S1, Table 1). Responses in female participants were greater than in male participants due to lower body weights in women (Supplementary Fig. S2 and Supplementary Table S2). Responses in participants with and without previous MDMA experiences did not differ (Supplementary Fig. S3 and Supplementary Table S3). The mean effect duration was 3.5, 4.2, 4.7, and 5.2 h after the administration of 125 mg R-MDMA, MDMA, S-MDMA, and 250 mg R-MDMA, respectively (Supplementary Table S4). MDMA, S-MDMA, and 250 mg R-MDMA induced comparable alterations of mind and mystical-type effects on the 5D-ASC and PES48/MEQ, respectively (Fig. 2, Supplementary Fig. S4, statistics in Supplementary Tables S5 and S6). R-MDMA and S-MDMA also similarly increased the 3D-ASC total score reflecting comparable psychedelic effects (Supplementary Table S5). On the AMRS, 250 mg R-MDMA induced significantly higher “Introversion” than MDMA, and S-MDMA induced more “emotional excitation” than R-MDMA (Supplementary Fig. S5, Supplementary Table S7).
Fig. 1. Acute subjective effects of 125 mg MDMA, 125 mg S-MDMA, 125 mg R-MDMA, and 250 mg R-MDMA on the Visual Analog Scale (VAS).
S-MDMA produced overall stronger subjective responses than MDMA, with significant differences in “bad drug effects,” “alteration of vision,” and “audio-visual synesthesia.” R-MDMA at both doses produced overall lower subjective effects than MDMA, with significant differences in “drug high,” “happy,” “content,” “talkative,” “open,” “trust,” and “I feel close to others.” The substances were administered at t = 0 h. The data are expressed as the mean ± SEM percentage of maximally possible scores in 24 participants. The corresponding maximal responses and statistics are shown in Table 1.
Table 1.
Mean values and statistics for the acute subjective effects of MDMA, S-MDMA, R-MDMA and placebo.
| Placebo | 125 mg R-MDMA | 250 mg R-MDMA | 125 mg MDMA | 125 mg S-MDMA | F4, 92 | P= | Pla - R-MDMA (125 mg) | Pla - R-MDMA (250 mg) | Pla - MDMA | Pla - S-MDMA | R-MDMA (125 mg) - R-MDMA (250 mg) | R-MDMA (125 mg) - MDMA | R-MDMA (125 mg) - S-MDMA | R-MDMA (250 mg) - MDMA | R-MDMA (250 mg) - S-MDMA | MDMA - S-MDMA | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | ||||||||||||||
| Visual Analog Scale (VAS, %max) | ||||||||||||||||||
| Unidirectional scales (0–100) | ||||||||||||||||||
| Any drug effect | ΔEmax | 4.8 ±2.1 | 42 ±7.3 | 66 ±6.1 | 77 ±5.3 | 90 ±3.1 | 68.92 | <0.001 | *** | *** | *** | *** | *** | *** | *** | NS | ** | NS |
| Good drug effect | ΔEmax | 7.9 ±4.1 | 43 ±7.1 | 68 ±6.6 | 78 ±5.1 | 90 ±3.8 | 54.97 | <0.001 | *** | *** | *** | *** | *** | *** | *** | NS | ** | NS |
| Bad drug effect | ΔEmax | 0.5 ±0.3 | 14 ±5.1 | 19 ±4.6 | 20 ±5.9 | 39 ±7.0 | 10.51 | <0.001 | NS | * | * | *** | NS | NS | *** | NS | * | * |
| I like the effect | ΔEmax | 7.7 ±3.8 | 47 ±7.1 | 68 ±6.6 | 81 ±5.0 | 91 ±3.6 | 60.12 | <0.001 | *** | *** | *** | *** | ** | *** | *** | NS | ** | NS |
| Stimulated | ΔEmax | 2.5 ±1.1 | 31 ±6.9 | 60 ±6.7 | 70 ±6.7 | 88 ±3.7 | 58.82 | <0.001 | *** | *** | *** | *** | *** | *** | *** | NS | *** | NS |
| Drug high | ΔEmax | 1.4 ±0.6 | 29 ±7.0 | 48 ±7.6 | 73 ±6.7 | 84 ±5.1 | 49.31 | <0.001 | *** | *** | *** | *** | * | *** | *** | ** | *** | NS |
| Fear | ΔEmax | 0.0 ±0.0 | 4.9 ±4.2 | 5.8 ±3.4 | 5.9 ±3.4 | 19 ±6.9 | 3.90 | 0.006 | NS | NS | NS | ** | NS | NS | * | NS | NS | NS |
