Abstract
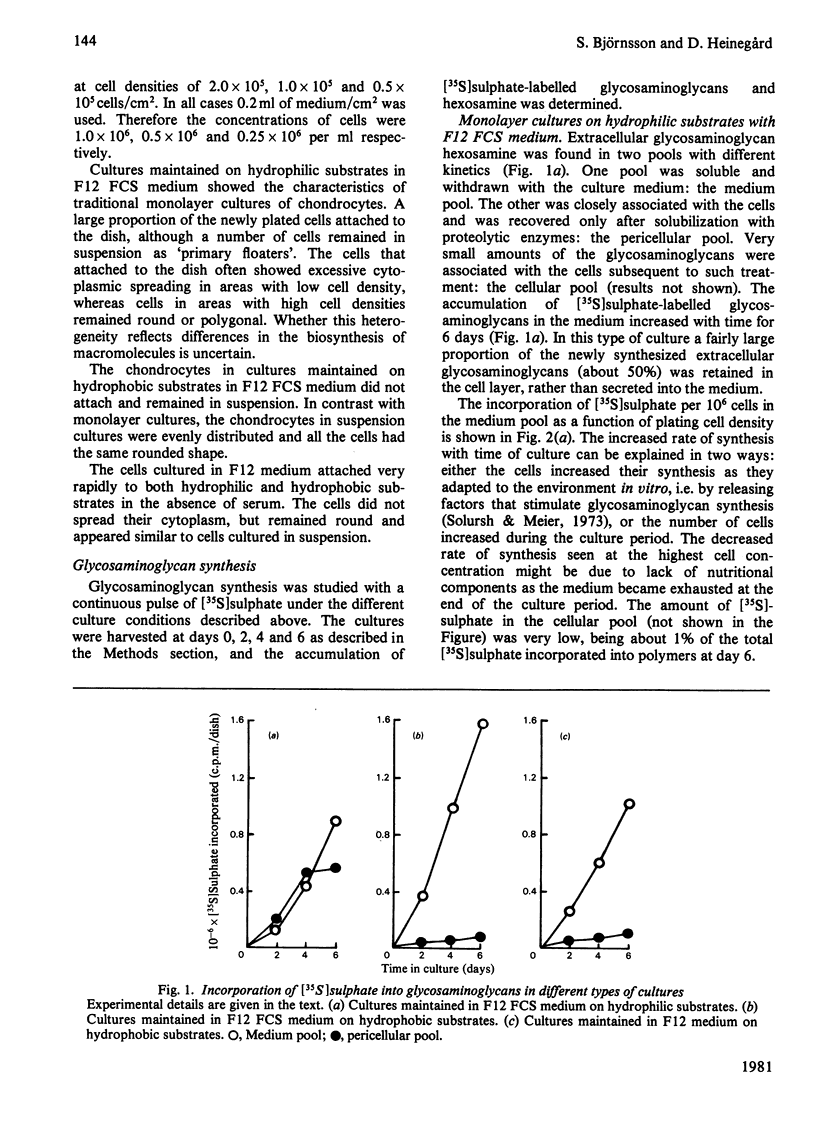
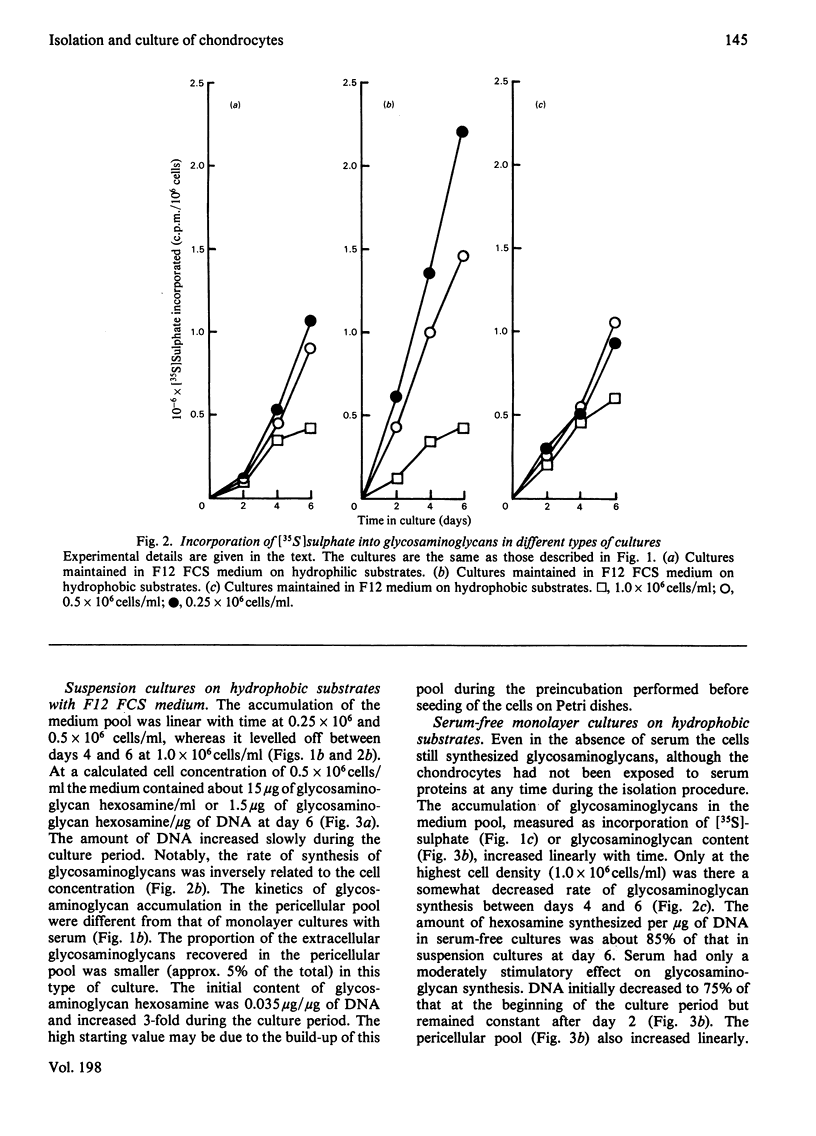
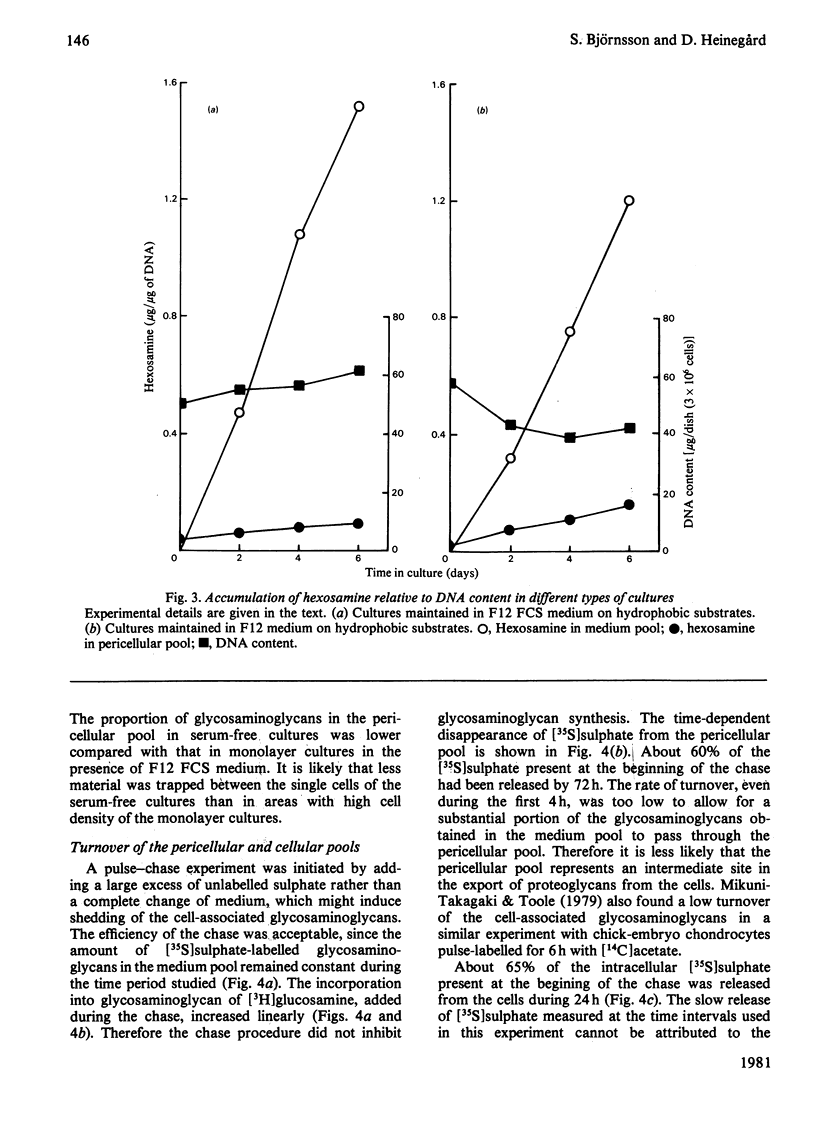
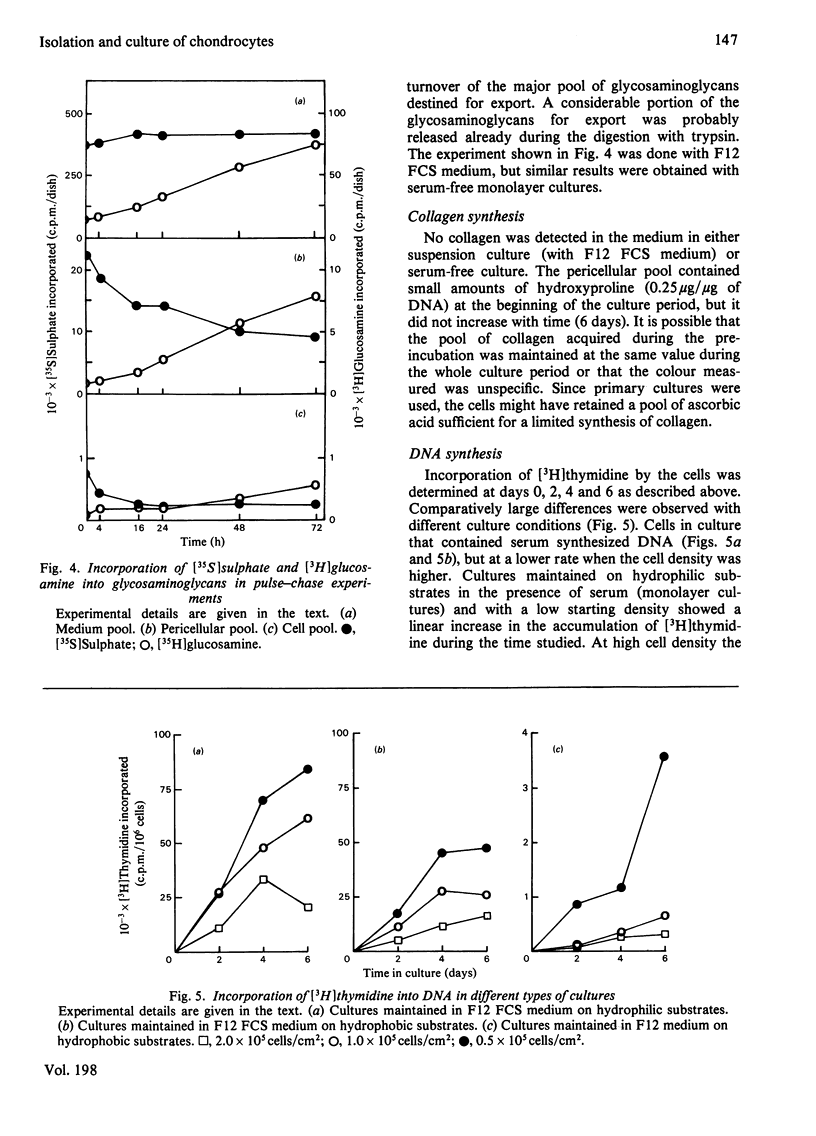
Large quantities of differentiated mammalian chondrocytes from normal hyaline cartilage were isolated after digestion of foetal bovine tracheas with collagenase. Incubation of the newly isolated cells for 1 day in the presence of dextran sulphate inhibited formation of cell aggregates during subsequent subculture in the absence of dextran sulphate. After incubation with dextran sulphate, the cells were plated in Ham's F12 medium with or without foetal calf serum on hydrophilic or hydrophobic Petri dishes. Chondrocytes cultured on hydrophilic substrates in the presence of serum attached to the substrate and showed cytoplasmic spreading. The cells did not attach to hydrophobic substrates in the presence of serum, but remained in suspension as single cells. In the absence of serum the chondrocytes attached to either substrate, but did not show any cytoplasmic spreading. By using labelling with [35S]sulphate and [3H]-thymidine it was shown that glycosaminoglycan synthesis did not require the presence of serum, whereas DNA synthesis required serum factors. Extracellular glycosaminoglycans were recovered