Abstract
A majority of human genes produce non-protein-coding RNA (ncRNA), and some have roles in development and disease. Neither ncRNA nor human skeletal muscle is ideally studied using short-read sequencing, so we used a customized RNA pipeline and network modelling to study cell-type specific ncRNA responses during muscle growth at scale. We completed five human resistance-training studies (n = 144 subjects), identifying 61% who successfully accrued muscle-mass. We produced 288 transcriptome-wide profiles and found 110 ncRNAs linked to muscle growth in vivo, while a transcriptome-driven network model demonstrated interactions via a number of discrete functional pathways and single-cell types. This analysis included established hypertrophy-related ncRNAs, including CYTOR—which was leukocyte-associated (false discovery rate [FDR] = 4.9 × 10−7). Novel hypertrophy-linked ncRNAs included PPP1CB-DT (myofibril assembly genes, FDR = 8.15 × 10−8), and EEF1A1P24 and TMSB4XP8 (vascular remodelling and angiogenesis genes, FDR = 2.77 × 10−5). We also discovered that hypertrophy lncRNA MYREM shows a specific myonuclear expression pattern in vivo. Our multi-layered analyses established that single-cell-associated ncRNA are identifiable from bulk muscle transcriptomic data and that hypertrophy-linked ncRNA genes mediate their association with muscle growth via multiple cell types and a set of interacting pathways.
Graphical Abstract
Graphical Abstract.
Introduction
Most genes in the human genome do not code for a protein (1) but instead are transcribed into non-coding RNAs (ncRNAs), including long non-coding RNAs (lncRNAs) (2). Early work demonstrated that the proportion of ncRNA genes increases with organismal complexity (3), and it is now recognized that ncRNAs participate in many key biological roles (4,5), including regulating genome organization and gene expression through nucleic and RNA–protein interactions (4,6). Further, ncRNAs can act as a surveillance mechanism to prevent the accumulation of aberrant products of protein translation (7), participate in epigenetic regulation (8,9), and several ncRNAs are directly implicated in human diseases (6,10,11,12,13). While ncRNAs classically have no-protein coding potential, emerging evidence suggests ncRNAs can harbour micro-peptides that have biological activity (14). Nevertheless, most ncRNAs have no characterized function and are not reliably detected in human muscle using short-read sequencing (15), highlighting a need for their accurate measurement and biological characterization.
Skeletal muscle accounts for ∼40% of total body mass (16), is paramount in various mechanical and metabolic functions, and is subject to age-related decline (17). Resistance exercise training (RT) results in muscle fibre hypertrophy, mitigating ageing-related loss of muscle (18). Yet not all humans robustly accrue skeletal muscle mass, even following prolonged supervised RT (19). This lack of response is indicative that the influence of RT on growth is often not readily discernable beyond the technical variation of a measurement instrument (20). Although exogenous factors—such as RT load or frequency (21) and ensuring adequate dietary protein provision (22)—modestly contribute to the load-induced hypertrophic phenotype, the intrinsic genomic characteristics of an individual are hypothesized to determine the bulk of the variability in hypertrophic response (23). Early human studies stratifying RT-induced hypertrophy identified a paradoxical gene signature consistent with less mammalian target of rapamycin complex 1 (mTORC1) activation in individuals with the greatest hypertrophy (24) or altered microRNA response profiles (19). Ultimately, studying individuals with the most robust responses to muscle loading may yield novel insights into causal regulators of adaptation and potentially identify therapeutic targets for treating muscle loss, including sarcopenia (25).
The current understanding of exercise transcriptomics is mostly limited to protein-coding genes; however, ncRNAs have begun to emerge as critical regulators of skeletal muscle growth. Recently, Wohlwend et al. (26) identified the lncRNA cytoskeleton regulator RNA (CYTOR) as regulated during muscle growth, negatively regulated with age and one that promoted fast skeletal muscle fibre expression in mice by sequestering the TEA domain transcription factor 1 (TEAD1). We discovered that the lncRNA, FRAIL1, was increased with muscle age (27), and more recently, increased FRAIL1 was found to negatively influence muscle strength and function (28). Additionally, Chronos is a skeletal muscle-enriched lncRNA that was shown to repress hypertrophic growth in vitro and in vivo by negatively regulating Bmp7 expression (29). Despite the emerging recognition that ncRNAs act as regulators of skeletal muscle development (30), myogenesis and musculoskeletal disease (31), most ncRNAs have no known function in adult muscle (1). The multi-nucleated and fibrous nature of skeletal muscle fibres makes it challenging to study using single-cell transcriptomics, and most single-cell technologies do not readily capture the non-coding transcriptome. In the present study, we generated five independent supervised exercise training studies (n = 144 subjects) and applied our customized bulk RNA profiling strategy (13,27,32), which more reliably detects ncRNAs in human muscle (15). Combined with pathway analyses (33,34), data-driven quantitative RNA network modelling (35) and selective spatial profiling, we were able to localize ncRNAs to specific cell types within muscle, providing insight into their biological role (36,37).
Materials and methods
Description of five independent clinical studies
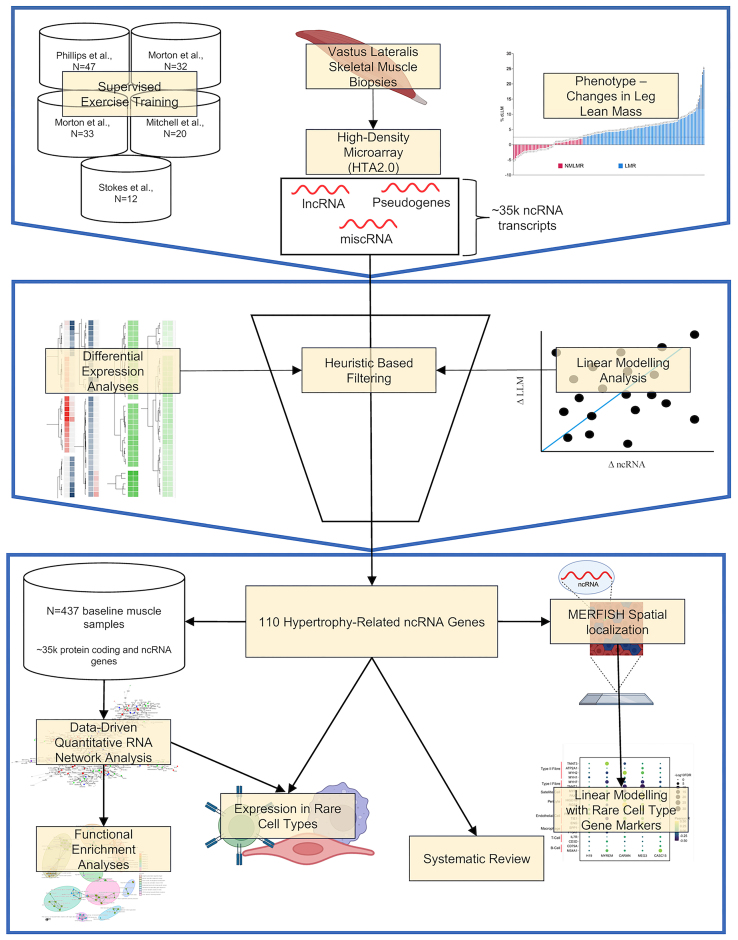
For a brief overview of the analysis, please refer to Figure 1. Each exercise intervention study utilized supervised training to load the thigh muscle and promote hypertrophy. It is known that a wide range of exercise protocols yield similar group average increases in muscle mass (20,21,24,38,39). Accordingly, despite variations in training experience and training load, the relative gains in lean mass in each study demonstrated the same average increase. Note that the global transcriptome profiles were partially utilized (other than 50 new transcriptome profiles) to validate a list of 141 protein-coding genes identified during the remodelling of lean mass with altered loading (25). All five studies were approved by local research ethics boards and complied with all ethical standards for research involving human participants set by the Declaration of Helsinki.
Figure 1.
Overview of the analysis pipeline. We used 144 participants from five supervised muscle-loading studies. We measured skeletal muscle hypertrophy (using DXA and MRI), and 88 individuals exhibited a change in skeletal muscle hypertrophy beyond the technical variation of our measurement instruments. RNA was extracted from skeletal muscle samples pre- and post-training, and we quantified ncRNA transcriptome (miscRNA, lncRNA and pseudogenes) using the Human Transcriptome Array 2.0. Using differential expression analyses, linear modelling analysis and heuristic-based filtering, we established 110 ncRNA genes are related to skeletal muscle hypertrophy. We used RNA network modelling analysis in baseline bulk skeletal muscle samples (n = 437) of protein-coding and ncRNA genes. We used a novel transcript spatial localization method to help facilitate the discovery of functional pathways and cell-type specific associations that several key ncRNA genes belong to.
