Abstract
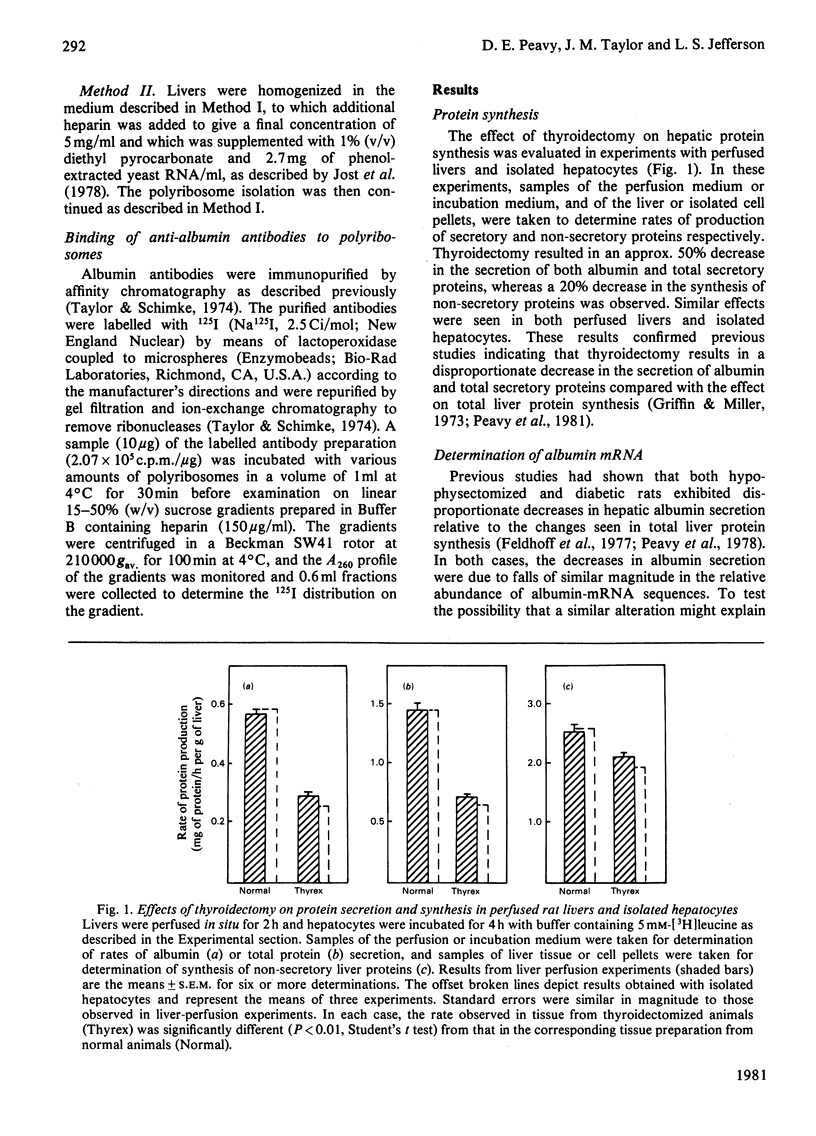
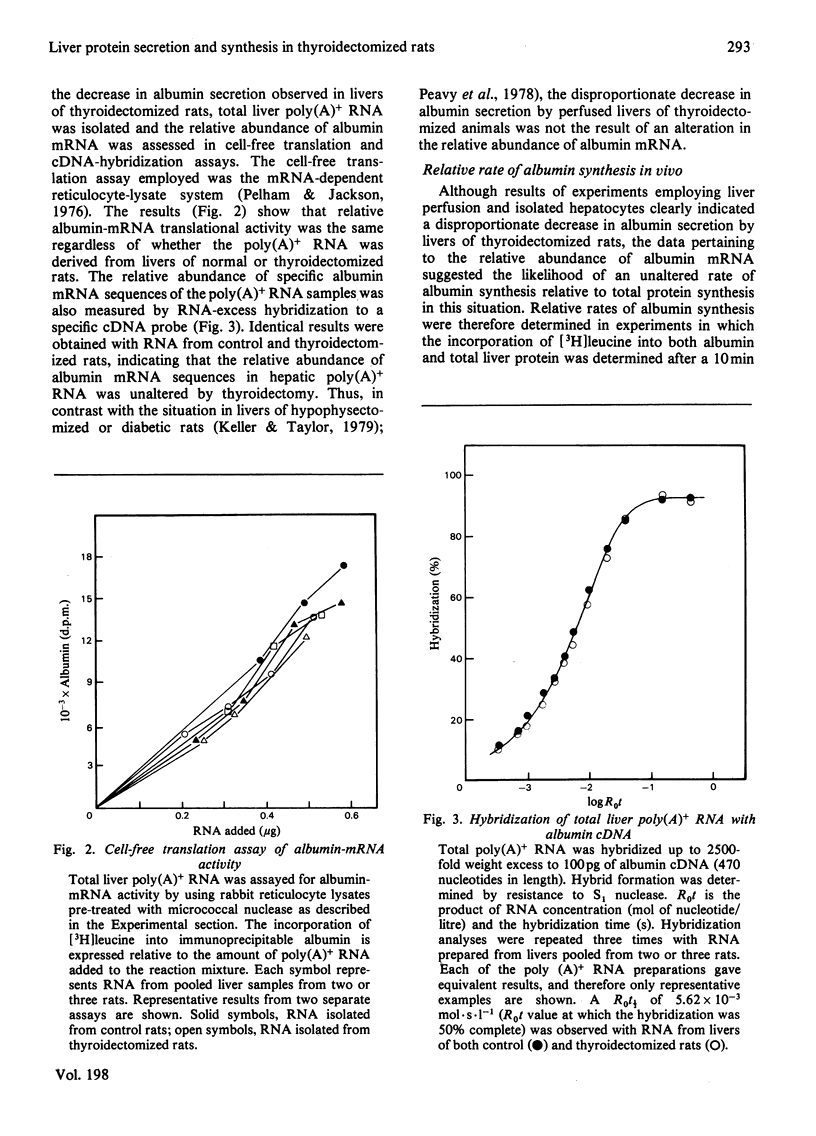
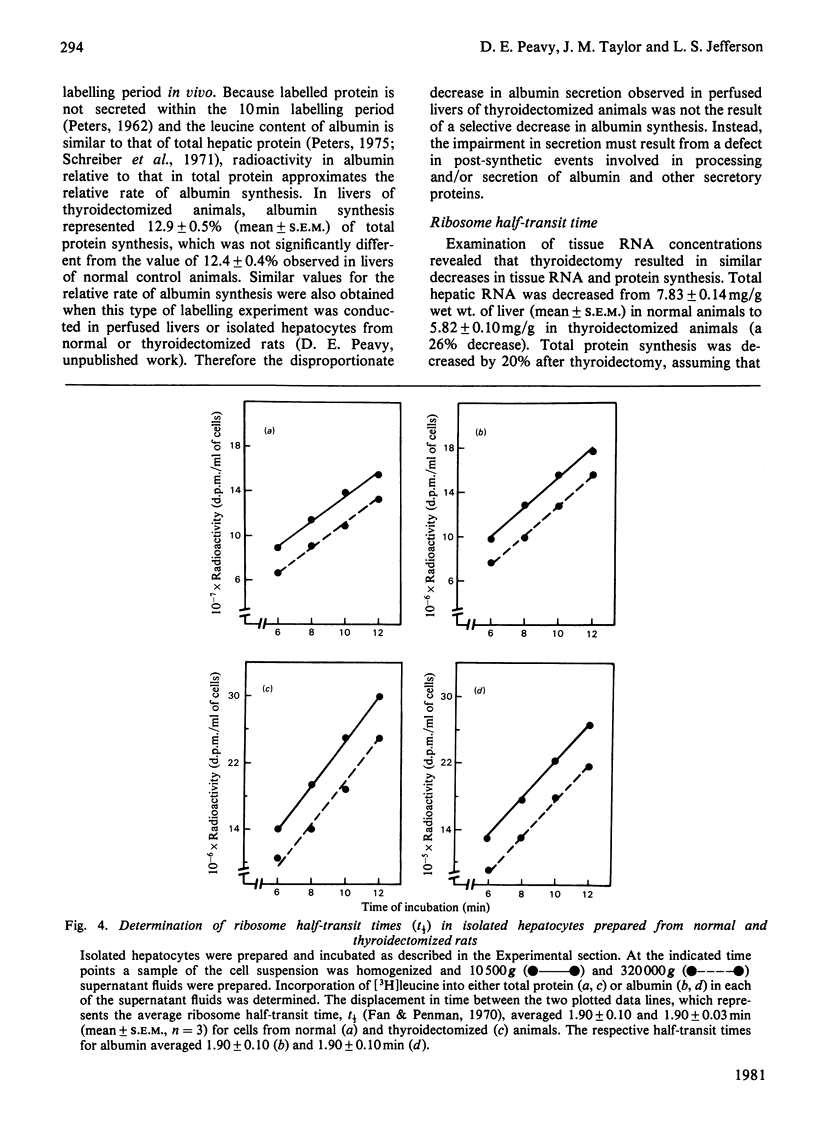
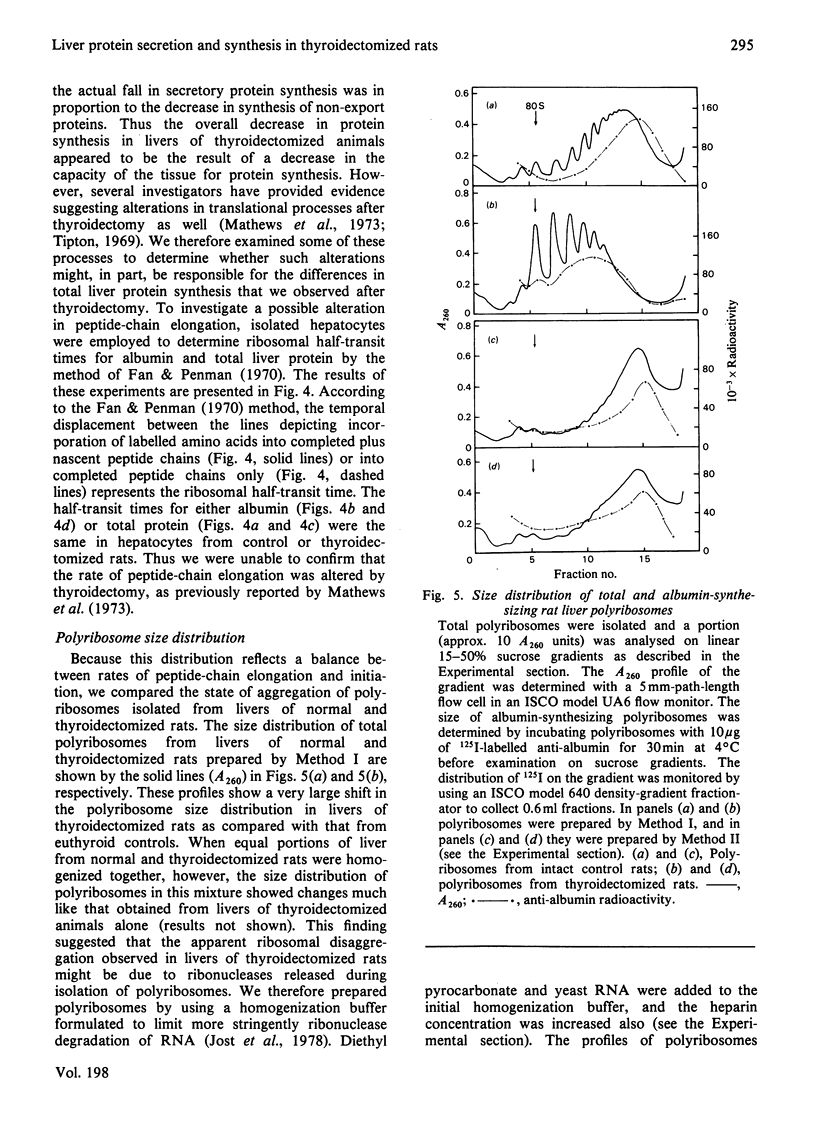
Perfused rat livers and isolated rat hepatocytes exhibited a 50% decrease in the secretion of both albumin and total secretory proteins after thyroidectomy. In contrast, synthesis of non-secretory proteins was decreased by only 20% from the rates observed in liver preparations from euthyroid rats. These observations suggested a disproportionate effect of thyroidectomy on the synthesis of secretory proteins compared with non-secretory proteins. Disproportionate decreases in the synthesis of albumin in other endocrine-deficient states such as hypophysectomy and diabetes had previously been shown to be associated with decreases of similar magnitude in the relative abundance of albumin-mRNA sequences. In contrast, thyroidectomy did not affect the activity or amount of albumin mRNA in total liver poly(A)-containing RNA when assayed by cell-free translation and by hybridization with complementary DNA, respectively. Furthermore, labelling experiments in vivo demonstrated that albumin synthesis represented 12.9 +/- 0.5% and 12.4 +/- 0.4% of total protein synthesis in livers of thyroidectomized and euthyroid rats respectively. Therefore the fall in secretion of albumin and total secretory protein after thyroidectomy did not appear to be a reflection of disproportionate decreases in the synthesis of these proteins. Instead, defects in steps involved in the post-synthetic processing and secretion of albumin are suggested. A number of comparisons, including ribosome half-transit times, the size distributions of total and albumin-synthesizing