Abstract
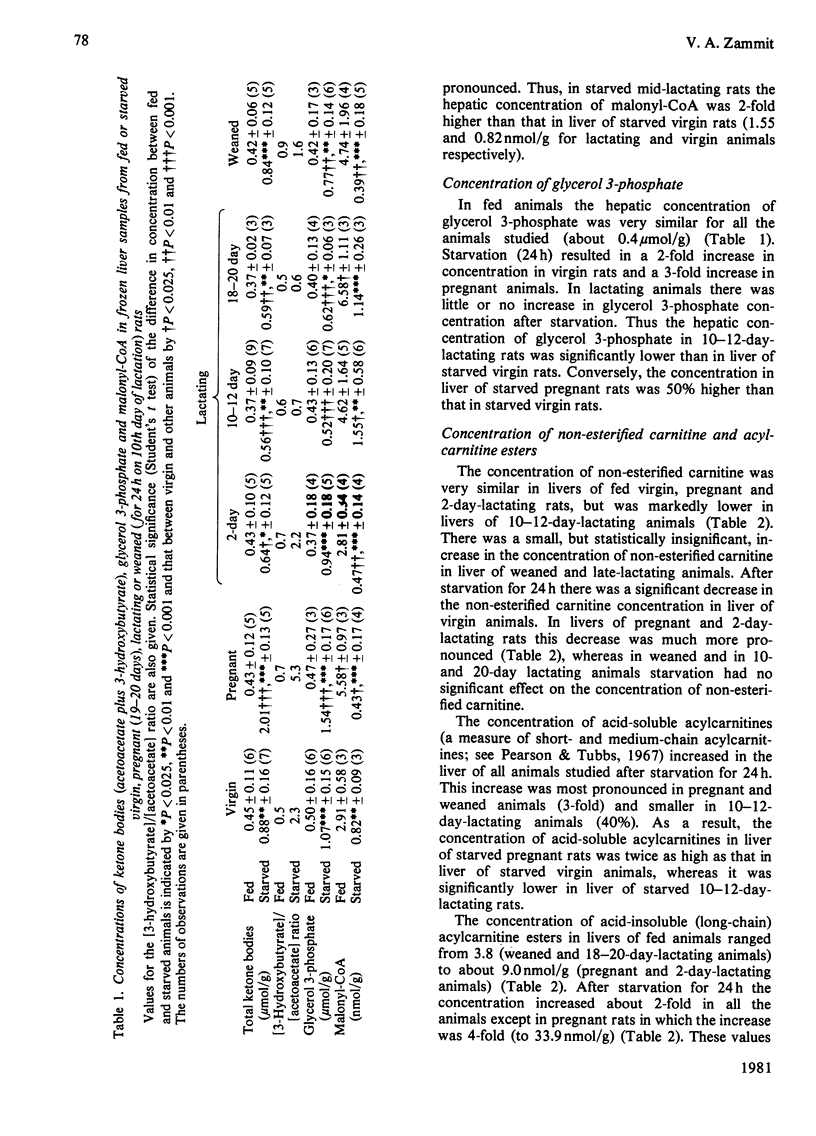
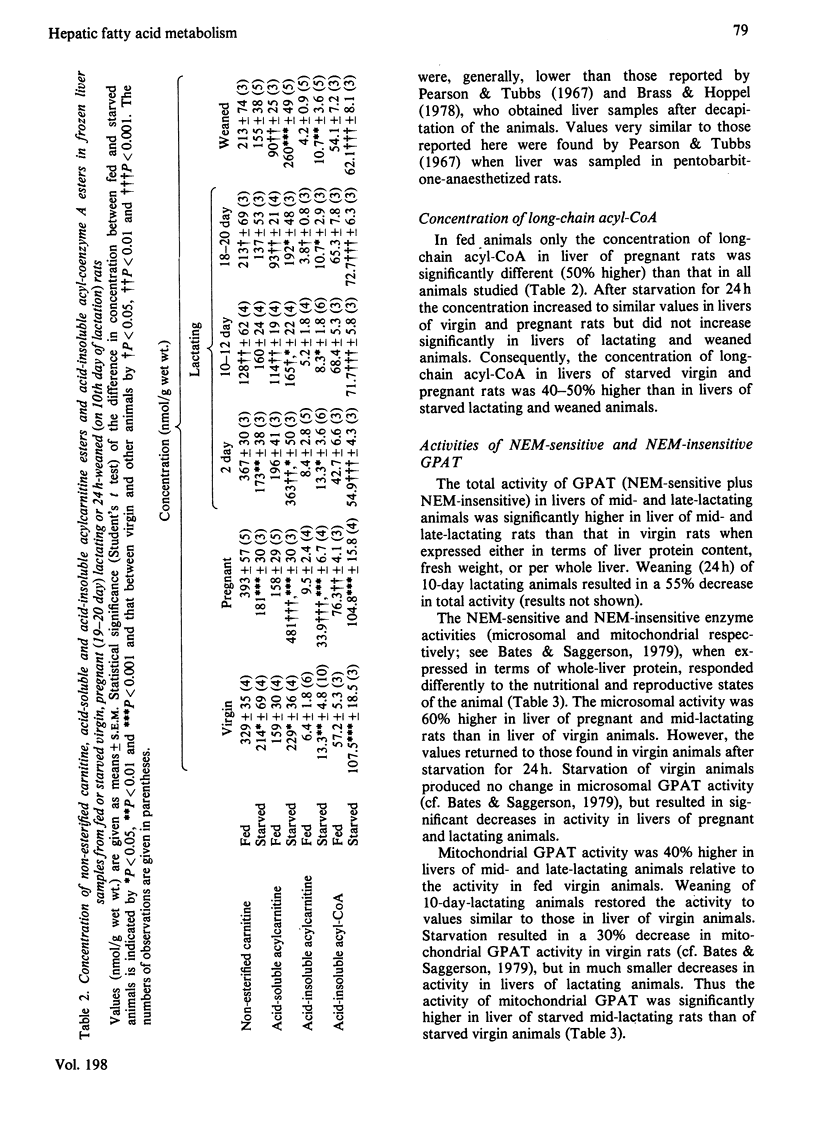
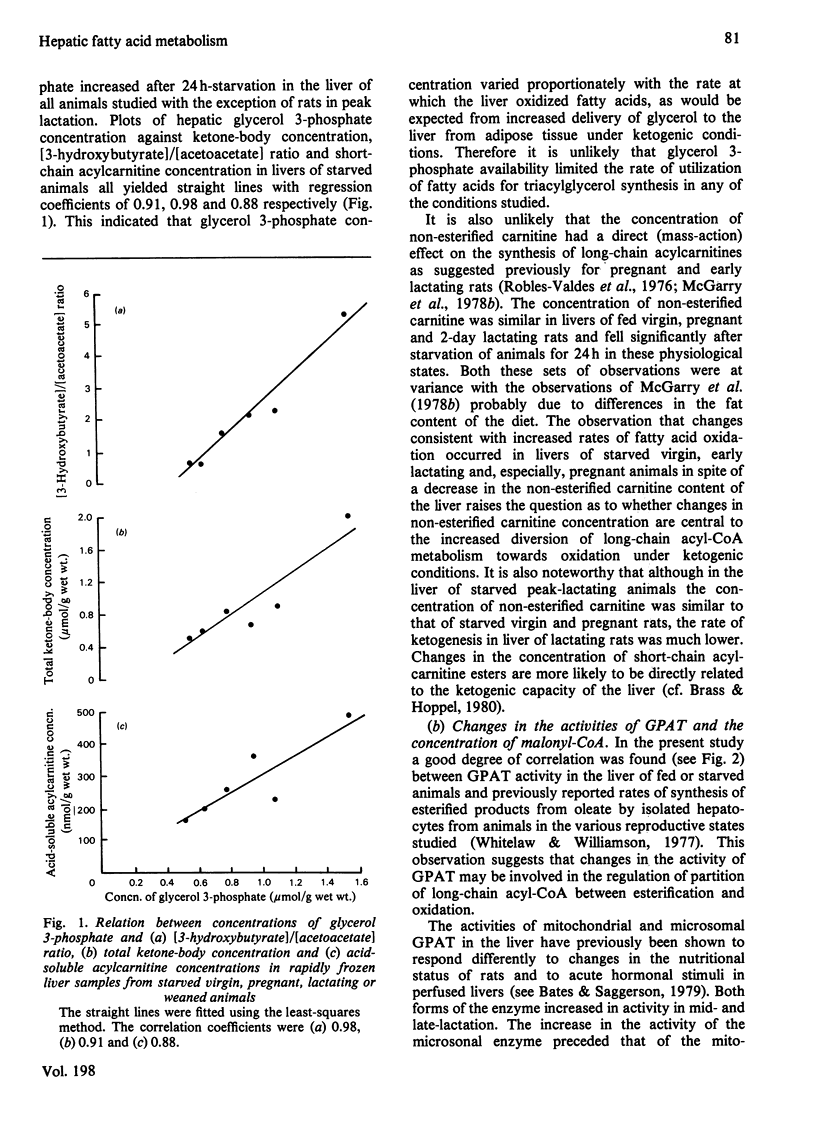
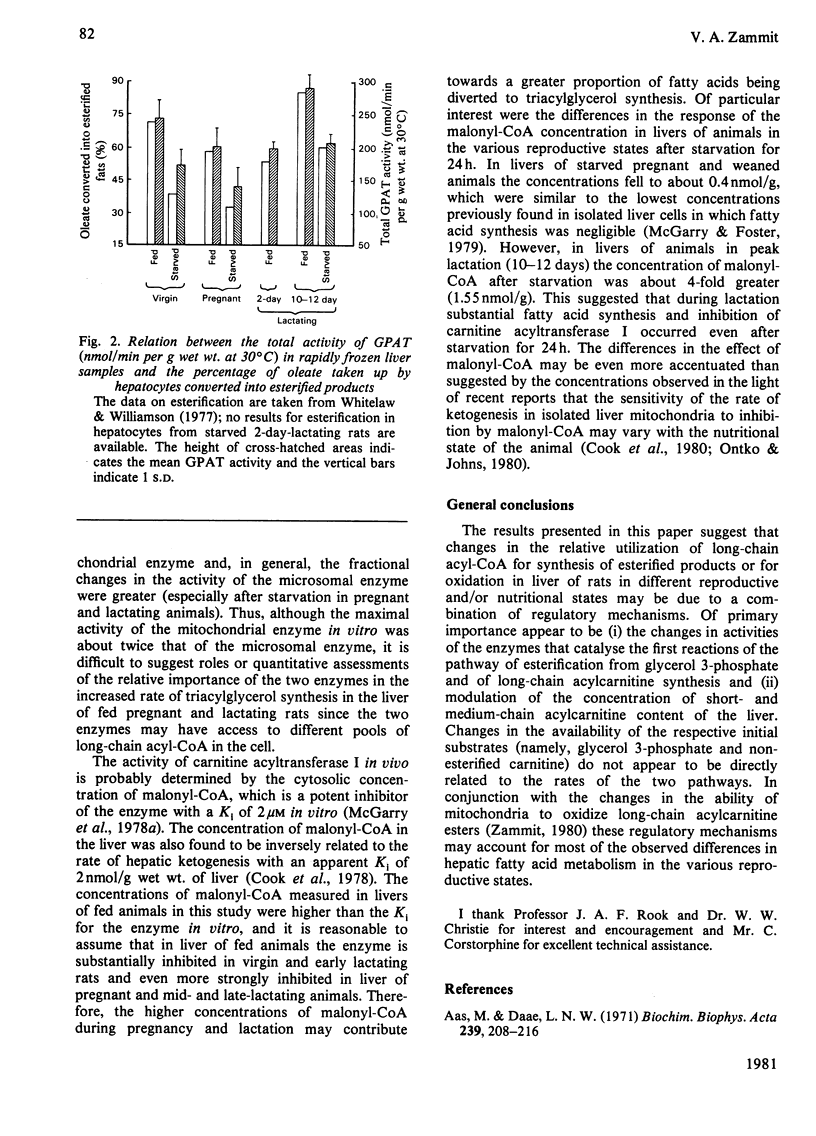
1. The concentrations of malonyl-CoA, glycerol 3-phosphate, non-esterified carnitine, acid-soluble and acid-insoluble acylcarnitines, acetoacetate, 3-hydroxybutyrate and acid-insoluble acyl-CoA were measured in rapidly-frozen liver samples from fed or starved (24h) virgin, pregnant (19–20 days), lactating (2, 10–12 and 18–20 days) and weaned (for 24h, on 10th day of lactation) rats. The activities of total and N-ethylmaleimide-sensitive and -insensitive glycerophosphate acyltransferase (acyl-CoA:sn-glycerol 3-phosphate O-acyltransferase; EC 2.3.1.15) were also measured. 2. The concentration of malonyl-CoA was significantly higher in liver of fed pregnant, mid- and late-lactating rats than in liver of fed virgin rats. After starvation for 24h hepatic malonyl-CoA concentrations were higher in mid-lactating rats and lower in pregnant and weaned rats than in virgin animals. 3. After starvation for 24h the hepatic concentrations of glycerol 3-phosphate, ketone bodies, acid-soluble acylcarnitines and the value for the [3-hydroxybutyrate]/[acetoacetate] ratio were all highest in pregnant rats, intermediate in virgin, 2-day lactating and weaned animals and lowest in mid- and late-lactating rats. The concentrations of acid-insoluble acylcarnitines also increased most in pregnant rats, after starvation. The concentration of acid-insoluble acyl-CoA increased equally after starvation in virgin and pregnant animals but did not increase significantly in all other animals studied. 