Study 1
Morton et al. (40) recruited recreationally active men (age: 22 ± 3 years; body mass index [BMI]: 26 ± 7 kg/m2). Each participant’s dominant leg was randomly assigned to a high-load, low-repetition (8–12 repetitions at ∼70–80% 1-repetition maximum [1RM]), RT protocol or a low-load, high-repetition (20–25 repetitions at ∼30–40% 1RM) RT protocol. The contralateral leg was assigned to the opposite condition providing two distinct interventions. Participants underwent RT three days a week (Monday, Wednesday and Friday) for 10 weeks. Each RT session consisted of three sets of unilateral knee extensions to volitional fatigue (the starting leg alternated between RT sessions). RT-induced skeletal muscle hypertrophy did not differ between allocation conditions. Participants also received 25 g of whey protein isolate supplement twice daily (morning or post-exercise and pre-sleep) for the duration of the study. A muscle biopsy was obtained at pre- and post-training for 32 participants. Unilateral leg lean mass (LLM) was quantified using dual-energy X-ray absorptiometry (DXA) pre- and post-training.
Study 2
Muscle biopsies originated from Morton et al. (39). Participants were active, with 4–5 years of RT experience, and then performed RT 4 days/week (Monday, Tuesday, Thursday and Friday) for 12 weeks (all the major muscle groups). Each session included five exercises performed for three sets to volitional failure. Participants were randomly assigned to complete either low-load, high-repetition (20–25 repetitions per set at ∼30–50% 1-RM) RT or high-load, low-repetition RT (8–12 repetitions per set at ∼75–90% 1-RM). Indices of muscle hypertrophy did not differ between the training groups. Participants consumed 30 g of protein after each exercise session. Biopsy tissue was available at baseline and following 12 weeks of RT for 33 participants. The average of bilateral LLM was assessed using DXA at pre- and post-training.
Study 3
Muscle biopsies originated from Phillips et al. (41). Sedentary participants performed high-intensity cycle training 3 days per week (Monday, Wednesday and Friday) for 6 weeks. Each session consisted of a 2 min warmup at 50 W followed by five sets of high-intensity cycling at 125% VO2 peak for 1 min. Each set was separated by 90 s of rest. Biopsy tissue was available at pre- and post-training for 47 participants, including 18 males and 29 females with an average age of 39 years (range 21–51 years) and a BMI of 31 kg/m2 (range 26–43 kg/m2). Unilateral upper LLM was assessed using DXA at baseline and 3 days following the final training session.
Study 4
Muscle biopsies originated from Mitchell et al. (42). Young, recreationally active men participated in whole-body RT 4 days per week for 16 weeks. RT sessions consisted of two upper-body and two lower-body training sessions per week. The program progressed from three sets of twelve repetitions to four sets of six repetitions of each exercise. The last set of each exercise was performed to volitional failure. Participants consumed 30 g of protein immediately after each exercise session. Biopsy tissue was available for 20 participants pre- and post-training. Unilateral thigh muscle volume was measured using Magnetic Resonance Imaging (MRI) pre- and post-training.
Study 5
Muscle biopsies originated from Stokes et al. (25). Recreationally active men (age: 21 ± 3 years; BMI: 24 ± 3 kg/m2) underwent unilateral leg extension and leg press RT thrice weekly (Monday, Wednesday and Friday) for 10 weeks. Specifically, each session consisted of three sets of 8–12 repetitions of leg extension and three sets of 8–12 repetitions on a leg press. The last set of RT was performed to volitional failure. Following each exercise session, participants ingested 25 g of whey protein isolate. Biopsy tissue was available for 12 participants pre- and post-training. Unilateral LLM was measured pre- and post-training using DXA.
Defining hypertrophy beyond typical laboratory variation
Changes in DXA LLM (or, in one case, quadriceps muscle volume by MRI) defined subjects that demonstrated a genuine increase in muscle mass (‘responders’). For the remaining subjects, we could not show they demonstrated training-induced hypertrophy (we do not claim they have no response). We used the technical variation threshold for DXA-derived changes in LLM (2.0–2.4%) (43,44) and MRI-derived changes in thigh muscle volume (2.3%) (45) as cut-off values for these two groups. For all individuals, the percentage change in LLM (or quadriceps muscle volume for MRI) post-exercise training was calculated. Critically, there was no association between baseline LLM and changes in LLM (Supplementary Figure S1 and Supplementary Table S1). Individuals who exhibited a ≥2.5% increase in LLM were defined as ‘LM Responders (LMR)’, whereas individuals who showed <2.0% change in LLM (or change in quadriceps muscle volume for (42) were defined as having ‘No measurable LM response (NMLMR)’. These two groups (LMR, N = 88; NMLMR, N = 50) were studied separately for evidence of differential noncoding gene expression (DE). Only 6 individuals fell within the range of reported precision error (2.0–2.4%) of the DXA (or MRI), and they were included in the linear modelling analysis.
Clinical data for quantitative network modelling
We used n = 437 transcriptome profiles from mainly sedentary subjects, aged 50 years or younger, to generate a human muscle tissue gene co-expression model using multiscale embedded gene co-expression network analysis (MEGENA) (35). A total of 35 079 protein-coding and non-coding genes were used as input, and the analysis time was ∼600 h on a 3.2 GHz 16-core Intel Xeon W-based Mac Pro (768GB 2933 MHz DDR4 RAM). Unlike most popular and fast network methods, MEGENA applies statistical thresholds to identify significant relationships at both the gene and network structure level, for example, false discovery rate (FDR) < 1% for gene-gene Pearson correlation coefficients, P < 0.05 for module significance, P < 0.01 for network connectivity, with 1000 permutations for adjusted values (FDR). We present the overall hierarchical organisation of the planar filtered networks using a sunburst plot, and individual module plots were produced using Fruchterman-Reingold force-directed plotting within MEGENA (35). Gene co-expression modules that contained our candidate ncRNA genes were labelled in the sunburst plot and studied further (see below).
RNA extraction and transcriptome profiling
In each case, RNA was extracted from ∼20 mg of muscle tissue (25,39,40,42) in 1000 ml of TRIzol, which was added to Lysing Matrix D tubes containing ceramic microbeads (MP Biomedicals, Solon, OH, USA) and homogenized using a FastPrep tissue homogeniser (MP Biomedicals, Solon, OH, USA). Approximately 200 ml of chloroform was added, and the tubes were hand-shaken vigorously for 15 s and incubated at room temperature for 5 min. Samples were then centrifuged at 12 000 g for 10 min at 4°C, and the upper aqueous phase containing RNA was transferred to an RNase-free tube. RNA was purified using the EZNA Total RNA Isolation kit (Omega Bio-Tek, Norcross, GA, USA). RNA was processed for transcriptome profiling using the GeneChip WT Plus Reagent Kit (no PCR) by processing to single-stranded sense DNA via a reverse transcription linear priming strategy that primes the poly-A and non-poly-A RNA. First- and second-strand cDNA synthesis was performed using ∼100 ng of RNA and a spike-in Poly-A control, followed by reverse transcription into chromosomal RNA (cRNA). cRNA was purified using magnetic beads and quantified using spectrophotometry (Nanodrop UV-Vis, Thermo Fisher Scientific). Approximately 15 mg of cRNA was then amplified and hydrolysed using RNase H (leaving single-stranded cDNA) and purified with magnetic beads. cDNA (5.5 mg) was then fragmented, labelled and hybridized to an array. The array was washed and stained using a GeneChip Fluidics Station 450 (Thermo Fisher) and scanned using a GeneChip Scanner 3000 7G (McMaster Genomic Core Facility, Hamilton). Samples from Phillips et al. (41) were processed using a Qiagen Tissuelyser and processed as above without the additional purification step (Jensen Laboratory, MPI, Horsholm, Denmark).