polyribosomes, and the fraction of RNA present as inactive ribosomes, provided evidence that the overall decrease in protein synthesis after thyroidectomy was not due to generalized alterations in translational processes. Instead, the decrease in total protein synthesis appeared to reflect the RNA content of the liver, which fell in proportion to th decrease in protein synthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALL E. G., KNOBIL E. Insulinlike activity of serum from normal and hypophysectomized monkeys. Endocrinology. 1963 Apr;72:658–661. doi: 10.1210/endo-72-4-658. [DOI] [PubMed] [Google Scholar]
- Coiro V., Braverman L. E., Christianson D., Fang S. L., Goodman H. M. Effect of hypothyroidism and thyroxine replacement on growth hormone in the rat. Endocrinology. 1979 Sep;105(3):641–646. doi: 10.1210/endo-105-3-641. [DOI] [PubMed] [Google Scholar]
- Dillmann W. H., Mendecki J., Koerner D., Schwartz H. L., Oppenheimer J. H. Triiodothyronine-stimulated formation of poly(A)-containing nuclear RNA and mRNA in rat liver. Endocrinology. 1978 Feb;102(2):568–575. doi: 10.1210/endo-102-2-568. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Feldhoff R. C., Taylor J. M., Jefferson L. S. Synthesis and secretion of rat albumin in vivo, in perfused liver, and in isolated hepatocytes. Effects of hypophysectomy and growth hormone treatment. J Biol Chem. 1977 Jun 10;252(11):3611–3616. [PubMed] [Google Scholar]
- González C., Montoya E., Jolín T. Effect of streptozotocin diabetes on the hypothalamic-pituitary-thyroid axis in the rat. Endocrinology. 1980 Dec;107(6):2099–2103. doi: 10.1210/endo-107-6-2099. [DOI] [PubMed] [Google Scholar]