4. The total concentration of carnitine was similar in livers of fed virgin, pregnant and 2-day lactating animals but fell markedly by the 10th day of lactation and remained low in late-lactating animals. The concentration of non-esterified carnitine followed the same pattern. After starvation for 24h the hepatic concentration of non-esterified carnitine decreased significantly in virgin, pregnant and 2-day lactating animals, but remained unchanged in mid- and late-lactating or weaned animals. 5. The activities of N-ethylmaleimide-sensitive and -insensitive glycerophosphate acyltransferase both increased significantly in livers of mid-lactating animals. After starvation for 24h the activity of the N-ethylmaleimide-insensitive O-acyltransferase decreased in livers of virgin, pregnant and mid-lactating animals, whereas the activity of the N-ethylmaleimide-sensitive O-acyltransferase was unchanged in virgin animals but decreased markedly in livers of pregnant and lactating rats. 6. The results are discussed in relation to the importance of different metabolic parameters in the regulation of long-chain acyl-CoA metabolism in the liver.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aas M., Daae L. N. Fatty acid activation and acyl transfer in organs from rats in different nutritional states. Biochim Biophys Acta. 1971 Jul 13;239(2):208–216. doi: 10.1016/0005-2760(71)90166-4. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Saggerson E. D. A study of the glycerol phosphate acyltransferase and dihydroxyacetone phosphate acyltransferase activities in rat liver mitochondrial and microsomal fractions. Relative distribution in parenchymal and non-parenchymal cells, effects of N-ethylmaleimide, palmitoyl-coenzyme A concentration, starvation, adrenalectomy and anti-insulin serum treatment. Biochem J. 1979 Sep 15;182(3):751–762. doi: 10.1042/bj1820751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynen A. C., Vaartjes W. J., Geelen M. J. Opposite effects of insulin and glucagon in acute hormonal control of hepatic lipogenesis. Diabetes. 1979 Sep;28(9):828–835. doi: 10.2337/diab.28.9.828. [DOI] [PubMed] [Google Scholar]