| Alteration of vision | ΔEmax | 3.0 ±1.6 | 24 ±6.9 | 37 ±6.7 | 54 ±7.8 | 74 ±6.7 | 32.23 | <0.001 | * | *** | *** | *** | NS | *** | *** | NS | *** | * |
| Alteration of sense of time | ΔEmax | 2.2 ±2.0 | 28 ±7.2 | 46 ±7.7 | 60 ±6.8 | 73 ±6.6 | 34.55 | <0.001 | ** | *** | *** | *** | NS | *** | *** | NS | ** | NS |
| Audio-visual synesthesia | ΔEmax | 4.6 ±4.2 | 15 ±5.9 | 31 ±6.8 | 29 ±6.8 | 53 ±8.3 | 16.01 | <0.001 | NS | ** | ** | *** | NS | NS | *** | NS | ** | ** |
| Ego dissolution | ΔEmax | 1.5 ±0.8 | 22 ±7.4 | 32 ±7.4 | 41 ±7.7 | 58 ±8.2 | 17.83 | <0.001 | * | *** | *** | *** | NS | NS | *** | NS | ** | NS |
| Bidirectional scales (−50 to 50) | ||||||||||||||||||
| Happy | ΔEmax | 3.9 ±2.2 | 14 ±3.5 | 24 ±3.8 | 34 ±3.3 | 38 ±3.4 | 32.25 | <0.001 | * | *** | *** | *** | NS | *** | *** | * | *** | NS |
| Content | ΔEmin | 4.8 ±2.2 | 19 ±3.4 | 30 ±3.6 | 39 ±2.8 | 44 ±2.5 | 46.83 | <0.001 | *** | *** | *** | *** | * | *** | *** | * | *** | NS |
| Talkative | ΔEmax | 2.8 ±1.6 | 13 ±3.2 | 22 ±3.9 | 35 ±3.6 | 37 ±3.9 | 30.55 | <0.001 | NS | *** | *** | *** | NS | *** | *** | ** | ** | NS |
| Open | ΔEmax | 4.3 ±2.3 | 15 ±3.4 | 28 ±3.9 | 40 ±2.9 | 42 ±2.9 | 50.64 | <0.001 | * | *** | *** | *** | ** | *** | *** | ** | *** | NS |
| Trust | ΔEmax | 4.0 ±2.0 | 16 ±3.5 | 24 ±4.1 | 40 ±3.1 | 44 ±2.3 | 48.93 | <0.001 | ** | *** | *** | *** | NS | *** | *** | *** | *** | NS |
| I feel close to others | ΔEmax | 0.8 ±0.4 | 14 ±3.1 | 23 ±3.9 | 34 ±3.3 | 41 ±3.4 | 43.83 | <0.001 | ** | *** | *** | *** | NS | *** | *** | ** | *** | NS |
| I want to be alone | ΔEmax | 0.2 ±0.2 | 6.1 ±2.6 | 10 ±3.4 | 6.9 ±2.7 | 12 ±3.4 | 4.15 | 0.004 | NS | * | NS | ** | NS | NS | NS | NS | NS | NS |
| I want to be with others | ΔEmax | 1.8 ±1.1 | 17 ±3.4 | 24 ±4.0 | 31 ±3.5 | 40 ±3.5 | 32.15 | <0.001 | *** | *** | *** | *** | NS | ** | *** | NS | *** | NS |
| Autonomic effects | ||||||||||||||||||
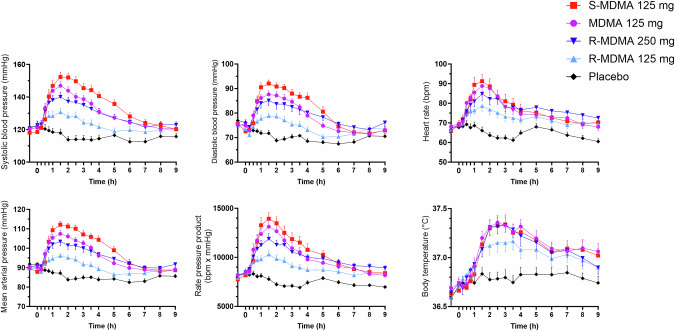
| Systolic blood pressure (mmHg) | Emax | 127 ±2.7 | 138 ±2.5 | 147 ±2.5 | 152 ±2.3 | 160 ±3.1 | 74.46 | <0.001 | *** | *** | *** | *** | *** | *** | *** | NS | *** | ** |
| Diastolic blood pressure (mmHg) | Emax | 78 ±0.8 | 84 ±1.1 | 91 ±1.6 | 92 ±1.3 | 97 ±1.6 | 62.90 | <0.001 | *** | *** | *** | *** | *** | *** | *** | NS | *** | *** |
| Mean arterial pressure (mmHg) | Emax | 94 ±1.0 | 101 ±1.4 | 108 ±1.8 | 111 ±1.3 | 117 ±1.9 | 93.43 | <0.001 | *** | *** | *** | *** | *** | *** | *** | NS | *** | *** |
| Heart rate (beats/min) | Emax | 75 ±1.3 | 87 ±2.6 | 93 ±3.4 | 95 ±3.5 | 100 ±3.3 | 21.50 | <0.001 | *** | *** | *** | *** | NS | NS | *** | NS | NS | NS |
| Rate pressure product (mmHg x bpm) | Emax | 9135 ±218 | 11565 ±476 | 12999 ±569 | 14018 ±662 | 15389 ±705 | 41.15 | <0.001 | *** | *** | *** | *** | NS | *** | *** | NS | *** | NS |
| Body temperature (°C) | Emax | 37.1 ±0.06 | 37.4 ±0.09 | 37.5 ±0.09 | 37.6 ±0.08 | 37.7 ±0.10 | 10.25 | <0.001 | NS | *** | *** | *** | NS | NS | * | NS | NS | NS |
| List of complaints (LC score) | ||||||||||||||||||
| Acute adverse effects | 0–9 h | 0.5 ±0.5 | 9.4 ±1.8 | 14 ±1.9 | 10 ±1.7 | 13 ±1.5 | 21.58 | <0.001 | *** | *** | *** | *** | NS | NS | NS | NS | NS | NS |
| Subacute adverse effects | 9–24 h | 0.1 ±0.5 | 6.7 ±1.9 | 8.2 ±1.5 | 5.4 ±1.3 | 7.2 ±1.4 | 8.95 | <0.001 | *** | *** | ** | *** | NS | NS | NS | NS | NS | NS |
| Subacute adverse effects | 24–72 h | 1.3 ±0.7 | 6.9 ±1.8 | 6.0 ±1.7 | 5.1 ±1.6 | 10 ±2.2 | 6.05 | <0.001 | * | NS | NS | *** | NS | NS | NS | NS | NS | NS |
| Becks depression-inventar (BDI) | ||||||||||||||||||