- Griffin E. E., Miller L. L. Effects of hypothyroidism, hyperthyroidism, and thyroxine on net synthesis of plasma proteins by the isolated perfused rat liver. Modulation of the response to insulin plus cortisol in the net synthesis of albumin, fibrinogen, 1-acid glycorprotein, 2-(acute phase) globulin, and haptoglobin. J Biol Chem. 1973 Jul 10;248(13):4716–4723. [PubMed] [Google Scholar]
- Halban P. A., Wollheim C. B. Intracellular degradation of insulin stores by rat pancreatic islets in vitro. An alternative pathway for homeostasis of pancreatic insulin content. J Biol Chem. 1980 Jul 10;255(13):6003–6006. [PubMed] [Google Scholar]
- Haschemeyer A. E. Rates of polypeptide chain assembly in liver in vivo: relation to the mechanism of temperature acclimation in Opsanus tau. Proc Natl Acad Sci U S A. 1969 Jan;62(1):128–135. doi: 10.1073/pnas.62.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolin T., Morreale de Escobar G., Escobar del Rey F. Differential effects in the rat thyroidectomy, propylthiouracil and other goitrogens on plasma insulin and thyroid weight. Endocrinology. 1970 Jul;87(1):99–110. doi: 10.1210/endo-87-1-99. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Pehling G., Panyim S., Ohno T. An improved method for isolation of active vitellogenin messenger RNA from chicken liver. Use of diethylpyrocarbonate. Biochim Biophys Acta. 1978 Feb 16;517(2):338–348. doi: 10.1016/0005-2787(78)90200-9. [DOI] [PubMed] [Google Scholar]
- Keller G. H., Taylor J. M. Effect of hypophysectomy and growth hormone treatment on albumin mRNA levels in the rat liver. J Biol Chem. 1979 Jan 25;254(2):276–278. [PubMed] [Google Scholar]
- Keller G. H., Taylor J. M. Effect of hypophysectomy on the synthesis of rat liver albumin. J Biol Chem. 1976 Jun 25;251(12):3768–3773. [PubMed] [Google Scholar]