- Brass E. P., Hoppel C. L. Carnitine metabolism in the fasting rat. J Biol Chem. 1978 Apr 25;253(8):2688–2693. [PubMed] [Google Scholar]
- Brass E. P., Hoppel C. L. Relationship between acid-soluble carnitine and coenzyme A pools in vivo. Biochem J. 1980 Sep 15;190(3):495–504. doi: 10.1042/bj1900495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D. N., Haavik A. G., Porter J. W. Comparative studies of the rat and pigeon liver fatty acid synthetases. Arch Biochem Biophys. 1968 Jul;126(1):141–154. doi: 10.1016/0003-9861(68)90568-7. [DOI] [PubMed] [Google Scholar]
- Cederblad G., Lindstedt S. A method for the determination of carnitine in the picomole range. Clin Chim Acta. 1972 Mar;37:235–243. doi: 10.1016/0009-8981(72)90438-x. [DOI] [PubMed] [Google Scholar]
- Cook G. A., King M. T., Veech R. L. Ketogenesis and malonyl coenzyme A content of isolated rat hepatocytes. J Biol Chem. 1978 Apr 25;253(8):2529–2531. [PubMed] [Google Scholar]
- Cook G. A., Otto D. A., Cornell N. W. Differential inhibition of ketogenesis by malonyl-CoA in mitochondria from fed and starved rats. Biochem J. 1980 Dec 15;192(3):955–958. doi: 10.1042/bj1920955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco J. P., Hoppel C. Hepatic mitochondrial function in ketogenic states. Diabetes, starvation, and after growth hormone administration. J Clin Invest. 1975 Jun;55(6):1237–1244. doi: 10.1172/JCI108042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge A. G. Regulation of the activity of acetyl coenzyme A carboxylase by palmitoyl coenzyme A and citrate. J Biol Chem. 1972 Nov 10;247(21):6946–6952. [PubMed] [Google Scholar]