| BDI Score | 24–72 h | 2.0 ±0.9 | 4.3 ±1.2 | 4.4 ±1.2 | 4.8 ±1.3 | 8.4 ±1.7 | 4.07 | 0.004 | NS | NS | NS | * | NS | NS | NS | NS | NS | NS |
| Symptom checklist 90 R (SCL-90-R) | ||||||||||||||||||
| GSI score | 24–72 h | 0.08 ±0.02 | 0.18 ±0.05 | 0.17 ±0.05 | 0.14 ±0.03 | 0.34 ±0.77 | 5.02 | 0.001 | NS | NS | NS | * | NS | NS | NS | NS | NS | NS |
| Hormones and markers | ||||||||||||||||||
| Oxytocin (pg/mL)α | ΔCmax | 2.9 ±1.9 | 48 ±13 | 178 ±22 | 296 ±33 | 436 ±40 | 66.2β | <0.001 | NS | *** | *** | *** | *** | *** | *** | ** | *** | *** |
| Cortisol (nmol/L) | ΔCmax | −156 ±27 | 140 ±28 | 249 ±26 | 285 ±26 | 398 ±27 | 81.90 | <0.001 | *** | *** | *** | *** | ** | ** | *** | NS | *** | * |
| Prolactin (µg/L) | ΔCmax | −6.5 ±2.0 | 13 ±4.5 | 35 ±5.5 | 39 ±7.1 | 53 ±8.5 | 32.20 | <0.001 | *** | *** | *** | *** | *** | ** | *** | NS | NS | ** |
NS not significant, ΔEmax maximal effect difference from baseline, ΔCmax maximal plasma concentrationfrom baseline, N = 24, αN = 20, β = F4,81.
*P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 2. Acute mystical-type experiences on the 5 Dimensions of Altered States of Consciousness (5D-ASC) scale.
MDMA, S-MDMA, and 250 mg R-MDMA induced comparable alterations of mind. The data are expressed as the mean ± SEM percentage of maximally possible scale scores in 24 participants. Statistics are shown in Supplementary Table S5.
Autonomic and adverse effects
Autonomic effects over time and related peak responses are shown in Fig. 3 and Table 1, respectively. S-MDMA induced higher increases in blood pressure than MDMA and R-MDMA. MDMA, S-MDMA, and 250 mg R-MDMA increased heart rate and body temperature comparably.
Fig. 3. Acute autonomic effects.
S-MDMA induced greater increases in blood pressure compared with MDMA and both R-MDMA doses. MDMA, S-MDMA, and 250 mg R-MDMA increased heart rate and body temperature comparably. The substances were administered at t = 0 h. The data are expressed mean ± SEM in 24 participants. The corresponding maximal responses and statistics are shown in Table 1.
All substances produced similar acute and subacute adverse effects on the List of Complaints (Table 1). Frequently reported adverse effects included fatigue, headache, decreased appetite, feeling dull, lack of concentration, and dry mouth (Supplementary Table S8). All substances nominally increased self-ratings of depressive mood on the BDI 1–3 days after substance administration. Significantly higher ratings were seen for S-MDMA compared with placebo, with no significant differences between active drug substances (Table 1). No severe adverse events were observed.
Endocrine effects
All substances increased plasma prolactin and cortisol compared with placebo. S-MDMA increased plasma prolactin more than MDMA and plasma oxytocin and cortisol more than MDMA and R-MDMA (Supplementary Fig. S6, Table 1).
Plasma drug concentrations
Pharmacokinetic parameters are shown in Table 2. Concentration-time curves are shown in Supplementary Figs. S7–S9. Elimination half-lives (t1/2) for S-MDMA and R-MDMA were 5.1 and 11 h, respectively, when racemic MDMA was administered. The half-life of S-MDMA was 4.1 h when it was administered alone. The half-life of R-MDMA was 12 and 14 h after administration of the 125 and 250 mg doses, respectively (Table 2).
Table 2.
Pharmacokinetic parameters based on non-compartmental analyses [geometric mean (95% CI), range], N = 24.