Computational processing of the HTA 2.0 array platform
We updated our 2017 process for annotating and processing the transcriptomic technology (13,15,25,27,32). Briefly, the HTA 2.0 array has 6.9 million 25-mer probes and the annotation of each probe is checked against the recent reference genome and transcriptomes (46,47) using R. To do this, we produce a standardized FASTA (with a unique label for each 25mer probe) file, a probe GC content file and a probe-level chip definition file (CDF). For the present study, the FASTA file was aligned against Grch38 - Gencode 43 (48) using the STAR aligner (49). Probes that uniquely map were combined to form groups of probes (probe-sets), and each probe-set relied on n > 3 probes (median = 66). In addition, the raw probe signal of a large number of diverse human muscle profiles (n = 1124, GSE154846) was analysed using the probe-level CDF and aroma.affymetrix (46), affy (50) and the affxparser (bioconductor.org/packages/affxparser/) packages. Probes with both a very low signal and a low coefficient of variation were removed to arrive at a final set of probes to be included in the CDF (13,15,25,27,32). This CDF was then used to summarize RNA expression from the GC-corrected CEL files produced from studies 1 to 5 and 437 pre-existing CEL files, after which iterative rank-order normalisation (IRON) (51) in the default mode was used to generate a normalised expression matrix. From the starting 6.9M probes, reannotation and summarization yielded a transcript level probe-set CDF used 5.23M probes to define 220.6K probe-sets prior to study-specific transcript signal filtering (Supplement Data S1). The custom CDF used in this study is deposited at GSE154846, along with new raw data produced for this study (GSE270823). This updated CDF can be utilized to extract any other human muscle data sets produced on the HTA 2.0 platform. We estimated that a total of 173 594 transcripts were annotated above the background signal using standard deviation-based filtering.
Differential expression analyses of ncRNA genes
For DE analyses, we used SAMR (52) and 10K permutations to estimate the FDR, a method that appears more robust than other P-value correction methods (53). Paired SAMR analyses were applied to the LMR group (176 paired RNA samples) and the NLMR group (100 paired RNA samples). We relied on an FDR cut-off of 5% and a ≥ |1.2| fold change to generate differentially expressed ncRNA lists. To focus on the most distinct observations between the LMR and NLMR groups, we applied the following heuristics. We prioritized ncRNA transcripts that displayed a fold change in opposite directions across our two response groups or where the differentially expressed ncRNA showed a fold change ratio between the groups ≥ |1.2|. Finally, to clarify if there were any ncRNAs differentially expressed at baseline between our groups (note baseline physiology could not distinguish LMR and NLMR groups), we utilized an unpaired SAMR analysis (using the same criteria for a differentially expressed transcript as described above).
Assessing the relationship between changes in gene expression and changes in lean mass
The linear relationship between changes in ncRNA transcript expression and changes in LLM (or change in quadriceps muscle volume for (42) was examined using a type III analysis of variance (R Core Team (2021). R: A language and environment for statistical computing (https://www.R-project.org/). We used the following linear model: changes in ncRNA transcript ∼ changes in LLM. A Pearson correlation coefficient (r) and a P-value were calculated for each transcript per study. Using the three largest cohort studies (39,40,41), we combined P-values using the Stouffer method, using the poolr R package (54). For transcripts that passed filters 1 and 2 (see below), FDRs were calculated (55) using multtest (56). ncRNA transcripts that were deemed to change linearly in proportion with delta lean mass passed all of the following filters: (i) 5-study median r (absolute) ≥0.2, (ii) consistent (direction) r value in 4/5 studies and (iii) an FDR < 10% (the median was FDR 8%).
Cell type-specific gene markers and MERSCOPE-based transcript localization
We calculated Pearson correlation coefficients and P-values (FDR adjusted) to establish the association of ncRNAs or gene co-expression modules with markers of type I and type II muscle fibres and rarer mononuclear cell types found in bulk skeletal muscle (satellite cells, endothelial cells, pericytes, macrophages, T cells and B cells). These analyses relied on a network model generated using 437 muscle biopsy samples in individuals aged 50 years or younger (GSE154846). We used the following myonuclei and mononuclear cell-specific gene markers: type I fibres (MYH7, TNNT1) (57,58,59), type II fibres (MYH1, MYH2, ATP2A1, TNNT3) (57,58,59), satellite cells (PAX7, MYF5) (57,60), endothelial cells (ENG, TIE1, PECAM1, APLNR), pericytes (RGS5, HIGD1B) (57,61), macrophages (F13A1, SPP1) (57), T cells (CD3D, IL7R) (57,62) and B cells (MS4A1, CD79A) (57,62). The MERSCOPE is a spatially resolved single-cell transcriptome profiling technology. As part of the process of designing and validating ongoing work in our laboratory, we produced images of the location of a small number of the ncRNAs identified in the present study. The MERSCOPE assay also included markers for muscle fibre types, satellite cells and endothelial cells (type I fibre [MYH7 fish probe] (57,58,59), type II fibres [ATP2A1 fish probe] (57,58,59), satellite cells [PAX7, MYF5 merfish probes] (57,60) and endothelial cells [ENG, TIE1, PECAM1, APLNR merfish probes]). The panel was used to profile three independent muscle samples originating from the present study, and some ncRNA data pertinent to the present results is presented. The gene list was sent to Vizgen Inc, and they selected 30–50 merfish probes per gene for profiling on the MERSCOPE. Briefly, each fresh frozen sample embedded in the optimal cutting temperature compound (OCT, Tissue-Tek, The Netherlands) was sectioned at a thickness of 10 um in a cryostat at −20°C and transferred onto the circular MERSCOPE glass slide (PN 2040001, Vizgen, USA) within the fiducial bead border, and prepared for transport (63,64). Tissue sections were fixed in pre-warmed 4% paraformaldehyde for 1 h at 37°C. After fixation, the tissue sections were washed in 1X PBS three times for 5 min at room temperature, permeabilized in 70% ethanol (molecular grade) at 4°C overnight. Then, Vizgen cell boundary staining kit (PN 10400009, Vizgen, USA) was applied to stain cell membranes on the tissue sections after a 1-h incubation in Blocking Solution mixed with RNase inhibitor (PN 20300100, Vizgen, USA). Afterwards, the tissue sections were incubated with Formamide Wash Buffer (PN 20300002, Vizgen, USA) at 37°C for 30 min and incubated in the custom-designed merfish panel at 37°C for ∼40 h. Then, the slide was incubated in the Formamide Wash Buffer at 47°C (30 min × 2), rinsed with the sample preparation buffer and stained with the DAPI and poly(T) Staining reagents, and the fiducial fluorescent beads (perimeter of scannable area). The probes were imaged using the MERSCOPE (Vizgen Core Laboratory, Boston, USA) with default settings (poly(T) and DAPI and Cell Boundary Channels ‘on’, while scan thickness was 10 μm). The raw images were converted to ‘vzg’ meta-output files. For ncRNAs included in both assays, we used the MERSCOPE visualizer software and cell-specific marker probes to evaluate the location of each ncRNA.
Functional enrichment pathway analysis
Enrichment analyses (e.g. gene ontology [GO]) (33) are used to uncover the functional biology of lists of genes, categorizing genes by their known biochemical functions (65). For each network module, enrichment analysis was executed using the web tool Database for Annotation, Visualization and Integrated Discovery (DAVID; https://david.ncifcrf.gov/) (33), versus a custom background file containing the protein-coding genes estimated to be expressed in skeletal muscle tissue in the present study (65). Most ncRNAs are not ontologically characterized and thus do not contribute to the enrichment analyses. Data-driven RNA network modelling methods are constructed based on experimentally derived gene co-expression similarities (35), and so provide a solution to categorizing ncRNAs using ‘guilt by association’. Gene co-expression modules with <500 genes and GO terms with an FDR ≤ 5.0 × 10−5 were selected for further study. The gene lists from the ncRNA-containing modules, along with the background list of expressed genes, were analysed using the compareCluster function (66,67) in the ClusterProfiler R package utlising the GOTERM_BP_ALL from DAVID. ClusterProfiler was used to carry out functional enrichment using over-representation analyses and combined with enrichment mapping (66) to establish links between distinct ncRNA-containing MEGENA modules. Note that not all gene identifiers from each module are mapped to the databases being used to generate these pathway analyses (a common problem).
