Abstract
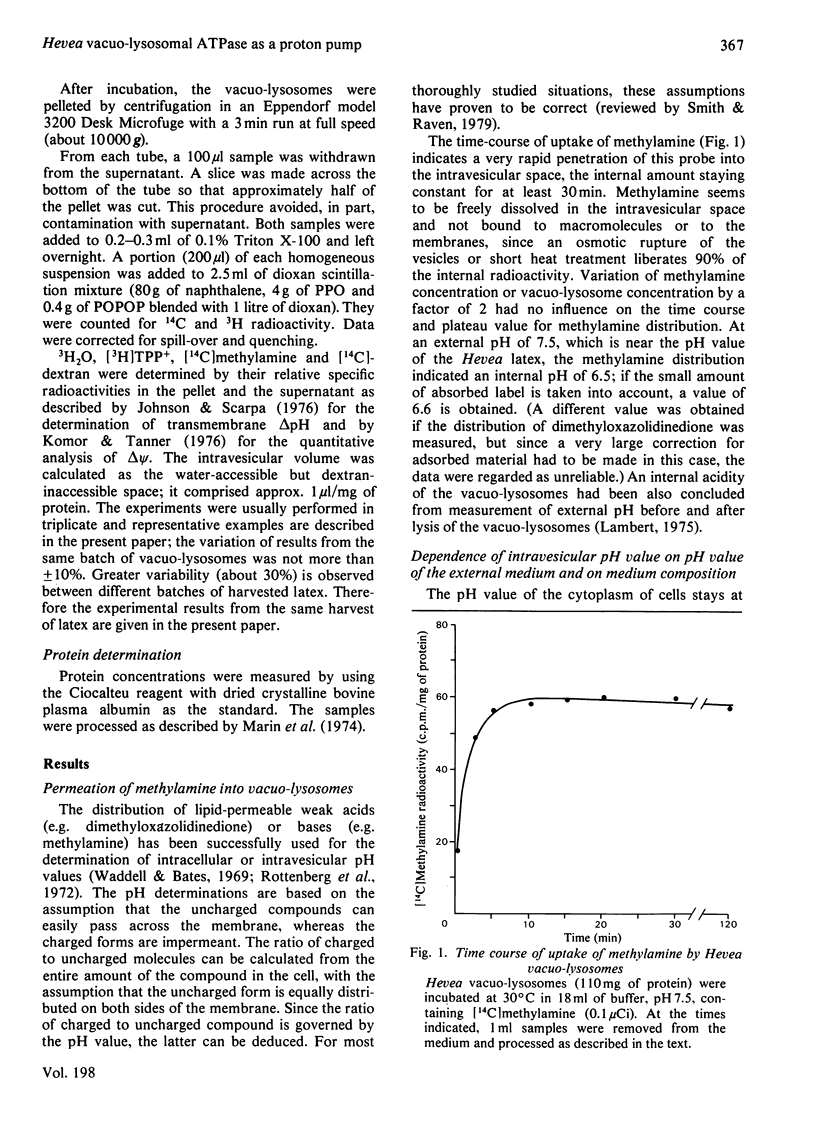
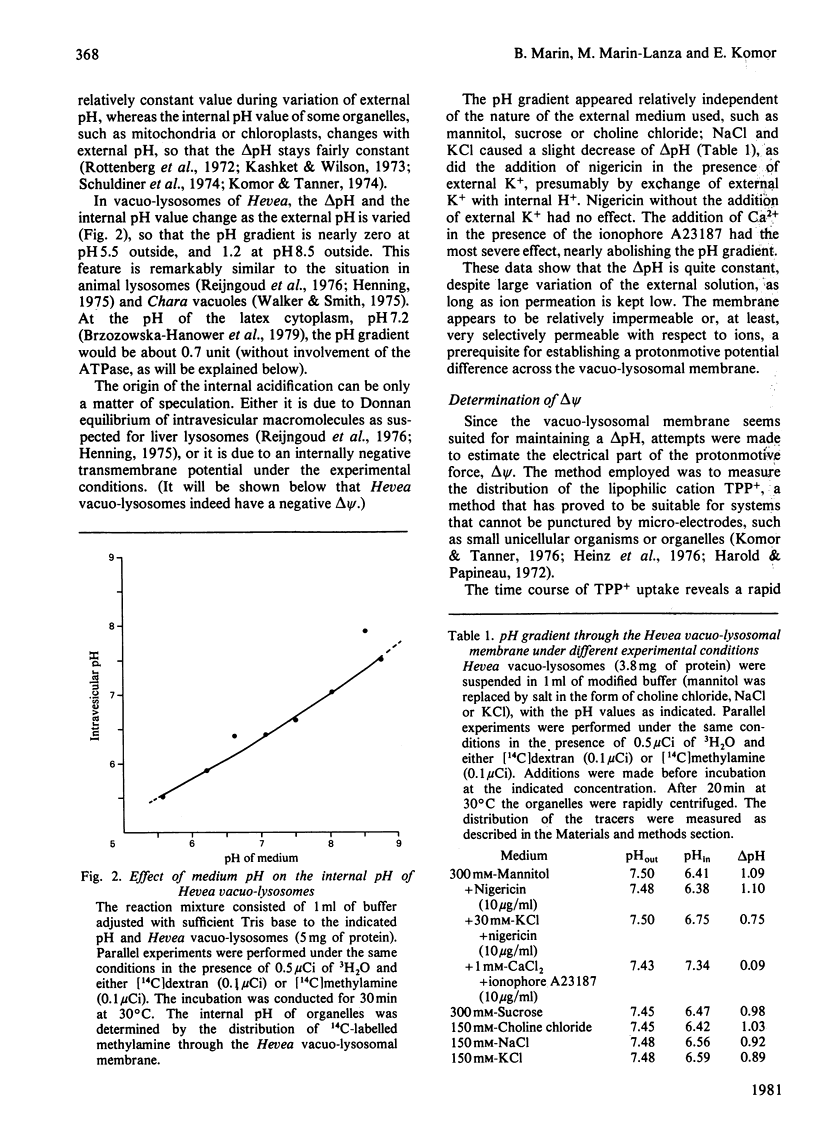
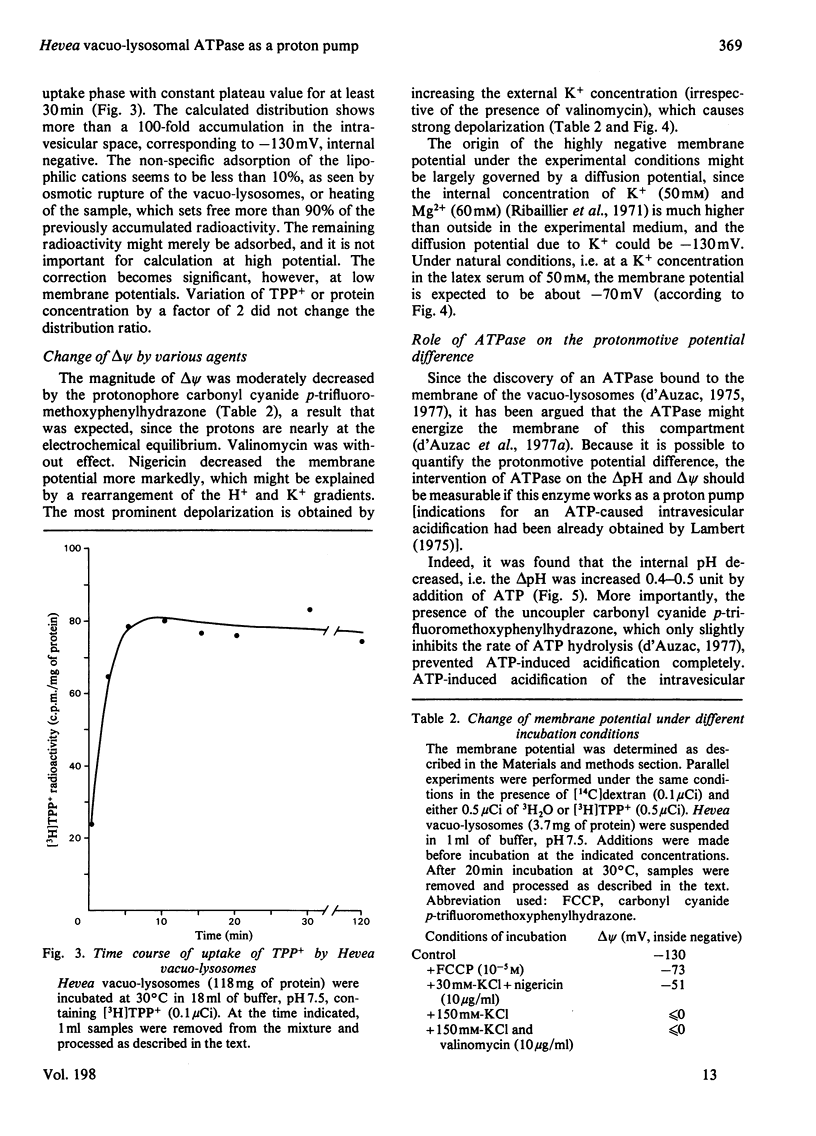
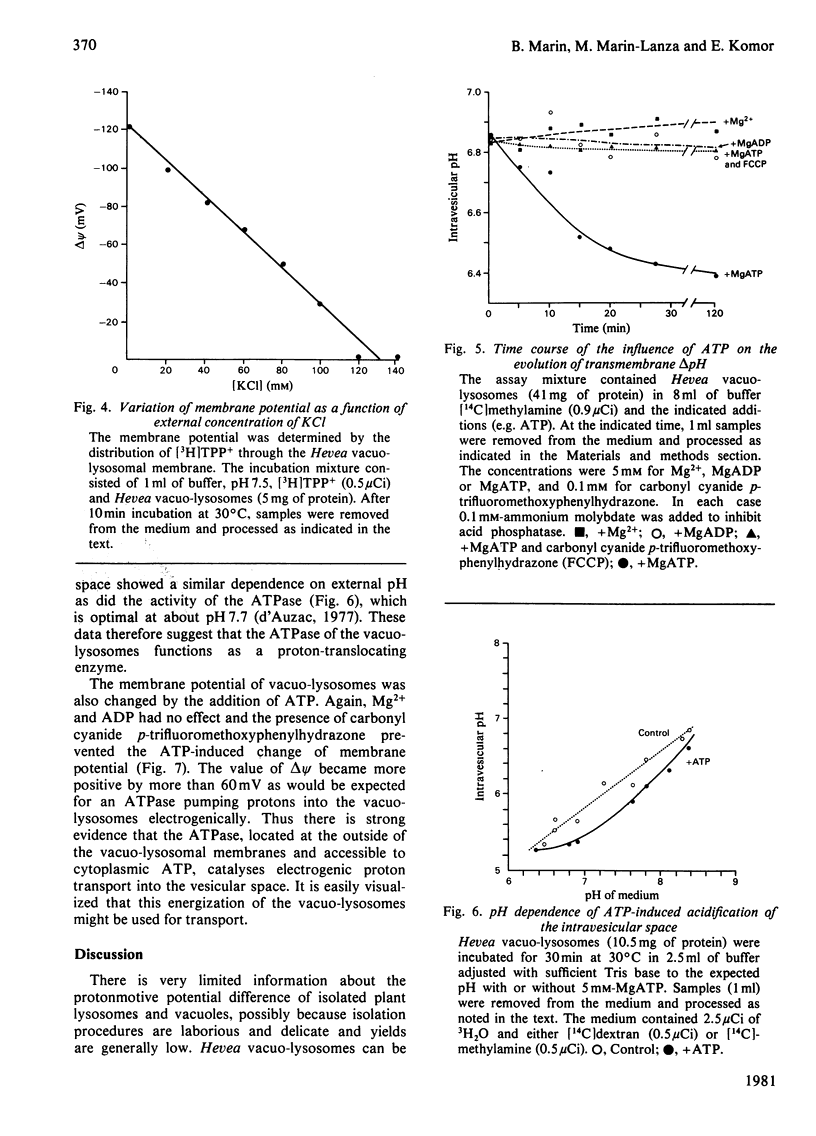
The vacuo-lysosomes of Hevea brasiliensis (rubber tree) constitute a suitable model system for the study of active transport and energization at the level of the membrane of plant vacuoles. The pH gradient (delta pH) and the membrane potential (delta psi) of vacuo-lysosomes were determined by means of the weak base methylamine and the lipophilic cation tetraphenylphosphonium. The values obtained depended strongly on the experimental conditions such as medium pH or K+ concentration. Under experimental conditions, i.e., pH 7.5 outside and low K+, the delta pH amounts to about 0.9 unit, interior acid, and the delta psi to -120 mV, interior negative. The delta psi is presumably caused by the imposed K+ gradient, and the internal acidification might be a consequence of the passive proton inflow along the electric field. This explanation is sustained by the ineffectiveness of carbonyl cyanide p-trifluoromethoxyphenylhydrazone in destroying the delta pH and delta psi, whereas higher K+ concentration decreased both. Under conditions existing in vivo, the membrane potential might be significantly lower. The presence of ATP increased the acidification of the intravesicular space by 