Abstract
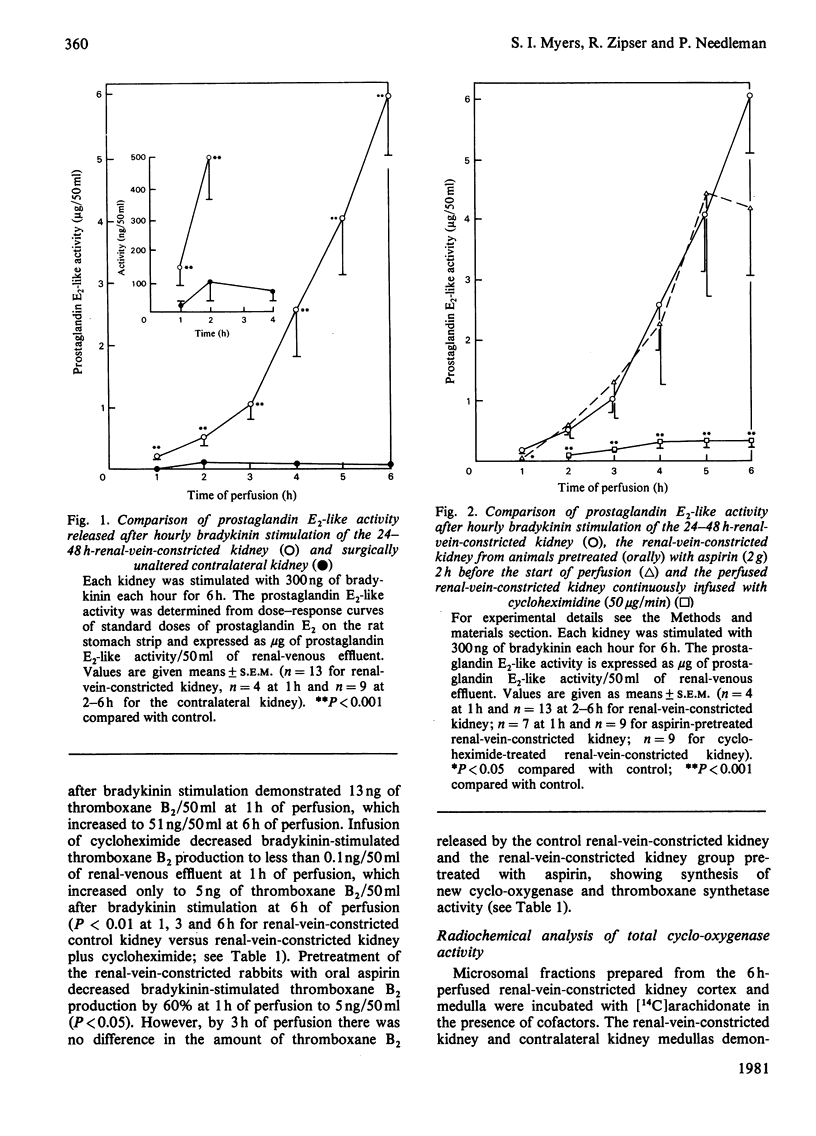
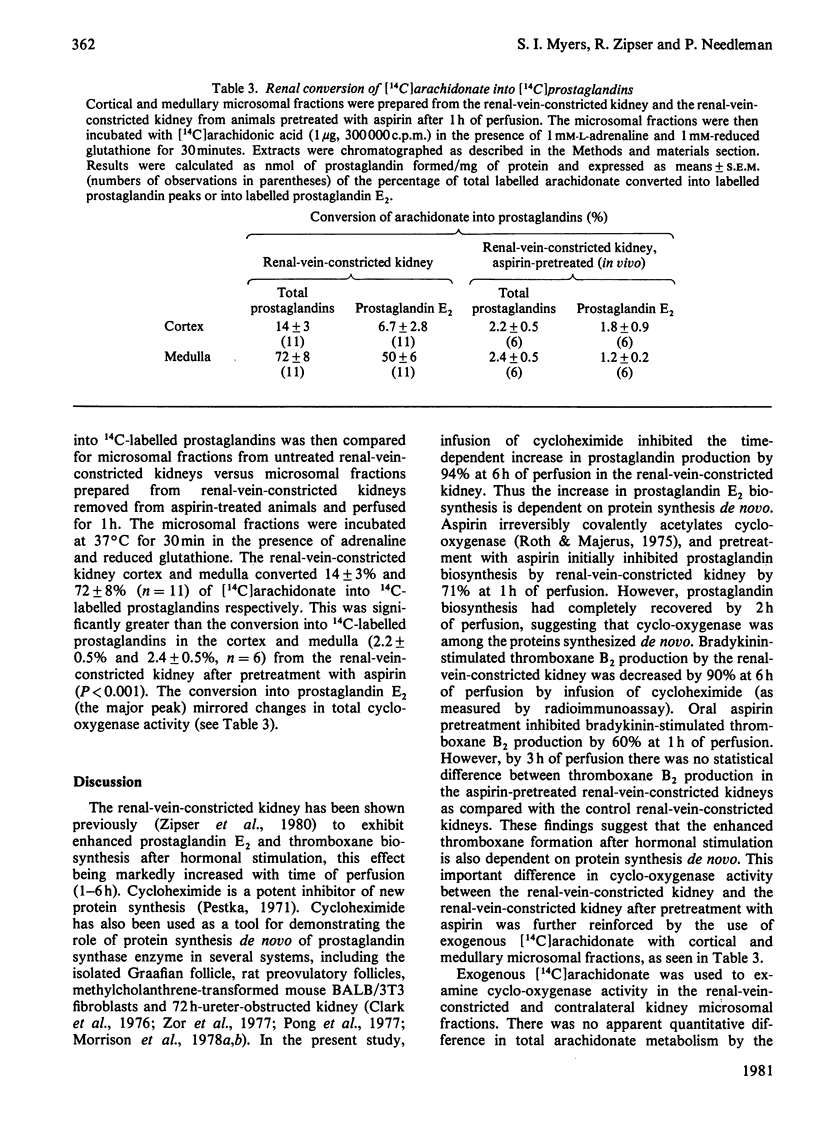
The ipsilateral kidney was removed from a rabbit 48h after unilateral partial renal-vein-constriction and was perfused with Krebs–Henseleit media at 37°C. Hourly administration of a fixed dose of bradykinin to the renal-vein-constricted kidney demonstrated a marked time-dependent increase in the release of bioassayable prostaglandin E2 and thromboxane A2 into the venous effluent as compared with the response of the contralateral control kidney. The renal-vein-constricted kidney produced up to 60 times more prostaglandin E2 in response to bradykinin after 6h of perfusion as compared with the contralateral kidney; thromboxane A2 was not demonstratable in the contralateral kidney. Inhibition of protein synthesis de novo in the perfused renal-vein-constricted kidney with cycloheximide lessened the hormone-stimulated increase in prostaglandin E2 by 94% and in thromboxane A2 by 90% at 6h of perfusion. Covalent acetylation of the renal cyclo-oxygenase by prior oral administration of aspirin to the rabbit inhibited initial bradykinin-stimulated prostaglandin E2 biosynthesis 71% at 1h of perfusion. However, there was total recovery from aspirin in the renal-vein-constricted kidney by 2h of perfusion after bradykinin stimulation. Total cyclo-oxygenase activity as measured by [14C]arachidonate metabolism to labelled prostaglandins by renal cortical and renal medullary microsomal fractions prepared from 6h-perfused kidneys demonstrated that renal-vein-constricted kidney-cortical cyclo-oxygenase activity was significantly greater than the contralateral-kidney-cortical conversion, whereas medullary arachidonate metabolism was comparable in both the renal-vein-constricted kidney