Results
Skeletal muscle hypertrophy following supervised exercise training
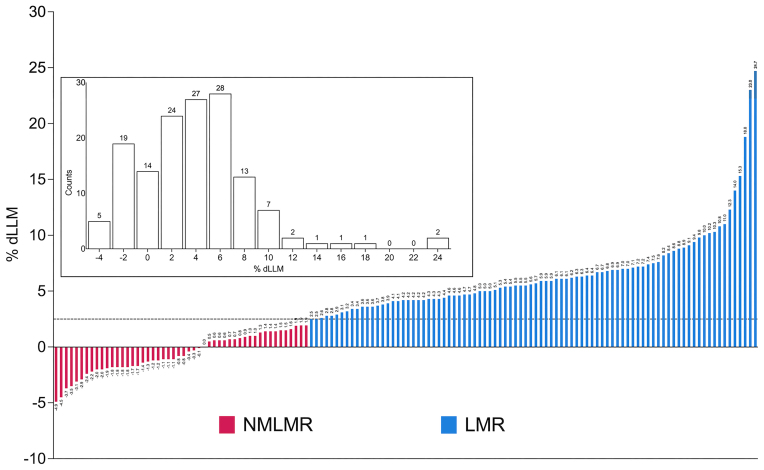
The average change in LLM (using DXA) across (25,39,40,41) was 3.4 ± 3.9% (range: −4.9% to 18.8%); the average change in quadriceps muscle volume using MRI (42) was 7.1 ± 7.0% (range: −1.8% to 24.7%). Out of 144 participants, 88 individuals (61% of the total sample) were defined as LMR, whereas 50 individuals (34% of the total sample) did not demonstrate LLM changes beyond typical measurement error (NMLMR group). Thus, the average change in LLM for the LMR group and the NMLMR group was 6.6 ± 3.9% (range: 2.5% to 24.7%) and −0.6 ± 1.8% (range: −4.9% to 1.9%), respectively (Figure 2). Baseline LLM status could not explain the observed variation in LLM gain (Supplementary Figure S1; the demographics of the individual studies can be found in Supplementary Table S1). We do not claim that the NMLMR subjects can not grow muscle tissue, just that we are unable to unequivocally assign any change in LLM beyond previously established method variation and we treated them as phenotypically divergent on this basis.
Figure 2.
Phenotypic overview of included studies. Waterfall plot showing the percent change in leg lean mass (% dLLM) for 138 participants used for differential expression analyses. The dotted line (2.5% dLLM) depicts the threshold for the precision error of the DXA and MRI. Individuals who exhibited a ≥2.5% dLLM (or quadriceps muscle volume for MRI) were allocated to the group: ‘Lean Mass Responders’ (LMR; n = 88), whereas individuals who showed <2.0% dLLM were assigned to the group called ‘No Measurable Lean Mass Response’ (NMLMR; n = 50). Each bar corresponds to one individual, and the number above each bar corresponds to the numerical value for dLLM%. Box Inset: Histogram depicts the number of individuals (144) falling into each %dLLM bin, and counts above each bar correspond to the number of individuals in that particular %dLLM bin. For a detailed overview of participant characteristics, please refer to Supplementary Table S1.
Overview of RNA molecules measured in bulk human skeletal muscle using high-density arrays
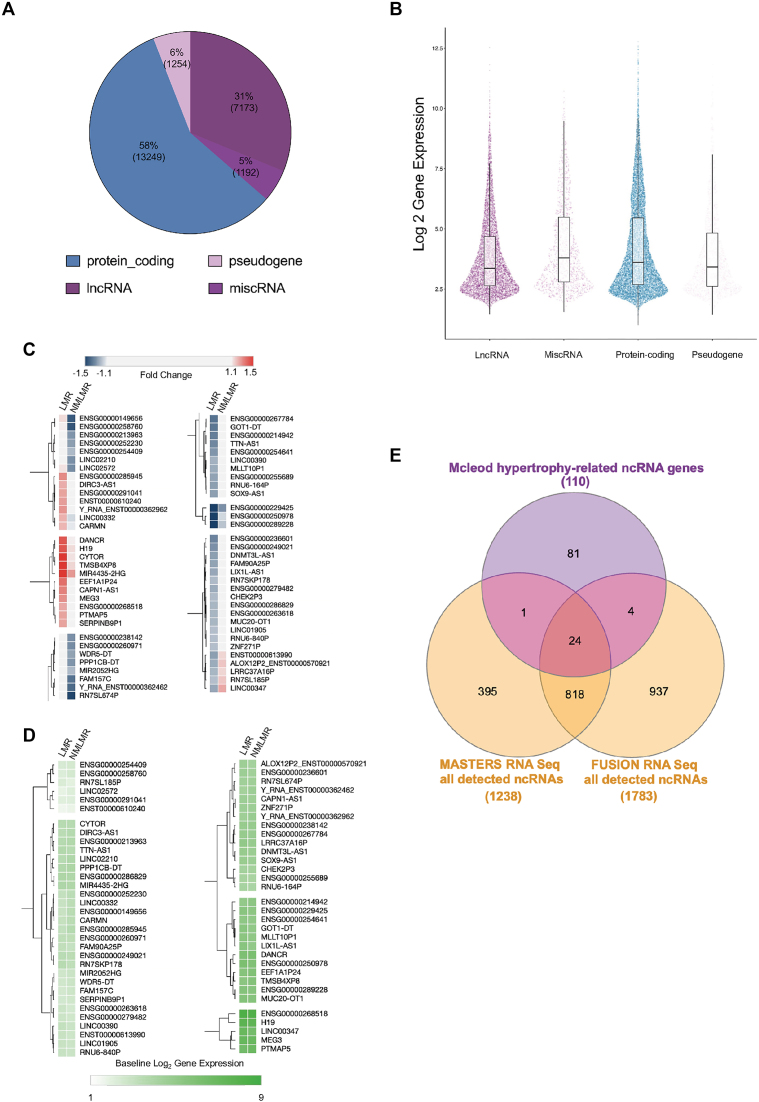
Approximately 58% of quantified genes were protein-coding after filtering out low signal (n = 13 249, Figure 3A). The majority of detectable ncRNA genes in skeletal muscle are annotated as ‘lncRNA’ molecules (7173 genes), which are broadly categorized as genes that are ≥200 bp in length. Other classes of ncRNAs included miscellaneous RNA (n = 1192; ncRNA genes that are <200 bp in length) and pseudogenes (n = 1254). Note that ncRNA genes are routinely described as ‘low-abundance’ genes (36) based on sequencing cDNA libraries. We find here that the expression profile of ncRNA genes is similar to protein-coding genes in human skeletal muscle (Figure 3B) using a library preparation method that does not rely on PCR and is not 3′ biased—consistent with a previous meta-analysis (15) from our group. The superior coverage with our array protocol over typical sequencing protocols most likely reflects, firstly, a more diverse cRNA library and, secondly, the lack of use of a competitive/counting quantification method, enabling more equitable detection of all RNA species (where array probes exist for that RNA—which in practice results in substantial greater coverage of the muscle transcriptome (15)).
Figure 3.
RNA molecules quantified on high-density arrays and establishing a 110 ncRNA gene signature associated with skeletal muscle hypertrophy. (A) Quantitative overview of the number of genes (2 SD filter applied to protein-coding genes and 1 SD filter to ncRNA genes) belonging to each class of RNA. (B) The distribution of the abundance (log 2 signal) of each RNA class is visualized using the median (horizontal line within the box), upper and lower interquartile ranges (upper and lower edges of the box), upper and lower whiskers (1.5× interquartile range), and the points correspond to an individual gene. (C) Heat plot that denotes fold changes for 65 ncRNA genes were differentially expressed and uniquely regulated in a manner that was distinct across the LMR group (50 genes) and the NMLMR group (15 genes). (D) The heat plot denotes the log2 baseline gene expression for the 65 uniquely regulated ncRNA genes. The heat plots (C and D) were created using Morpheus (https://clue.io/Morpheus), and genes were hierarchically clustered using Euclidean distance (linkage method: complete). (E) A total of 110 hypertrophy-related ncRNA genes were contrasted to all consistently detected ncRNA genes from two short-read RNA sequencing data sets profiling skeletal muscle (MASTERS ≈ 34 million reads, n = 136 (71); FUSION ≈ 60 million reads, n = 278 (70) using a Venn diagram tool (https://www.interactivenn.net/; (127)).
Establishing a set of ncRNA genes related to skeletal muscle hypertrophy
One hundred ninety-three ncRNA genes were significantly regulated in the LMR group (Supplementary Data S2), whereas 50 genes were significantly regulated in the NMLMR group (Supplementary Data S3). To enable us to focus on the most robust results, we applied heuristic filtering (see ‘Materials and methods’ section) to identify those ncRNA that demonstrated the most distinct pattern of regulation between our two groups (13,68,69). This analysis revealed a subset of 50 ncRNA genes that were specifically regulated just in the LMR group and so may be directly linked with hypertrophy. Fifteen ncRNA genes were uniquely regulated in the NMLMR group (Figure 3C), and these may be associated with processes that impair growth or aim (unsuccessfully) to overcome negative influences on growth.