- Keller G. H., Taylor J. M. Synthesis of a complementary DNA to rat liver albumin mRNA. Biochem Biophys Res Commun. 1977 Jul 11;77(1):328–334. doi: 10.1016/s0006-291x(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Khairallah E. A., Mortimore G. E. Assessment of protein turnover in perfused rat liver. Evidence for amino acid compartmentation from differential labeling of free and tRNA-gound valine. J Biol Chem. 1976 Mar 10;251(5):1375–1384. [PubMed] [Google Scholar]
- Kurtz D. T., Sippel A. E., Feigelson P. Effect of thyroid hormones on the level of the hepatic mRNA for alpha2u globulin. Biochemistry. 1976 Mar 9;15(5):1031–1036. doi: 10.1021/bi00650a013. [DOI] [PubMed] [Google Scholar]
- Martial J. A., Baxter J. D., Goodman H. M., Seeburg P. H. Regulation of growth hormone messenger RNA by thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 May;74(5):1816–1820. doi: 10.1073/pnas.74.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews R. W., Oronsky A., Haschemeyer A. E. Effect of thyroid hormone on polypeptide chain assembly kinetics in liver protein synthesis in vivo. J Biol Chem. 1973 Feb 25;248(4):1329–1333. [PubMed] [Google Scholar]
- Morrissey J. J., Cohn D. V. Secretion and degradation of parathormone as a function of intracellular maturation of hormone pools. Modulation by calcium and dibutyryl cyclic AMP. J Cell Biol. 1979 Dec;83(3):521–528. doi: 10.1083/jcb.83.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS T., Jr The biosynthesis of rat serum albumin. II. Intracellular phenomena in the secretion of newly formed albumin. J Biol Chem. 1962 Apr;237:1186–1189. [PubMed] [Google Scholar]