and contralateral kidney. These data suggest that perfusion of a renal-vein-constricted kidney initiates a time-dependent induction of synthesis of prostaglandin-producing enzymes, which appear to be primarily localized in the renal cortex. The presence of the synthetic capacity to generate very potent vasodilator and vasoconstrictor prostaglandins in the renal cortex suggests that these substances could mediate or modulate changes in renal vascular resistance in pathological states.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benabe J. E., Klahr S., Hoffman M. K., Morrison A. R. Production of thromboxane A2 by the kidney in glycerol-induced acute renal failure in the rabbit. Prostaglandins. 1980 Mar;19(3):333–347. doi: 10.1016/0090-6980(80)90069-6. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Marsh J. M., LeMaire W. J. The role of protein synthesis in the stimulation by LH of prostaglandin accumulation in rat preovulatory follicles in vitro. Prostaglandins. 1976 Aug;12(2):209–216. doi: 10.1016/0090-6980(76)90116-7. [DOI] [PubMed] [Google Scholar]
- Eckenfels A., Vane J. R. Prostaglandins, oxygen tension and smooth muscle tone. Br J Pharmacol. 1972 Jul;45(3):451–462. doi: 10.1111/j.1476-5381.1972.tb08101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S. H., Vane J. R. Prostaglandins: their disappearance from and release into the circulation. Nature. 1967 Dec 2;216(5118):868–873. doi: 10.1038/216868a0. [DOI] [PubMed] [Google Scholar]
- Morrison A. R., Moritz H., Needleman P. Mechanism of enhanced renal prostaglandin biosynthesis in ureter obstruction. Role of de novo protein synthesis. J Biol Chem. 1978 Nov 25;253(22):8210–8212. [PubMed] [Google Scholar]