Notably, only two ncRNA genes were differentially expressed between the two groups prior to supervised training (Supplementary Data S4), demonstrating that these baseline ncRNA transcriptomic profiles, much like the physiological characteristics, did not distinguish LMR from NMLMR groups (Figure 3D). In addition to DE analyses applied to the two post-hoc categorical groups (i.e. LMR group versus NMLMR group), linear analysis was used to determine if there was a linear relationship between changes in ncRNA gene expression and changes in LLM, using all 144 subjects. This analysis identified 46 ncRNA genes with a modest relationship to gain in LLM (median r = 0.26; median FDR = 8%; Supplementary Figure S2A and Supplementary Data S5). Combined with the DE analyses, a final list of 110 hypertrophy-related ncRNA genes (Supplementary Data S6) was considered further. Notably, we identified most of the known muscle hypertrophy-related ncRNA genes (e.g. CYTOR, MYREM, H19 and MEG3), which now have some established biochemical roles in skeletal muscle (Supplementary Table S2). The rest of the hypertrophy ncRNAs had no prior associated function in skeletal muscle (Supplementary Table S2). Cardiac mesoderm enhancer-associated non-coding RNA (CARMN) was the only ncRNA gene that displayed a linear association with changes in LLM (Supplementary Figure S2B–F) and was differentially expressed in the LMR group. Critically, only 30% of the hypertrophy ncRNA genes are consistently detected in skeletal muscle by RNA sequencing (70,71) (Figure 3E and Supplementary Data S7), and only five ncRNA genes (CASC15, MYREM, DANCR, MIR4435-2HG, MEG3) were reported as being regulated by RT when using RNAseq (71).
Spatial distribution of ncRNA genes in human skeletal muscle
There are several multi-plex true single-cell spatial technologies (63,64,72), and we present here data from the MERSCOPE single-cell spatial workflow to provide the visual location of a few hypertrophy ncRNA genes, along with markers of type I and type II muscle fibres, satellite cells and endothelial cells (57). The prototype MERSCOPE assay (for an overview of the three samples used for MERSCOPE-MERFISH, please refer to Supplementary Figure S3) showed reasonable agreement with the bulk expression data from the same biopsy sample (r = 0.78–0.81). In our bulk transcriptomic data, MYREM (MYBPC2 cis regulating lncRNA enhancer of myogenesis (ENSG00000268518) expression was positively associated with type II fibre markers (e.g. TNNT3: r = 0.37; FDR = 2.57 × 10−14; Figure 4A) (57) and negatively associated with type I fibre markers (e.g. MYH7:r = –0.33; FDR = 1.45 × 10−11; Figure 4A) (57). Visually, MYREM is concentrated beneath the basal lamina and was not observed in nuclei with gene markers of satellite cells (PAX7, MYF5) or endothelial cells (APLNR, TIE1, KDR, ENG), indicating that MYREM is a novel marker of mature myofibril nuclei in humans (Figure 4B), especially in type II fibres. This interpretation is supported by recent data from Chang et al. (73) demonstrating that MYREM increases the transcriptional expression of MYBPC2, a type II isoform of skeletal muscle myosin-binding protein C (74,75).
Figure 4.
Examples of spatially resolved hypertrophy-related ncRNA genes in human skeletal muscle. (A) Dot plot depicting the association between the expression of hypertrophy-related ncRNA genes—that were used for MERFISH—and gene markers for skeletal muscle fibre types and rare mononuclear cell types, using bulk transcriptomic data (n = 437). The colour of the dot corresponds to the Pearson correlation coefficient, and the size of the dot is proportional to the –log10 FDR. (B) High-level image of type I (MYH7) and type II (ATP2A1) FISH probes on a human skeletal muscle cross-section. Box inset shows magnified region of skeletal muscle cross-section with MERFISH probes for MYREM, as well as MERFISH probes for endothelial cell protein-coding gene markers (APLNR, TIE1, KDR, ENG) and satellite cell protein-coding gene markers (MYF5, PAX7). The spatial distribution of (C) H19, (D) MEG3, (E) CARMN and (F) CASC15, all with the same magnification, location and protein-coding MERFISH probes as box inset in (B).
H19 is an imprinted lncRNA, and its pre-training abundance in human muscle is associated with training-induced changes in maximal aerobic capacity (76). Spatially, it was ubiquitous throughout human skeletal muscle (Figure 4C) without any strong associations with fibre type markers or mononuclear cell types (Figure 4A). In contrast, Maternally Expressed Gene 3 (MEG3), another imprinted lncRNA, was co-localized with satellite cell gene markers (PAX7 and MYF5; Figure 4D), and its expression was significantly correlated with PAX7 expression (r = 0.51; FDR = 2.48 × 10−29; Figure 4A) in the bulk transcriptomic data. Indeed, a previous report in mice (77) found that MEG3 is enriched in satellite cells and modulates myoblast differentiation and proliferation, impacting skeletal muscle regeneration.
CARMN had a low average expression in bulk human skeletal muscle (4.4 log2 units), implying it may not be expressed by mature muscle cells. Figure 4E demonstrates that CARMN appears located near endothelial cell gene markers (APLNR, TIE1, KDR, ENG), and we noted that it was positively associated with endothelial cell gene markers (e.g. TIE1: r = 0.33; FDR = 3.26 × 10−12; Figure 4A) in our bulk transcriptomic data. Previous work claimed that CARMN is a vascular smooth muscle cell-specific lncRNA (78). Although vascular smooth muscle cells are absent from muscle capillaries (61), pericytes are associated with endothelial cells in skeletal muscle and have been proposed to regulate endothelial cell proliferation and differentiation (79). Notably, in our data, CARMN expression is most strongly associated with pericyte gene markers (e.g. RGS5: r = 0.50; FDR = 4.31 × 10−27; Figure 4A), which leads us to hypothesize that CARMN might modulate the role of pericytes during skeletal muscle growth. Many reports of cell-unique patterns of lncRNA expression come from a failure to appreciate the limitations of RNAseq cDNA library production (80). Like CARMN expression, cancer susceptibility candidate 15 (CASC15) also has a low signal in bulk skeletal muscle (Figure 4F). CASC15 is an intergenic lncRNA, and small interfering knockdown experiments have shown that CASC15 contains tumour-promoting properties in melanoma cell lines (81), and we find greater expression of CASC15 is associated with human muscle growth in vivo.
Data-driven RNA network modelling of ncRNA and protein-coding genes demonstrates that the hypertrophy-related ncRNAs represent functionally interacting pathways
Given most ncRNAs have no known function despite representing half the genome, inference methods are required to highlight potential molecular functions. We applied time-consuming data-driven quantitative network modelling to the muscle coding and non-coding transcriptome (n = 437) and identified statistically robust co-expression modules that contained hypertrophy-related ncRNAs. There were 136 significant co-expression modules that contained at least one hypertrophy-related ncRNA (Figure 5A). Twelve of these modules were enriched in GO biological processes, including cell migration, contractile fibre, inner mitochondrial membrane complex, RNA processing and MHC protein complex processes (Table 1; Supplementary Data S8–S10). Application of a network-based enrichment method (67) to the co-expression modules independently identified by MEGENA, identifies that the hypertrophy ncRNA-containing modules interact at least two levels (Figure 5B). Firstly, genes belonging to modules contribute to more than one distinct biological function (pie-chart structures) both within and across functional groups (e.g. regulation of cell activation and positive regulation of cytokine production). Secondly, functional group labels highlight muscle tissue level processes (e.g. muscle structural development and vascular development) or the involvement of low abundance cells (e.g. lymphocyte activation). The genes driving these significant overlapping interactions are listed in Supplementary Data S11.
Figure 5.
Discovery of interacting functional pathways that contain hypertrophy-related ncRNA genes. (A) Sunburst plot denoting the hierarchical organization of ncRNA containing co-expression network modules constructed from 437 resting skeletal muscle biopsy samples (<50 years old), using MEGENA (35). Each concentric ring corresponds to a cluster of co-expressed genes, with the size of the ring being proportional to the number of genes. Filled concentric rings are modules that contain at least one of the 110 hypertrophy-related ncRNA genes. (B) Using the compareCluster function (66,67) in the ClusterProfiler R package and the GOTERM_BP_ALL from DAVID as the ontology database, and the ∼13.4K protein coding genes expressed in muscle, we established the relationships between hypertrophy ncRNA containing modules. A q-value of 1% was utilized as a filter for presenting significant ontologies. Pie-charts illustrate when genes from more than one network contribute to an individual significant ontology category, while some ontology categories extend across distinct grouped functions. Further, each network can contain multiple ontologies which also show overlap within and across groups of functional processes. Finally, there is evidence that interacting groups of functions occur at the level of immune cell types. This analysis demonstrates that the ncRNAs interact at both the pathway and cell interaction level in a coordinated manner.
Table 1.