- Palmiter R. D. Regulation of protein synthesis in chick oviduct. II. Modulation of polypeptide elongation and initiation rates by estrogen and progesterone. J Biol Chem. 1972 Nov 10;247(21):6770–6780. [PubMed] [Google Scholar]
- Peavy D. E., Taylor J. M., Jefferson L. S. Correlation of albumin production rates and albumin mRNA levels in livers of normal, diabetic, and insulin-treated diabetic rats. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5879–5883. doi: 10.1073/pnas.75.12.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy D. E., Taylor J. M., Jefferson L. S. Protein synthesis in perfused rat liver following thyroidectomy and hormone treatment. Am J Physiol. 1981 Jan;240(1):E18–E23. doi: 10.1152/ajpendo.1981.240.1.E18. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Redman C. M., Yu S., Banerjee D., Morris H. P. In vitro synthesis and secretion of albumin by Morris hepatomas 5123C and 7800. Cancer Res. 1979 Jan;39(1):101–111. [PubMed] [Google Scholar]
- Schreiber G., Urban J., Zähringer J., Reutter W., Frosch U. The secretion of serum protein and the synthesis of albumin and total protein in regenerating rat liver. J Biol Chem. 1971 Jul 25;246(14):4531–4538. [PubMed] [Google Scholar]
- Seo H., Vassart G., Brocas H., Refetoff S. Triiodothyronine stimulates specifically growth hormone mRNA in rat pituitary tumor cells. Proc Natl Acad Sci U S A. 1977 May;74(5):2054–2058. doi: 10.1073/pnas.74.5.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenai R., Wallis M. Biosynthesis and degradation of prolactin in the rat anterior pituitary gland. Time course of incorporation of label in vitro and evidence for rapid degradation. Biochem J. 1979 Sep 15;182(3):735–743. doi: 10.1042/bj1820735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Schimke R. T. Specific binding of albumin antibody to rat liver polysomes. J Biol Chem. 1974 Jun 10;249(11):3597–3601. [PubMed] [Google Scholar]