- Morrison A. R., Nishikawa K., Needleman P. Thromboxane A2 biosynthesis in the ureter obstructed isolated perfused kidney of the rabbit. J Pharmacol Exp Ther. 1978 Apr;205(1):1–8. [PubMed] [Google Scholar]
- Morrison A. R., Nishikawa K., Needleman P. Unmasking of thromboxane A2 synthesis by ureteral obstruction in the rabbit kidney. Nature. 1977 May 19;267(5608):259–260. doi: 10.1038/267259a0. [DOI] [PubMed] [Google Scholar]
- Needleman P., Wyche A., Bronson S. D., Holmberg S., Morrison A. R. Specific regulation of peptide-induced renal prostaglandin synthesis. J Biol Chem. 1979 Oct 10;254(19):9772–9779. [PubMed] [Google Scholar]
- Nishikawa K., Morrison A., Needleman P. Exaggerated prostaglandin biosynthesis and its influence on renal resistance in the isolated hydronephrotic rabbit kidney. J Clin Invest. 1977 Jun;59(6):1143–1150. doi: 10.1172/JCI108738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pong S. S., Hong S. L., Levine L. Prostaglandin production by methylcholanthrene-transformed mouse BALB/3T3. Requirement for protein synthesis. J Biol Chem. 1977 Feb 25;252(4):1408–1413. [PubMed] [Google Scholar]
- Roth G. J., Majerus P. W. The mechanism of the effect of aspirin on human platelets. I. Acetylation of a particulate fraction protein. J Clin Invest. 1975 Sep;56(3):624–632. doi: 10.1172/JCI108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipser R., Myers S., Needleman P. Exaggerated prostaglandin and thromboxane synthesis in the rabbit with renal vein constriction. Circ Res. 1980 Aug;47(2):231–237. doi: 10.1161/01.res.47.2.231. [DOI] [PubMed] [Google Scholar]
- Zor U., Strulovici B., Lindner H. R. Stimulation by cyclic GMP of prostaglandin E production in isolated Graafian follicles. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1086–1091. doi: 10.1016/0006-291x(77)90967-6. [DOI] [PubMed] [Google Scholar]