| Cmax (ng/mL) | tmax (h) | t1/2 (h) | AUC24 (ng·h/mL) | AUC∞ (ng·h/mL) | CL/F (L/h) | Vz/F (L) | |
|---|---|---|---|---|---|---|---|
| 125 mg (±)-MDMA | |||||||
| (±)-MDMA | 290 (263–320) | 2.9 (2.5–3.5) | 8.7 (7.6–10) | 3274 (2881–3722) | 4007 (3390–4738) | 31 (26–37) | 392 (364–422) |
| 180–408 | 1.5–7.0 | 4.6–16 | 1659–5209 | 1735–7591 | 16–72 | 273–530 | |
| (±)-MDA | 14 (12–17) | 6.8 (5.9–7.7) | 14 (11–18) | 231 (197–271) | 340 (231–500) | 368 (250–540) | 7492 (6114–9181) |
| 6.8–28 | 3.0–9.0 | 8.0–29 | 95–455 | 112–1145 | 109–1115 | 4376–12,862 | |
| (±)-HMMA | 141 (112–177) | 2.9 (2.5–3.4) | 12 (11–13) | 1666 (1332–2084) | 2274 (1814–2851) | 55 (44–69) | 943 (750–1186) |
| 49–431 | 1.5–7.0 | 7.7–18 | 563–4876 | 648–6070 | 21–193 | 304–2456 | |
| S-MDMA | 123 (111–137) | 2.8 (2.4–3.4) | 5.1 (4.7–5.5) | 1051 (933–1186) | 1111 (977–1263) | 56 (49–64) | 413 (379–450) |
| 72–189 | 1.5–7.0 | 3.5–7.4 | 567–1571 | 574–1710 | 37–109 | 292–595 | |
| S-MDA | 12 (10–14) | 6.3 (5.5–7.3) | 11 (9.3–13) | 158 (128–197) | 230 (187–283) | 272 (221–334) | 4311 (3632–5119) |
| 5.8–24 | 3.0–9.0 | 7.0–17 | 37–315 | 144–449 | 139–433 | 2294–6726 | |
| R-MDMA | 167 (151–184) | 3.3 (2.7–4.1) | 11 (9.1–13) | 2224 (1944–2544) | 2995 (2436–3681) | 21 (17–26) | 327 (301–356) |
| 100–232 | 2.0–9.0 | 5.1–24 | 1092–3539 | 1166–6410 | 9.8–54 | 231–456 | |
| R-MDA | 4.2 (3.6–5.0) | 14 (11–18) | 72 (58–89) | ||||
| 2.4–11 | 7.0–24 | 13–171 | |||||
| 125 mg S-MDMA | |||||||
| S-MDMA | 239 (215–265) | 2.8 (2.3–3.4) | 4.1 (3.6–4.6) | 1869 (1659–2106) | 1917 (1680–2187) | 65 (57–74) | 382 (349–418) |
| 137–413 | 1.0–8.0 | 2.3–7.4 | 949–2862 | 954–3051 | 41–131 | 253–606 | |
| S-MDA | 21 (18–25) | 5.6 (5.0–6.3) | 8.0 (6.9–9.2) | 261 (209–326) | 349 (296–411) | 358 (304–422) | 4126 (3443–4944) |
| 9.0–46 | 3.0–9.0 | 4.0–12 | 62–608 | 196–761 | 164–637 | 2045–8142 | |
| HMMA | 175 (145–211) | 3.6 (3.2–4.1) | 7.7 (6.8–8.8) | 1955 (1666–2294) | 2293 (1973–2665) | 55 (47–63) | 609 (491–754) |
| 71–421 | 1.5–7.0 | 4.8–13 | 822–3581 | 989–3768 | 33–126 | 249–1564 | |
| 125 mg R-MDMA | |||||||
| R-MDMA | 335 (305–368) | 3.2 (2.7–3.8) | 12 (11–14) | 4775 (4249–5366) | 6869 (5803–8132) | 18 (15–22) | 328 (298–361) |
| 209–463 | 2.0–7.0 | 6.6–32 | 2307–6916 | 2559–14771 | 8.5–49 | 199–550 | |
| R-MDA | 8.2 (6.8–9.8) | 16 (13–20) | 146 (121–175) | ||||
| 3.4–18 | 7.0–24 | 61–337 | |||||
| HMMA | 142 (105–191) | 2.3 (1.8–2.8) | 19 (17–22) | 1631 (1300–2045) | 2956 (2369–3688) | 42 (34–53) | 1181 (943–1479) |
| 28–551 | 0.8–6.0 | 12–39 | 500–5026 | 730–7100 | 18–171 | 376–4297 | |
| 250 mg R-MDMA | |||||||
| R-MDMA | 694 (638–755) | 3.6 (2.9–4.3) | 14 (13–16) | 10,087 (9113–11,164) | 15754 (13,939–17,805) | 16 (14–18) | 329 (302–358) |
| 501–975 | 1.5–8.0 | 10–28 | 5770–15,780 | 10,049–29,136 | 8.6–25 | 203–466 | |
| R-MDA | 16 (13–19) | 22 (19–25) | 273 (228–327) | ||||
| 7.7–41 | 8.0–24 | 120–725 | |||||
| HMMA | 162 (128–203) | 2.8 (2.3–3.4) | 18 (16–21) | 2020 (1672–2441) | 3559 (2929–4325) | 70 (58–85) | 1840 (1513–2239) |
| 60–539 | 1.5–8.0 | 7.2–35 | 908–5211 | 1450–8484 | 29–172 | 807–3958 | |
AUC area under the plasma concentration-time curve, AUC∞ AUC from time zero to infinity, AUC24 from time 0 to 24, CL/F apparent total clearance, Cmax maximum observed plasma concentration, T1/2 plasma half-life, Tmax time to reach Cmax, 95%CI 95% confidence interval, Vz/F apparent volume of distribution.
Correlations
Correlations between the drug plasma concentrations and subjective, cardiovascular, cortisol, and prolactin responses are shown in Supplementary Figs. S10–S13, respectively.
Blinding
Participants could not distinguish effects of the active substances (Supplementary Table S9) after the treatment session or at the end-of-study visit. Placebo was correctly identified by 83% of participants after the study session.