- Taylor J. M., Schimke R. T. Synthesis of rat liver albumin in a rabbit reticulocyte cell-free protein-synthesizing system. J Biol Chem. 1973 Nov 25;248(22):7661–7668. [PubMed] [Google Scholar]
- Taylor J. M., Tse T. P. Isolation of rat liver albumin messenger RNA. J Biol Chem. 1976 Dec 10;251(23):7461–7467. [PubMed] [Google Scholar]
- Tipton S. R. Some effects of thyroid hormone on polyribosomes in rat liver. Ala J Med Sci. 1969 Jul;6(3):259–265. [PubMed] [Google Scholar]
- Tolman E. L., Schworer C. M., Jefferson L. S. Effects of hypophysectomy on amino acid metabolism and gluconeogenesis in the perfused rat liver. J Biol Chem. 1973 Jul 10;248(13):4552–4560. [PubMed] [Google Scholar]
- Towle H. C., Dillmann W. H., Oppenheimer J. H. Messenger RNA content and complexity of euthyroid and hypothyroid rat liver. J Biol Chem. 1979 Apr 10;254(7):2250–2257. [PubMed] [Google Scholar]
- Towle H. C., Mariash C. N., Oppenheimer J. H. Changes in the hepatic levels of messenger ribonucleic acid for malic enzyme during induction by thyroid hormone or diet. Biochemistry. 1980 Feb 5;19(3):579–585. doi: 10.1021/bi00544a029. [DOI] [PubMed] [Google Scholar]
- Uenoyama K., Ono T. Synthesis of albumin by the free polyribosomes in 5123 hepatoma. Biochim Biophys Acta. 1972 Sep 29;281(1):124–129. doi: 10.1016/0005-2787(72)90194-3. [DOI] [PubMed] [Google Scholar]
- Zaninovich A. A., Brown T. J., Boado R., Bromage N. R., Matty A. J. Thyroxine metabolism in diabetic rats. Acta Endocrinol (Copenh) 1977 Oct;86(2):336–343. doi: 10.1530/acta.0.0860336. [DOI] [PubMed] [Google Scholar]