0.5pH unit to a delta pH of up to 1.4 and shifts the membrane potential at least 60mV to a more positive value. The change of the protonmotive potential did not occur with ADP; the pH-dependence of the change was identical with the pH-dependence of a vacuo-lysosomal membrane-bound ATPase, and the effect of ATPase was prevented by the presence of the uncoupler carbonyl cyanide p-trifluoromethoxyphenylhydrazone. The change of protonmotive potential difference, brought about by the ATPase, was at least 90 mV. This is evidence that a vacuo-lysosomal ATPase in plants can function as an electrogenic proton pump that transfers protons into the vacuo-lysosomal space.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. I. The membrane potential. J Membr Biol. 1972;8(1):27–44. doi: 10.1007/BF01868093. [DOI] [PubMed] [Google Scholar]
- Heinz E., Geck P., Pietrzyk C. Driving forces of amino acid transport in animal cells. Ann N Y Acad Sci. 1975 Dec 30;264:428–441. doi: 10.1111/j.1749-6632.1975.tb31501.x. [DOI] [PubMed] [Google Scholar]
- Henning R. pH gradient across the lysosomal membrane generated by selective cation permeability and Donnan equilibrium. Biochim Biophys Acta. 1975 Aug 20;401(2):307–316. doi: 10.1016/0005-2736(75)90314-4. [DOI] [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A. Internal pH of isolated chromaffin vesicles. J Biol Chem. 1976 Apr 10;251(7):2189–2191. [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Proton-coupled accumulation of galactoside in Streptococcus lactis 7962. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2866–2869. doi: 10.1073/pnas.70.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Tanner W. The determination of the membrane ptoential of Chlorella vulgaris. Evidence for electrogenic sugar transport. Eur J Biochem. 1976 Nov 1;70(1):197–204. doi: 10.1111/j.1432-1033.1976.tb10970.x. [DOI] [PubMed] [Google Scholar]
- Komor E., Tanner W. The hexose-proton cotransport system of chlorella. pH-dependent change in Km values and translocation constants of the uptake system. J Gen Physiol. 1974 Nov;64(5):568–581. doi: 10.1085/jgp.64.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh R. A., Branton D. Isolation of Vacuoles from Root Storage Tissue of Beta vulgaris L. Plant Physiol. 1976 Nov;58(5):656–662. doi: 10.1104/pp.58.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin B., Trouslot P. Some evidence for the occurrence of ribonucleic acid in the lutoid fraction (lysosomal compartment) from Hevea brasiliensis latex. Biochem J. 1974 Nov;143(2):479–481. doi: 10.1042/bj1430479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron R., Kanner B. I., Schuldiner S. The role of a transmembrane pH gradient in 5-hydroxy tryptamine uptake by synaptic vesicles from rat brain. FEBS Lett. 1979 Feb 15;98(2):237–240. doi: 10.1016/0014-5793(79)80190-8. [DOI] [PubMed] [Google Scholar]
- Moreau F., Jacob J. L., Dupont J., Lance C. Electron transport in the membrane of lutoids from the latex of Hevea brasiliensis. Biochim Biophys Acta. 1975 Jul 8;396(1):116–124. doi: 10.1016/0005-2728(75)90194-2. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Allison V. P. Proton translocation of the bovine chromaffin-granule membrane. Biochem J. 1978 Mar 15;170(3):661–672. doi: 10.1042/bj1700661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijngoud D. J., Oud P. S., Kás J., Tager J. M. Relationship between medium pH and that of the lysosomal matrix as studied by two independent methods. Biochim Biophys Acta. 1976 Oct 5;448(2):290–302. doi: 10.1016/0005-2736(76)90243-1. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Grunwald T., Avron M. Determination of pH in chloroplasts. I. Distribution of ( 14 C) methylamine. Eur J Biochem. 1972 Jan 31;25(1):54–63. doi: 10.1111/j.1432-1033.1972.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Padan E., Rottenberg H., Gromet-Elhanan Z., Avron M. Delta pH and membrane potential in bacterial chromatophores. FEBS Lett. 1974 Dec 15;49(2):174–177. doi: 10.1016/0014-5793(74)80505-3. [DOI] [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]