Summary of biologically relevant significant RNA co-expression modules containing hypertrophy-related ncRNA genes
| Network | Non-coding candidate | Module size | Module biotype composition | Biological processes | Cellular components |
|---|---|---|---|---|---|
| 1 (Figure 6) | CYTOR | 60 | 98% protein-coding, 2% lncRNA, 0% miscRNA, 0% pseudogene | Lymphocyte activation (FDR = 4.96 × 10−7; FE = 6.62). Leukocyte migration (FDR = 1.05 × 10−6; FE = 10.61). Leukocyte chemotaxis (FDR = 1.56 × 10−5; FE = 13.42) | Plasma membrane part (FDR = 1.53 × 10−9; FE = 3.44). Integral component of plasma membrane (FDR = 3.02 × 10−7; FE = 4.39). Intrinsic component of plasma membrane (FDR = 4.83 × 10−7; FE = 4.18) |
| 2 (Figure 7) | PPP1CB-DT | 52 | 81% protein-coding, 17% lncRNA, 0% miscRNA, 2% pseudogene | Myofibril assembly (FDR = 8.15 × 10−8; FE = 47.52). Cellular component assembly involved in morphogenesis (FDR = 8.15 × 10−8; FE = 30.68). Striated muscle contraction (FDR = 8.15 × 10−8; FE = 22.15) | Myofibril (FDR = 5.84 × 10−10; FE = 19.75). Contractile fiber (FDR = 5.84 × 10−10; FE = 19.02). Sarcomere (FDR = 2.72 × 10−9; FE = 19.86) |
| 3 (Figure 8) | EEF1A1P24, TMSB4XP8 | 370 | 95% protein-coding, 2% lncRNA, 0% miscRNA, 4% pseudogene | Cell migration (FDR = 5.05 × 10−12; FE = 2.64) Cardiovascular system development (FDR = 2.28 × 10−9; FE = 3.11) Vasculature development (FDR = 2.28 × 10−9; FE = 3.11) | Extracellular exosome (FDR = 3.96 × 10−6; FE = 1.90) Plasma membrane part (FDR = 3.96 × 10−6; FE = 1.74) Intrinsic component of plasma membrane (FDR = 2.20 × 10−5; FE = 2.02) |
| 4 (Supplementary Figure S5) | MYREM | 105 | 85% protein-coding, 11% lncRNA, 3% miscRNA, 1% pseudogene | N/S | Mitochondrial inner membrane (FDR = 3.24 × 10−5; FE = 5.99) |
| 5 | FAM157C | 224 | 79% protein-coding, 27% lncRNA, 3% miscRNA, 5% pseudogene | Positive regulation of T cell-mediated cytotoxicity (FDR = 2.93 × 10−5; FE = 26.14) Antigen processing and presentation of exogenous peptide antigen via MHC class I (FDR = 2.93 × 10−5; FE = 23.76) Antigen processing and presentation of endogenous peptide antigen via MHC class I (FDR = 2.93 × 10−5; FE = 32.67) | MHC protein complex (FDR = 1.35 × 10−8; FE = 38.15) Intrinsic component of endoplasmic reticulum membrane (FDR = 3.32 × 10−7; FE = 8.60) An integral component of the lumenal side of the endoplasmic reticulum membrane (FDR = 3.33 × 10−5; FE = 31.20) |
| 6 | DNMT3L-AS1, LINC01792, LINC01122, ENSG00000273674, ENSG00000258760 | 305 | 43% protein-coding, 45% lncRNA, 9% miscRNA, 3% pseudogene | N/S | Intermediate filament (FDR = 4.77 × 10−6; FE = 14.37) Intermediate filament cytoskeleton (FDR = 1.94 × 10−5; FE = 11.26) |
| 7 | LINC02210 | 385 | 83% protein-coding, 10% lncRNA, 3% miscRNA, 4% pseudogene | Nucleic acid metabolic process (FDR = 1.48 × 10−7; FE = 1.57) RNA processing (FDR = 1.01 × 10−5; FE = 2.41). RNA metabolic process (FDR = 1.31 × 10−5; FE = 1.53) | Nuclear lumen (FDR = 5.08 × 10−11; FE = 1.68) Nuclear part (FDR = 1.99 × 10−10; FE = 1.61). Intracellular organelle lumen (FDR = 1.99 × 10−10; FE = 1.54) |
| 8 | MIR4435-2HG | 112 | 90% protein-coding, 4% lncRNA, 1% miscRNA, 5% pseudogene | Antigen processing and presentation of exogenous peptide antigen via MHC class II (FDR = 1.26 × 10−5; FE = 42.62) Peptide antigen assembly with MHC class II protein complex (FDR = 1.26 × 10−5; FE = 70.46) | Integral component of lumenal side of endoplasmic reticulum membrane (FDR = 9.01 × 10−9; FE = 54.08) Lumenal side of endoplasmic reticulum membrane (FDR = 9.01 × 10−9; FE = 54.08) MHC class II protein complex (FDR = 6.36 × 10−8; FE = 62.26) |
| 9 | PAXIP1-AS2 | 86 | 90% protein-coding, 5% lncRNA, 2% miscRNA, 3% pseudogene | N/S | Nucleoplasm (FDR = 2.61 × 10−8; FE = 2.33) Nuclear lumen (FDR = 2.22 × 10−7; FE = 2.10) Nuclear part (FDR = 2.22 × 10−7; FE = 2.03) |
| 10 | RNU6-840P, RNU6-164P, Y_RNA_ENST00000362962 | 387 | 22% protein-coding, 9% lncRNA, 59% miscRNA, 10% pseudogene | N/S | Mitochondrial respiratory chain (FDR = 9.90 × 10−5; FE = 17.06) Inner mitochondrial membrane protein complex (FDR = 9.90 × 10−5; FE = 11.92) |
| 11 | RNU6-936P | 428 | 15% protein-coding, 4% lncRNA, 79% miscRNA, 2% pseudogene | RNA processing (FDR = 8.34 × 10−11; FE = 5.44). | Spliceosomal snRNP complex (FDR = 1.05 × 10−5; FE = 21.16) Small nuclear ribonucleoprotein complex (FDR = 1.05 × 10−5; FE = 19.70) |
| 12 | ZNF271P | 146 | 87% protein-coding, 6% lncRNA, 3% miscRNA, 3% pseudogene | Nucleic acid metabolic process (FDR = 6.02 × 10−7; FE = 1.85). | Nucleus (FDR = 1.15 × 10−5; FE = 1.50) Centrosome (FDR = 9.18 × 10−5; FE = 3.74) |
Biologically relevant modules contained at least one of the 110 hypertrophy-related ncRNA genes, <500 co-expressed genes, and had an FDR of ≤ 5.0 × 10−5 for at least one biological process and/or cellular component. For brevity, only three enriched GO terms per category are shown; for a complete list of all enriched GO terms, please refer to Supplementary Data S10.
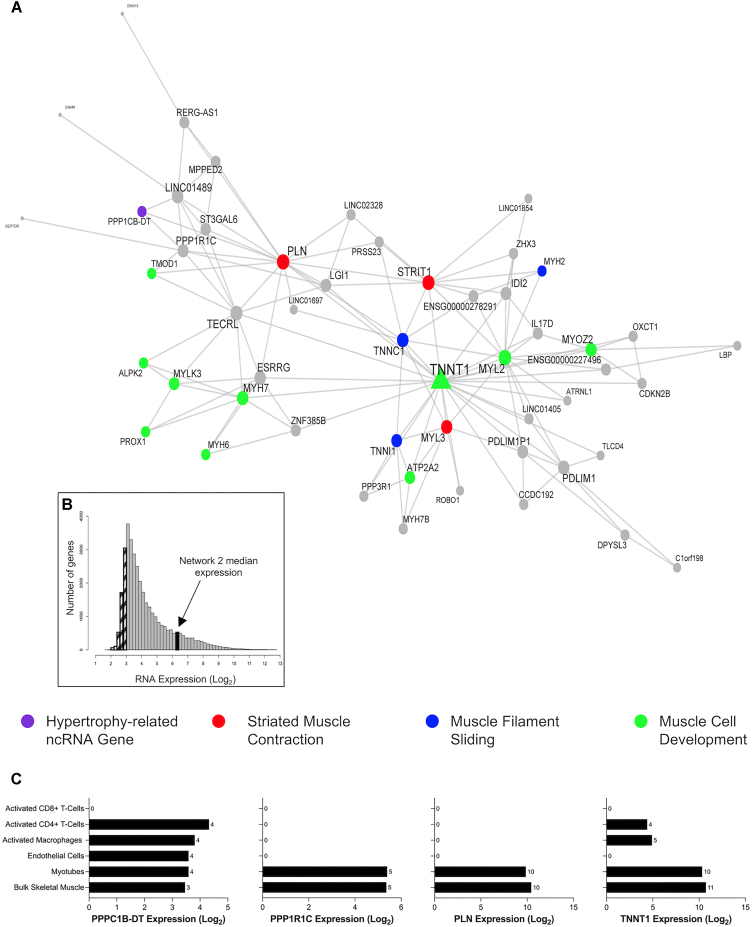
We next explored, in fine detail, some of the key MEGENA networks. Network 1 contained 60 genes, including CYTOR—a ncRNA known to regulate hypertrophy in model systems (Figure 6A and Table 1)—and the network represented immune-related processes e.g. leukocyte migration (FDR = 1.05 × 10−6; fold enrichment [FE] = 10.61), lymphocyte activation (FDR = 4 × 10−7; FE = 6.62) and leukocyte chemotaxis (FDR = 1.56 × 10−5; FE = 13.42). The hub gene of this module was lymphocyte cytosolic protein 1 (LCP1 or L-plastin), which has a direct role in aggregating actin filaments into parallel bundles that is critical for T-cell activation, migration and stability (82). LCP1 expression was positively correlated with CYTOR expression (r = 0.49) (Supplementary Figure S3). FCER1G, which encodes the Fc Epsilon Receptor Ig protein, facilitates the activation of natural killer cell lymphocytes, and it was also positively correlated with CYTOR (r = 0.66; Supplementary Figure S4A). The median expression of the network genes in skeletal muscle is very low (3.7 log2 units; Figure 6B), indicating that this module of co-expressed genes is likely expressed in the small number of immune cells found in muscle tissue—illustrating the sensitivity of our transcriptomic methods. To further investigate, we reprocessed publicly available data from the same laboratory platform, obtained from human primary immune cells (macrophages [GSE124106], CD8 + T cells [GSE117230], CD4 + T cells [GSE62921] and endothelial cells [GSE236137]), and our own in vitro human myotube data (27). We noted that LCP1, FCER1G and SLC31A2 are mostly expressed in immune cells (macrophages, CD8 + and/or CD4 + T-cells), compared with other cell types found in skeletal muscle tissue samples (Figure 6C). This trend is observed for most genes in the LCP1 module (Supplementary Figure S4B and C). Thus, CYTOR regulation is linked to successful muscle hypertrophy in humans and is found in an immune-cell-related module.