Discussion
The present controlled study was the first to directly compare acute effects of MDMA, S-, and R-MDMA. As hypothesized, S-MDMA induced greater subjective stimulation than R-MDMA. However, at the doses used S-MDMA also had greater effects than R-MDMA on many other mood scales. Contrary to our hypothesis, R-MDMA did not produce greater psychedelic effects than S-MDMA. We observed overall comparable effects of MDMA, S-MDMA, and R-MDMA with regard to effect strength and quality of the responses with minor differences. Specifically, S-MDMA induced overall slightly stronger effects and significantly greater bad drug effects, visual alterations, and synesthesia on the VAS, comparable psychedelic- and mystical-type alterations of mind on the 5D-ASC and MEQ, and comparable mood effects on the AMRS compared with MDMA. S-MDMA produced greater increases in blood pressure, cortisol, and prolactin compared with MDMA and was the only substance to significantly induce depressive symptoms 1–3 days after administration. The higher 250 mg R-MDMA dose produced lower subjective effects on most VASs, comparable psychedelic-like alterations on the 5D-ASC and MEQ, and more introversion on the AMRS compared with MDMA and S-MDMA.
Evidence from animal studies and human reports indicates that both enantiomers of MDMA are active and produce differential effects or are even reportedly needed to synergistically produce the full MDMA experience [13, 16, 17]. Based on animal data, we expected that S-MDMA and racemic MDMA would be overall equipotent in inducing stimulant-type and adverse effects in humans [9, 13, 16, 33] and thus selected the same dose of 125 mg S-MDMA and MDMA for the present comparison. However, other self-administration data in humans indicated that a 100 mg dose of S-MDMA induced similar “intoxication” to 125 mg racemic MDMA [21]. The present findings confirm a slightly higher potency of S-MDMA compared with MDMA and indicate that a 100 mg dose of S-MDMA would be equivalent to a 125 mg dose of racemic MDMA. Thus, the overall slightly greater subjective and cardiostimulant effects of S-MDMA in the present study may mainly reflect the 25% greater potency of S-MDMA compared with MDMA rather than any qualitative differences between S-MDMA and MDMA.
Nevertheless, supporting our primary hypothesis, S-MDMA exhibited more cardio- and psychostimulant effects than MDMA and R-MDMA in the present study, consistent with animal data [11]. The stronger increase in blood pressure in response to S-MDMA compared with R-MDMA may reflect the higher potency of S-MDMA to interact with the norepinephrine-transporter and release norepinephrine compared with R-MDMA [4, 34]. Additionally, S-MDMA was the only substance to significantly produce depressed mood ratings 1–3 days after drug administration, which could reflect greater transient serotonin depletion [35]. In the present study, we also observed significantly higher ratings of “drug high” after the administration of S-MDMA compared with R-MDMA. S-MDMA was found to be more potent than R-MDMA in maintaining self-administration in rhesus monkeys [17], and S-MDMA but not R-MDMA reinstated responding for amphetamine, indicative of greater abuse liability [12, 19]. S-MDMA may be more addictive in humans than R-MDMA, but we cannot exclude the possibility that the small differences between substances in the present study are dose-dependent rather than substance-dependent.
R-MDMA was expected to elicit more psychedelic-like effects compared with S-MDMA because of its higher potency to stimulate 5-HT2A receptors [8]. However, in the present study, R-MDMA did not produce more psychedelic-like effects on the 5D-ASC or PES48/MEQ than S-MDMA or MDMA. Thus, we could not confirm our hypothesis that R-MDMA induces more psychedelic-like effects than S-MDMA at the doses used, although a higher dose of R-MDMA would need to be investigated. On the other hand, on the VAS, S-MDMA produced greater alterations of vision and greater audio-visual synesthesia than MDMA and R-MDMA, effects that would both be considered characteristic of psychedelics [36].
The therapeutic efficacy of MDMA might be enhanced by its ability to promote prosocial behaviors, foster openness, and facilitate a stronger therapeutic bond between the patient and therapist [2, 37, 38]. Animal studies found increases in social interaction in response to MDMA and higher doses of R-MDMA but only weak or no prosocial effects of S-MDMA [15, 39]. In the present first study in humans, all substances increased VAS ratings of “talkative,” “open,” “trust,” “I feel close to others,” and “I want to be with others” compared with placebo, but S-MDMA induced higher ratings on all these scales compared with R-MDMA at both doses. All substances produced comparable increases in ratings of feelings of “connectedness” on the PES48 compared with placebo. Thus, the present findings do not indicate greater prosocial effects of R-MDMA compared with MDMA or S-MDMA.
Oxytocin has overlapping social cognitive effects with MDMA [2, 40–42] and contributes to acute subjective effects of MDMA [1]. Cortisol and prolactin could be considered biomarkers of the serotonergic activity of MDMA [43]. In the present study, all substances increased circulating levels of oxytocin, cortisol, and prolactin. S-MDMA produced greater increases in oxytocin and cortisol compared with R-MDMA. S-MDMA also released prolactin at least as effectively as R-MDMA, in contrast to a study in rhesus monkeys [10]. The present findings align with stronger stimulation of the serotonin system by S-MDMA compared with R-MDMA at the doses used in the present study and are consistent with the greater serotonergic potency (but not selectivity) of S-MDMA compared with R-MDMA [4, 34].
Animal studies reported no hyperthermic effects of R-MDMA in mice or rats [14–16]. However, we found similar minimal increases in body temperature after S-MDMA and R-MDMA in the present human study.
Based on preliminary human data, the potency of R-MDMA was considered lower than MDMA and S-MDMA, with an effective dose “that might lie in the vicinity of 300 mg” [21]. Subjective effects of the R-MDMA doses that were used in the present study were lower than the 125 mg MDMA and 125 mg S-MDMA doses and indicate that a 300 mg dose may induce a comparable overall response to 125 mg MDMA or 100 mg S-MDMA. Thus, we would consider S-MDMA to be 1.25-fold more potent than MDMA and R-MDMA to be 2.4-fold less potent than MDMA. The in vitro potency of S-MDMA to release norepinephrine [34] or interact with the norepinephrine transporter was 4-fold higher compared with R-MDMA, predicting an approximately 4-fold higher potency in vivo [44].