in two pools: the medium pool and the pericellular pool, the latter being isolated by proteolytic digestion. The kinetics of these pools differed, depending on the presence or absence of serum and the type of substrate used. The turnover of the pericellular pool was studied in a pulse-chase experiment. At the end of the chase (72 h), only 60% of the material in the pericellular pool had been metabolized.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONOPOULOS C. A., GARDELL S., SZIRMAI J. A., DETYSSONSK E. R. DETERMINATION OF GLYCOSAMINOGLYCANS (MUCOPOLYSACCHARIDES) FROM TISSUE ON THE MICROGRAM SCALE. Biochim Biophys Acta. 1964 Mar 2;83:1–19. doi: 10.1016/0926-6526(64)90045-x. [DOI] [PubMed] [Google Scholar]
- Abbott J., Holtzer H. The loss of phenotypic traits by differentiated cells. 3. The reversible behavior of chondrocytes in primary cultures. J Cell Biol. 1966 Mar;28(3):473–487. doi: 10.1083/jcb.28.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremerskov V. Dextran sulphate inhibits cell adhesion in tissue culture. Nat New Biol. 1973 Dec 12;246(154):174–174. doi: 10.1038/newbio246174a0. [DOI] [PubMed] [Google Scholar]
- Coon H. G., Cahn R. D. Differentiation in vitro: effects of Sephadex fractions of chick embryo extract. Science. 1966 Sep 2;153(3740):1116–1119. doi: 10.1126/science.153.3740.1116. [DOI] [PubMed] [Google Scholar]