- Halperin M. L., Robinson B. H., Fritz I. B. Effects of palmitoyl CoA on citrate and malate transport by rat liver mitochondria. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1003–1007. doi: 10.1073/pnas.69.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. A., Williamson D. H. Measurements of substrate uptake by mammary gland of the rat. Biochem J. 1972 Oct;129(5):1171–1173. doi: 10.1042/bj1291171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund H., Borrebaek B., Bremer J. Regulation of palmitate esterification/oxidation by glucagon in isolated hepatocytes: the role of alpha-glycerophosphate concentration. Biochim Biophys Acta. 1980 Dec 5;620(3):364–371. doi: 10.1016/0005-2760(80)90128-9. [DOI] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. Regulation of fat metabolism of the liver. Nature. 1967 Aug 12;215(5102):716–718. doi: 10.1038/215716a0. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. In support of the roles of malonyl-CoA and carnitine acyltransferase I in the regulation of hepatic fatty acid oxidation and ketogenesis. J Biol Chem. 1979 Sep 10;254(17):8163–8168. [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. Characteristics of fatty acid oxidation in rat liver homogenates and the inhibitory effect of malonyl-CoA. Biochim Biophys Acta. 1978 Sep 28;530(3):305–313. doi: 10.1016/0005-2760(78)90150-9. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Robles-Valdes C., Foster D. W. Role of carnitine in hepatic ketogenesis. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4385–4388. doi: 10.1073/pnas.72.11.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Takabayashi Y., Foster D. W. The role of malonyl-coa in the coordination of fatty acid synthesis and oxidation in isolated rat hepatocytes. J Biol Chem. 1978 Nov 25;253(22):8294–8300. [PubMed] [Google Scholar]