Pharmacokinetics of R- and S-MDMA in humans have only been described after the administration of racemic MDMA [45–47]. After MDMA administration, R-MDMA had higher plasma concentrations (Cmax and area under the curve) and an extended half-life compared with S-MDMA [45–47]. The present study confirmed the greater plasma exposure and longer elimination half-life of R-MDMA compared with S-MDMA after the administration of racemic MDMA. Additionally, the present study characterized pharmacokinetics of S-MDMA and R-MDMA in the absence of interactions with the other enantiomer. The elimination half-life of S-MDMA was 4.1 h when it was administered alone but 5.1 h when it was administered with R-MDMA in the form of racemic MDMA. The elimination half-life of R-MDMA was 12 and 14 h for the 125 and 250 mg doses of pure R-MDMA, respectively, indicating an increase with dose. Additionally, the formation of R-MDA from R-MDMA was dose-proportional, whereas the formation of HMMA from R-MDMA decreased with higher doses of R-MDMA. Although the dose of R-MDMA was doubled from 125 mg to 250 mg, the HMMA concentration did not double as well. Altogether, the data confirm that R-MDMA inhibits CYP2D6, thereby inhibiting its own inactivation to HMMA [48] similar to MDMA [49]. The present findings that the half-life of S-MDMA becomes shorter when it is administered without the R-enantiomer and that the HMMA concentrations were elevated when S-MDMA was administered compared with when R-MDMA was administered, indicating potentially less inhibition of CYP2D6 by S-MDMA.
We also showed that MDMA and MDA in humans did not undergo chiral inversion [32]. Thus, although HMMA was not enatioselectively measured, it can be assumed that only S- and R-HMMA are formed after S- and R-MDMA administration, respectively.
The present study has several strengths. A relatively large study sample (n = 24) and powerful within-subjects comparisons were used in a randomized double-blind design. Excellent blinding between S-MDMA, R-MDMA, and MDMA was confirmed. Two doses of the main substance of interest, R-MDMA, were included. We also included equal numbers of male and female participants. We used a wide range of internationally established psychometric outcome measures. Plasma concentrations were determined at close intervals in all participants and analyzed with validated achiral and chiral methods [32].
Notwithstanding its strengths, the present study also has limitations. To avoid too many exposures to MDMA, we had to limit the use of doses for each substance. We used only one dose of S-MDMA and only two doses of R-MDMA and failed to use exactly equivalent doses of the different substances. Doses of 100 mg S-MDMA and 300 mg R-MDMA would have been more equivalent. Consequently, we cannot confirm whether the observed differences between substances were attributable to the use of non-equivalent doses or qualitative properties of the substances. The study used a highly controlled hospital setting and included only healthy volunteers. People in different environments and patients with psychiatric disorders may respond differently to these substances. The outcome measures might not have been sufficiently sensitive to capture all aspects of the substance experience and very subtle differences between acute effects of MDMA and its enantiomers.
Conclusion
In conclusion, the present study found that racemic MDMA, S-MDMA, and R-MDMA induced overall similar qualitative subjective and adverse effects when dosed equivalently. S-MDMA may have slightly greater stimulant-like properties than MDMA and R-MDMA. The results indicate dose-equivalence with regard to overall acute effects of 125 mg MDMA, 100 mg S-MDMA, and 300 mg R-MDMA. The pharmacokinetic findings indicate that R-MDMA dose-dependently inhibits CYP2D6 and thus its own inactivation and the inactivation of S-MDMA when administered as racemic MDMA. Overall, the present findings do not presently indicate relevant beneficial effects of R-MDMA or S-MDMA over MDMA in substance-assisted therapy in patients.
Supplementary information
Acknowledgements
The authors thank Livio Erne, Albiona Nuraj, Sophie Dierbach, Mélusine Humbert-Droz, Leonie Duss, and Diana Noorshams for help with conducting the study, Patrick Vizeli for help with statistics, Beatrice Vetter for help with assessing the plasma substance concentrations, and Michael Arends for proofreading the manuscript.
Author contributions
IS and MEL designed the research. IA, IS, DL, DR, AK, NV, and AE performed the research. IS, DL, and MEL analyzed the data. IS and MEL wrote the manuscript with input from all other authors. All authors gave final approval to the manuscript.
Funding
This work was supported by the Swiss National Science Foundation (grant no. 32003B_185111 to MEL), the University Hospital Basel, and Mind Medicine, Inc. Open access funding provided by University of Basel.
Competing interests
MEL is a consultant for Mind Medicine, Inc. The other authors declare no conflicts of interest. Knowhow and data associated with this work are owned by the University Hospital Basel and were licensed by Mind Medicine, Inc. Mind Medicine, Inc., had no role in planning or conducting the present study or writing the publication.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Isabelle Straumann, Isidora Avedisian.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-024-01972-6.