Figure 6.
CYTOR is the only ncRNA gene co-expressed in network 1 and is biologically associated with immune cell-related functions. (A) A biologically enriched gene co-expression network (network 1; Table 1), which contains the hypertrophy-related ncRNA gene, CYTOR. This network of genes is related to immunological biological processes, such as lymphocyte activation, leukocyte migration and leukocyte chemotaxis. Node and label size are proportional to node degree value within each network, and triangle symbols denote genes that are hubs of the cluster of co-expressed genes. (B) A frequency plot of the median log2 expression of 35k genes in resting bulk skeletal muscle. Hatched bars reflect gene signals most likely indistinguishable from background signal (15). The black bar denotes the median log2 expression of all 60 genes apart from network 1. (C) The log2 expression of several network 1 genes (CYTOR, SLC31A2, FCER1G, LCP1) in bulk skeletal muscle, human myotubes, endothelial cells, activated macrophages, CD8 + T cells and CD4 + T cells.
The next network (network 2) investigated (Figure 7A and Table 1) centred around the slow skeletal muscle troponin isoform, TNNT1. This module was composed of genes robustly co-expressed in muscle cells, median expression of 6.4 log2 units (Figure 7 and Supplementary Figure S5A). Nested within this module was a hypertrophy-related ncRNA gene, phosphatase 1 catalytic subunit beta divergent transcript (PPP1CB-DT; Figure 7A). Based on DAVID analyses, the network was enriched in biological processes related to myofibril assembly (FDR = 8.15 × 10−8; FE = 47.52), striated muscle contraction (FDR = 8.15 × 10−8; FE = 47.52), muscle filament sliding (FDR = 8.15 × 10−8; FE = 22.5), and the ontology label ‘transition between fast and slow fibre’ (FDR = 2.80 × 10−7; FE = 217.81) above the muscle transcriptome. Attractin-like-1 (ATRNL1) is in this network, and we previously identified (25) ATRNL1 was correlated with human skeletal muscle growth in a targeted analysis. The module also contained estrogen-related receptor gamma (ESRRG), which contributes to exercise-mediated remodelling of skeletal muscle function (83,84). In mouse models, ESRRG promotes an increased number of oxidative muscle fibres and is considered a regulator of oxidative metabolism (85). The expression levels of most genes in this module are positively associated with markers of type I fibres and negatively associated with markers of type II fibres (Supplementary Figure S5B and C). Collectively, the TNNT1 module most likely reflects genes that enhance the oxidative capacity of skeletal muscle, a common occurrence with RT in humans (86,87).
Figure 7.
A highly expressed gene co-expression network in skeletal muscle is related to muscle remodelling processes. (A) Network 2 is composed of 52 genes and contains the hypertrophy-related ncRNA gene, phosphatase 1 catalytic subunit beta divergent transcript (PPP1CB-DT). This network of genes is related to skeletal muscle remodelling processes, such as striated muscle contraction, muscle filament sliding and muscle cell development. Node and label size are proportional to node degree value within each network, and triangle symbols denote genes that are hubs of the cluster of co-expressed genes. (B) A frequency plot of the median log2 expression of 35k genes in resting bulk skeletal muscle. Hatched bars reflect gene signals most likely indistinguishable from background signal (15). The black bar denotes the median log2 expression of all 52 genes apart from network 2. (C) The log2 expression of several network 2 genes (PPPC1B-DT, PP1R1C, PLN, TNNT1) in bulk skeletal muscle, human myotubes, endothelial cells, activated macrophages, CD8 + T cells and CD4 + T cells.
A final module we considered in detail (370 genes; Figure 8 and Table 1; Supplementary Data S9 and S10) was centred around MAN1A1 and the ATLASTIN GTPase 3 (ATL3) genes, the latter of which are a constitutive endoplasmic reticulum fusion protein (88). Loss-of-function mutations in ATL3 lead to muscle weakness (89). This large module was enriched in GO terms related to microvascular remodelling, e.g., angiogenesis (FDR = 2.77 × 10−5; FE = 3.55), blood vessel development (FDR = 6.36 × 10−8; FE = 2.97), regulation of vasculature development (FDR = 1.66 × 10−6; FE = 3.71) and circulatory system development (FDR = 1.66 × 10−8; FE = 2.55). Network-based enrichment analysis (67) identified that these processes were directly linked to the muscle remodelling pathways (Figure 5B). EEF1A1P24 and TMSB4XP8 are two hypertrophy-related ncRNA genes in this module, and both are pseudogenes (Figure 8). Interestingly, they are part of a correlative cluster of several other pseudogenes (median R = 0.60; EEF1A1P9, EEF1A1P17, EEF1A1P25, EEF1A1P22, EEF1A1P16, EEF1A1P6, TMSB4XP4, TMSB4XP2; Figure 8 box inset). Pseudogenes are defined as copies of parent genes that are either retro-transposed counterparts or processed mRNAs (i.e. processed pseudogenes) or harbour mutations derived from gene duplication (i.e. unprocessed pseudogenes) (90). In recent years, these genes have been identified as potential decoys for microRNAs and modulators of the sensitivity of the related protein-coding genes to inhibition (91).
Figure 8.
RNA network related to vascular remodelling harbours a pair of pseudogenes related to skeletal muscle hypertrophy. This network is one of the largest gene co-expression RNA networks (370 genes), and a significant proportion of genes are associated with cell migration, vasculature development, and regulation of angiogenesis. Embedded in this network are a cluster of pseudogenes (EEF1A1P9, EEF1A1P24, EEF1A1P17, EEF1A1P25, EEF1A1P22, EEF1A1P16, EEF1A1P6, TMSB4XP4, TMSB4XP2, TMSB4XP8; black box), two of which are hypertrophy-related ncRNA genes (TMSB4XP8 and EEF1A1P24, purple). Node and label size are proportional to node degree value within each network, and triangle symbols denote genes that are hubs of the cluster of co-expressed genes.
Discussion
The present study is a comprehensive profile of the human skeletal muscle ncRNA transcriptome, which successfully identified numerous hypertrophy ncRNA candidates embedded in tissue growth pathways, often in a cell-specific manner and that they represent modules of integrated overlapping pathways. Most of the 110 ncRNA genes linked to successful hypertrophy would not have been identified had we relied on a typical RNA sequencing workflow. The use of data-driven quantitative RNA network modelling in independent data, along with network-based pathway enrichment and single-cell spatial transcript profiles allowed us to identify the functional pathways and cell types these ncRNA genes operated within. We established that type II myofibril-associated MYREM demonstrates specific myonuclear domain expression in vivo in human muscle. This work also added to the growing body of evidence that local immune-related responses, mitochondrial remodelling, and angiogenesis are all critically important contributors to successful human skeletal muscle hypertrophy (23,92,93).