- Ontko J. A., Johns M. L. Evaluation of malonyl-CoA in the regulation of long-chain fatty acid oxidation in the liver. Evidence for an unidentified regulatory component of the system. Biochem J. 1980 Dec 15;192(3):959–962. doi: 10.1042/bj1920959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson D. J., Tubbs P. K. Carnitine and derivatives in rat tissues. Biochem J. 1967 Dec;105(3):953–963. doi: 10.1042/bj1050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Valdes C., McGarry J. D., Foster D. W. Maternal-fetal carnitine relationship and neonatal ketosis in the rat. J Biol Chem. 1976 Oct 10;251(19):6007–6012. [PubMed] [Google Scholar]
- Vavrecka M., Mitchell M. P., Hübscher G. The effect of starvation on the incorporation of palmitate into glycerides and phospholipids of rat liver homogenates. Biochem J. 1969 Nov;115(2):139–145. doi: 10.1042/bj1150139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Wasfi I., Weinstein I., Heimberg M. Hepatic metabolism of [1-14C]oleate in pregnancy. Biochim Biophys Acta. 1980 Sep 8;619(3):471–481. doi: 10.1016/0005-2760(80)90099-5. [DOI] [PubMed] [Google Scholar]
- Whitelaw E., Williamson D. H. Effects of lactation of ketogenesis from oleate or butyrate in rat hepatocytes. Biochem J. 1977 Jun 15;164(3):521–528. doi: 10.1042/bj1640521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H. Recent developments in ketone-body metabolism. Biochem Soc Trans. 1979 Dec;7(6):1313–1321. doi: 10.1042/bst0071313. [DOI] [PubMed] [Google Scholar]
- Zammit V. A. The effect of glucagon treatment and starvation of virgin and lactating rats on the rates of oxidation of octanoyl-L-carnitine and octanoate by isolated liver mitochondria. Biochem J. 1980 Aug 15;190(2):293–300. doi: 10.1042/bj1900293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol A. The effect of fasting on the acylation of carnitine and glycerophosphate in rat liver subcellular fractions. Biochim Biophys Acta. 1974 Jul 25;357(1):14–23. doi: 10.1016/0005-2728(74)90107-8. [DOI] [PubMed] [Google Scholar]