References
- 1.Atila C, Holze F, Murugesu R, Rommers N, Hutter N, Varghese N, et al. Oxytocin in response to MDMA provocation test in patients with arginine vasopressin deficiency (central diabetes insipidus): a single-centre, case-control study with nested, randomised, double-blind, placebo-controlled crossover trial. Lancet Diabetes Endocrinol. 2023;11:454–64. [DOI] [PubMed] [Google Scholar]
- 2.Hysek CM, Schmid Y, Simmler LD, Domes G, Heinrichs M, Eisenegger C, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affect Neurosci. 2014;9:1645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell, Ot’alora GM JM, van der Kolk B, Shannon S, Bogenschutz M, Gelfand Y, et al. MDMA-assisted therapy for moderate to severe PTSD: a randomized, placebo-controlled phase 3 trial. Nat Med. 2023;29:2473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verrico CD, Miller GM, Madras BK. MDMA (ecstasy) and human dopamine, norepinephrine, and serotonin transporters: implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology. 2007;189:489–503. [DOI] [PubMed] [Google Scholar]
- 5.Hiramatsu M, Cho AK. Enantiomeric differences in the effects of 3,4-methylenedioxymethamphetamine on extracellular monoamines and metabolites in the striatum of freely-moving rats: an in vivo microdialysis study. Neuropharmacology. 1990;29:269–75. [DOI] [PubMed] [Google Scholar]
- 6.Steele TD, Nichols DE, Yim GK. Stereochemical effects of 3,4-methylenedioxymethamphetamine (MDMA) and related amphetamine derivatives on inhibition of uptake of [3H]monoamines into synaptosomes from different regions of rat brain. Biochem Pharm. 1987;36:2297–303. [DOI] [PubMed] [Google Scholar]
- 7.Forsling ML, Fallon JK, Shah D, Tilbrook GS, Cowan DA, Kicman AT, et al. The effect of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) and its metabolites on neurohypophysial hormone release from the isolated rat hypothalamus. Br J Pharm. 2002;135:649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nash JF, Roth BL, Brodkin JD, Nichols DE, Gudelsky GA. Effect of the R(-) and S(+) isomers of MDA and MDMA on phosphatidyl inositol turnover in cultured cells expressing 5-HT2A or 5-HT2C receptors. Neurosci Lett. 1994;177:111–5. [DOI] [PubMed] [Google Scholar]
- 9.Fantegrossi WE, Kiessel CL, De la Garza R 2nd, Woods JH. Serotonin synthesis inhibition reveals distinct mechanisms of action for MDMA and its enantiomers in the mouse. Psychopharmacology. 2005;181:529–36. [DOI] [PubMed] [Google Scholar]
- 10.Murnane KS, Fantegrossi WE, Godfrey JR, Banks ML, Howell LL. Endocrine and neurochemical effects of 3,4-methylenedioxymethamphetamine and its stereoisomers in rhesus monkeys. J Pharm Exp Ther. 2010;334:642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murnane KS, Murai N, Howell LL, Fantegrossi WE. Discriminative stimulus effects of psychostimulants and hallucinogens in S(+)-3,4-methylenedioxymethamphetamine (MDMA) and R(-)-MDMA trained mice. J Pharm Exp Ther. 2009;331:717–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitts EG, Curry DW, Hampshire KN, Young MB, Howell LL. (±)-MDMA and its enantiomers: potential therapeutic advantages of R(-)-MDMA. Psychopharmacology. 2018;235:377–92. [DOI] [PubMed] [Google Scholar]
- 13.Young R, Glennon RA. MDMA (N-methyl-3,4-methylenedioxyamphetamine) and its stereoisomers: similarities and differences in behavioral effects in an automated activity apparatus in mice. Pharm Biochem Behav. 2008;88:318–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frau L, Simola N, Plumitallo A, Morelli M. Microglial and astroglial activation by 3,4-methylenedioxymethamphetamine (MDMA) in mice depends on S(+) enantiomer and is associated with an increase in body temperature and motility. J Neurochem. 2013;124:69–78. [DOI] [PubMed] [Google Scholar]
- 15.Curry DW, Young MB, Tran AN, Daoud GE, Howell LL. Separating the agony from ecstasy: R(-)-3,4-methylenedioxymethamphetamine has prosocial and therapeutic-like effects without signs of neurotoxicity in mice. Neuropharmacology. 2018;128:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fantegrossi WE, Godlewski T, Karabenick RL, Stephens JM, Ullrich T, Rice KC, et al. Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (“ecstasy”) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology. 2003;166:202–11. [DOI] [PubMed] [Google Scholar]
- 17.Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G. 3,4-Methylenedioxymethamphetamine (MDMA, “ecstasy”) and its stereoisomers as reinforcers in rhesus monkeys: serotonergic involvement. Psychopharmacology. 2002;161:356–64. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Woolverton WL. Estimating the relative reinforcing strength of (±)-3,4-methylenedioxymethamphetamine (MDMA) and its isomers in rhesus monkeys: comparison to (+)-methamphetamine. Psychopharmacology. 2007;189:483–8. [DOI] [PubMed] [Google Scholar]
- 19.McClung J, Fantegrossi W, Howell LL. Reinstatement of extinguished amphetamine self-administration by 3,4-methylenedioxymethamphetamine (MDMA) and its enantiomers in rhesus monkeys. Psychopharmacology. 2010;210:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vizeli P, Liechti ME. Safety pharmacology of acute MDMA administration in healthy subjects. J Psychopharmacol. 2017;31:576–88. [DOI] [PubMed] [Google Scholar]
- 21.Anderson GM 3rd, Braun G, Braun U, Nichols DE, Shulgin AT. Absolute configuration and psychotomimetic activity. NIDA Res Monogr. 1978;22:8–15. [PubMed] [Google Scholar]
- 22.Janke W, Debus G. Die Eigenschaftswörterliste. Hogrefe: Göttingen; 1978.