- KURODA Y. STUDIES ON CARTILAGE CELLS IN VITRO. II. CHANGES IN AGGREGATION AND IN CARTILAGE-FORMING ACTIVITY OF CELLS MAINTAINED IN MONOLAYER CULTURES. Exp Cell Res. 1964 Jul;35:337–348. doi: 10.1016/0014-4827(64)90100-4. [DOI] [PubMed] [Google Scholar]
- Madsen K., Lohmander S. Production of cartilage-typic proteoglycans in cultures of chondrocytes from elastic cartilage. Arch Biochem Biophys. 1979 Aug;196(1):192–198. doi: 10.1016/0003-9861(79)90566-6. [DOI] [PubMed] [Google Scholar]
- Mikuni-Takagaki Y., Toole B. P. Shedding of hyaluronate from the cell surface of Rous sarcoma virus-transformed chondrocytes. J Biol Chem. 1979 Sep 10;254(17):8409–8415. [PubMed] [Google Scholar]
- Müller P. K., Lemmen C., Gay S., Gauss V., Kühn K. Immunochemical and biochemical study of collagen synthesis by chondrocytes in culture. Exp Cell Res. 1977 Aug;108(1):47–55. [PubMed] [Google Scholar]
- Nevo Z., Horwitz A. L., Dorfmann A. Synthesis of chondromucoprotein by chondrocytes in suspension culture. Dev Biol. 1972 May;28(1):219–228. doi: 10.1016/0012-1606(72)90139-x. [DOI] [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Shipman C., Jr Evaluation of 4-(2-hydroxyethyl)-1-piperazineëthanesulfonic acid (HEPES) as a tissue culture buffer. Proc Soc Exp Biol Med. 1969 Jan;130(1):305–310. doi: 10.3181/00379727-130-33543. [DOI] [PubMed] [Google Scholar]
- Solursh M., Meier S. A conditioned medium (CM) factor produced by chondrocytes that promotes their own differentiation. Dev Biol. 1973 Feb;30(2):279–289. doi: 10.1016/0012-1606(73)90089-4. [DOI] [PubMed] [Google Scholar]
- Srivastava V. M., MaleMud C. J., Sokoloff L. Chondroid expression by lapine articular chondrocytes in spinner culture following monolayer growth. Connect Tissue Res. 1974;2(2):127–136. doi: 10.3109/03008207409152098. [DOI] [PubMed] [Google Scholar]
- Stegemann H., Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967 Nov;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Wiebkin O. W., Muir H. Synthesis of proteoglycans by suspension and monolayer cultures of adult chondrocytes and de novo cartilage nodules-the effect of hyaluronic acid. J Cell Sci. 1977;27:199–211. doi: 10.1242/jcs.27.1.199. [DOI] [PubMed] [Google Scholar]
- von der Mark K., von der Mark H. Immunological and biochemical studies of collagen type transition during in vitro chrondrogenesis of chick limb mesodermal cells. J Cell Biol. 1977 Jun;73(3):736–747. doi: 10.1083/jcb.73.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]