- 23.Studerus E, Gamma A, Vollenweider FX. Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS One. 2010;5:e12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stocker K, Hartmann M, Ley L, Becker AM, Holze F, Liechti ME. The revival of the psychedelic experience scale: revealing its extended-mystical, visual, and distressing experiential spectrum with LSD and psilocybin studies. J Psychopharmacol. 2024;38:80–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology. 2006;187:268–83. [DOI] [PubMed] [Google Scholar]
- 26.Barrett FS, Johnson MW, Griffiths RR. Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. J Psychopharmacol. 2015;29:1182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zerssen DV. Die Beschwerden-Liste. Münchener Informationssystem. Psychis: München; 1976.
- 28.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. [DOI] [PubMed] [Google Scholar]
- 29.Derogatis LR, Rickels K, Rock AF. The SCL-90 and the MMPI: a step in the validation of a new self-report scale. Br J Psychiatry. 1976;128:280–9. [DOI] [PubMed] [Google Scholar]
- 30.Holze F, Avedisian I, Varghese N, Eckert A, Liechti ME. Role of the 5-HT2A receptor in acute effects of LSD on empathy and circulating oxytocin. Front Pharm. 2021;12:711255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holze F, Ley L, Muller F, Becker AM, Straumann I, Vizeli P, et al. Direct comparison of the acute effects of lysergic acid diethylamide and psilocybin in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacology. 2022;47:1180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luethi D, Rudin D, Straumann I, Thomann J, Avedisian I, Liechti ME, et al. Derivatization-free determination of chiral plasma pharmacokinetics of MDMA and its enantiomers. J Chromatogr B Anal Technol Biomed Life Sci. 2024;1238:124123. [DOI] [PubMed] [Google Scholar]
- 33.Paulus MP, Geyer MA. The effects of MDMA and other methylenedioxy-substituted phenylalkylamines on the structure of rat locomotor activity. Neuropsychopharmacology. 1992;7:15–31. [PubMed] [Google Scholar]
- 34.Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, et al. 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharm. 2003;63:1223–9. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt CJ, Levin JA, Lovenberg W. In vitro and in vivo neurochemical effects of methylenedioxymethamphetamine on striatal monoaminergic systems in the rat brain. Biochem Pharm. 1987;36:747–55. [DOI] [PubMed] [Google Scholar]
- 36.Straumann I, Ley L, Holze F, Becker AM, Klaiber A, Wey K, et al. Acute effects of MDMA and LSD co-administration in a double-blind placebo-controlled study in healthy participants. Neuropsychopharmacology. 2023;48:1840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mithoefer MC, Grob CS, Brewerton TD. Novel psychopharmacological therapies for psychiatric disorders: psilocybin and MDMA. Lancet Psychiatry. 2016;3:481–8. [DOI] [PubMed] [Google Scholar]
- 38.Kirkpatrick MG, Lee R, Wardle MC, Jacob S, de Wit H. Effects of MDMA and intranasal oxytocin on social and emotional processing. Neuropsychopharmacology. 2014;39:1654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitts EG, Minerva AR, Chandler EB, Kohn JN, Logun MT, Sulima A, et al. 3,4-Methylenedioxymethamphetamine increases affiliative behaviors in squirrel monkeys in a serotonin 2A receptor-dependent manner. Neuropsychopharmacology. 2017;42:1962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61:731–3. [DOI] [PubMed] [Google Scholar]
- 41.Hysek CM, Domes G, Liechti ME. MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacology. 2012;222:293–302. [DOI] [PubMed] [Google Scholar]
- 42.Kirkpatrick MG, Francis SM, Lee R, de Wit H, Jacob S. Plasma oxytocin concentrations following MDMA or intranasal oxytocin in humans. Psychoneuroendocrinology. 2014;46:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seibert J, Hysek CM, Penno CA, Schmid Y, Kratschmar DV, Liechti ME, et al. Acute effects of 3,4-methylenedioxymethamphetamine and methylphenidate on circulating steroid levels in healthy subjects. Neuroendocrinology. 2014;100:17–25. [DOI] [PubMed] [Google Scholar]
- 44.Luethi D, Liechti ME. Monoamine transporter and receptor interaction profiles in vitro predict reported human doses of novel psychoactive stimulants and psychedelics. Int J Neuropsychopharmacol. 2018;21:926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steuer AE, Schmidhauser C, Tingelhoff EH, Schmid Y, Rickli A, Kraemer T, et al. Impact of cytochrome P450 2D6 function on the chiral blood plasma pharmacokinetics of 3,4-Methylenedioxymethamphetamine (MDMA) and its phase I and II metabolites in humans. PLoS One. 2016;11:e0150955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steuer AE, Schmidhauser C, Schmid Y, Rickli A, Liechti ME, Kraemer T. Chiral plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine and its phase I and II metabolites following controlled administration to humans. Drug Metab Dispos. 2015;43:1864–71. [DOI] [PubMed] [Google Scholar]
- 47.Lo Faro AF, Sprega G, Beradinelli D, Tini A, Poyatos L, Papaseit E, et al. Development of enantioselective high-performance liquid chromatography-tandem mass spectrometry method for the quantitative determination of 3,4-methylenedioxy-methamphetamine (MDMA) and its phase-1 metabolites in human biological fluids. J Pharm Biomed Anal. 2024;238:115768. [DOI] [PubMed] [Google Scholar]
- 48.Schmid Y, Vizeli P, Hysek CM, Prestin K, Meyer zu Schwabedissen HE, Liechti ME. CYP2D6 function moderates the pharmacokinetics and pharmacodynamics of MDMA in a controlled study in healthy subjects. Pharmacogenet Genom. 2016;26:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peiro AM, Farre M, Roset PN, Carbo M, Pujadas M, Torrens M, et al. Human pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) after repeated doses taken 2 h apart. Psychopharmacology. 2013;225:883–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.