The benefits of high-density array profiling to study the skeletal muscle ncRNA transcriptome
A majority of recent skeletal muscle transcriptome studies have relied on short-read RNA sequencing (28,70,71,94–96). We have shown that short-read RNA sequencing profiling of human skeletal muscle detects only ∼20% of constitutively expressed ncRNAs and demonstrates enormous inter-study heterogeneity (70,71) (see Figure 3E). In the present study we estimate that >7000 ncRNA genes are detected in every skeletal muscle sample (Figure 3A), consistent with our previous work (13,15,27,32). Notably, only 30% of the 110 hypertrophy ncRNA genes were consistently detected in two short-read RNA sequencing studies of skeletal muscle growth (Figure 3E). Stochastic properties of sequencing and the reliance on poly-A enrichment strategies (ncRNAs are deficient in poly-A tails) means that most ncRNAs are not detected. A striking example is H19, a hypertrophy and exercise-related ncRNA that is abundantly expressed in human skeletal muscle (Supplementary Data S8), yet a recent RNA sequencing study indicated that H19 was not ‘robustly expressed’ in human skeletal muscle (28). In 2012, we identified H19 as being part of a baseline gene expression signature predictive of the cardiovascular adaptive response to exercise training (76). More recent work demonstrated that the H19 locus encodes several miRNAs that regulate protein translation initiation factors implicated in protein synthesis (97). Interestingly, H19 directly interacts with the C-terminal domain of dystrophin, blocking its degradation (98). Dystrophin links the internal cytoskeleton to the extracellular matrix (99), and transcriptome responses to exercise are linked to Dystrophin status (68). In Duchenne Muscular Dystrophy (DMD), H19 interaction with dystrophin is impaired, resulting in the degradation of dystrophin and muscle degeneration (98). Clearly, all of this evidence indicates that H19 is a crucially important lncRNA in muscle (with wide-spread expression, Figure 4C) and directly contributes to regulating tissue remodelling.
Identifying the spatial localization of ncRNA genes aids in generating functional hypotheses
Skeletal muscle is a conglomeration of cells dominated by large multinucleated myofibers while containing mononucleated cells, including satellite cells, endothelial cells, immune cells and pericytes. There is growing support that these rarer cells contribute to skeletal muscle remodelling and that perturbation of their role is related to muscle dysfunction (57,100,101). Using merfish probes, we illustrated that our hypertrophy ncRNA MYREM was localised near the periphery of skeletal muscle fibres and co-stained with DAPI indicative of mature myonuclei (102). Previous in vitro work showed that MYREM was markedly abundant in the nuclei of myotubes compared with the nuclei of myoblasts (37). Our data indicate that MYREM appears to be a novel marker of human myonuclei in vivo (absent from cells expressing endothelial or satellite cell marker genes). Nuclear lncRNAs participate in vital processes, such as chromatin organisation, transcriptional regulation and RNA processing (103). MYREM interacts with the RNA-binding protein (RBP), heterogeneous nuclear ribonucleoprotein L (HNRNPL) to enhance the transcription of the fast-twitch isoform skeletal muscle myosin-binding protein C2 (MYBPC2). MYBPC2 is involved during the later stages of the myogenic program, whereby mononucleated myoblasts fuse into multinucleated myotubes, and the myosin heavy chain proteins are expressed and function in muscle contraction (104). Interestingly, network modelling indicated that MYREM is co-expressed with a mitochondrial-inner membrane gene expression module (Supplementary Figure S6) containing genes such as ATP5F1D, NDUFA3, but also the protein-translation initiation factor EIF3K (105). Remodelling of the mitochondria during hypertrophy-induced myogenesis may support increased energetic-related mechanisms (e.g. translation (106)), explaining why a myogenesis lncRNA is co-expressed with mitochondrial-related genes and given this may mostly reflect the physiological loading of type II muscle fibres.
To our knowledge, we are the first to report that MEG3 localizes rather selectively to muscle satellite cells in human skeletal muscle (Figure 4D), while MEG3 has been reported to be nuclear located in other cell types (37). MEG3 is a maternally imprinted gene known to have tumour-suppressive properties in various cancers (107). Interestingly, Gtl2, the mouse ortholog of MEG3 (108), targets the polycomb repressive complex 2 to epigenetically silence a variety of genes from the Dlk1-Dio3 locus, including the protein-coding transmembrane gene, Dlk1 (109). A muscle-specific dlk1 knockout in mice has altered satellite cell lineage progression following skeletal muscle injury (110), and overexpression of dlk1 is associated with pathological hypertrophy (111,112). Taken together, the spatial location, network and whole tissue bulk correlation analysis indicates that several hypertrophy-related ncRNA genes in skeletal muscle are likely mediating their influence via rare cell types (by abundance) in skeletal muscle.
Data-driven network analysis provides biological insight into novel ncRNAs
Most of the hypertrophy-related ncRNAs in the present study had no previous roles in skeletal muscle biology (Supplementary Table S2). For example, PPP1CB-DT was co-expressed in a module of genes related to contractile fibre remodelling (Figure 7). PPP1CB-DT is a divergent ncRNA to PPP1CB, a protein-coding gene for one of three catalytic subunits of protein phosphatase 1 (PP1), which is a serine/threonine-specific protein phosphatase that plays major roles in regulating skeletal muscle contractility (113). Divergent ncRNAs are transcribed in the opposite direction from the promoters of protein-coding genes (114) and may affect adjacent protein-coding genes through direct regulation in cis (115) or by participating in chromatin modifications (116). We also noted that a pair of hypertrophy-related pseudogenes, EEF1A1P24 and TMSB4XP8, were found to be regulated in a vascular remodelling and angiogenesis module (Figure 8). The angiogenic molecular program is tightly coupled to skeletal muscle growth (25,117), and enhancing angiogenesis (by aerobic conditioning) may augment growth (118). TMSB4XP8 is a thymosin beta 4-X (TMSB4X) pseudogene, an actin-binding protein with known roles in cell migration and angiogenesis (119). In addition, EEF1A1P24 is a processed pseudogene for the protein-coding gene, eukaryotic elongation factor 1 (EEF1A), which plays an important role in delivering the amino-acyl tRNA to the ribosome during protein synthesis (120). Until recently a broad misunderstanding is that pseudogenes are non-functional counterparts to their related genes (90). Pseudogenes exert biological functions in a DNA (facilitating chromatin structure remodelling or gene conversion (90)), RNA (competitive endogenous RNA networks (121)) and/or via protein (some pseudogenes contain protein-coding potential (6)) dependent manner. We demonstrated, using network-based pathway modelling on large-scale quantitative network models of independent muscle transcriptomes (n = 437), that a group defined as ‘blood muscle morphogenesis and development’ directly connected the angiogenesis MEGENA modules with muscle tissue development (Figure 5B). These vascular-related ncRNAs may aid modification of microvascular flow during exercise (122) or feeding (123), which helps contribute to meeting the demands of increased rates of protein synthesis and overall a key role for the vascular system modulating muscle hypertrophy is now apparent (25,117,118).
RNA networks reveal novel roles of ncRNA genes in skeletal muscle immunomodulation
The immune system also appears to be a novel regulator of skeletal muscle remodelling (101). Our ncRNA analyses highlight biological processes distinct from our previously described protein-coding hypertrophy signature (25), such as immune-cell-related functions (Supplementary Figure S7). For example, MIR4435-2HG (a miRNA host gene) was co-expressed in a module dominated by immune-related processes (e.g. peptide antigen assembly with MHC class II protein complex; Table 1; Supplementary Data S10), and MIR4435-2HG has most recently described as a gene called ‘Morrbid’. Morrbid tightly regulates the lifespan and turnover of myeloid cells (e.g. neutrophils and eosinophils) by regulating the transcription of pro-apoptotic genes (94,124). Notably, CYTOR was also co-expressed in an immune-dominated protein-coding RNA module (Figure 6), with robust expression in macrophages, CD8 + T cells and CD4 + T cells (Supplementary Figure S4B). To date, the characterization of CYTOR in mouse muscle tissue has focused on muscle cells and not other cell types found within muscle tissue (94). In muscle cells, CYTOR sequesters the TEAD1 transcription factor (94), which can transcriptionally regulate the small peptide Apelin that regulates paracrine communication between muscle cells and endothelial cells (125). Apelin is a feature of the protein-coding pathways associated with human muscle growth. We find that CYTOR was widely expressed across multiple cell types (Figure 6C), suggesting that CYTOR can impact skeletal muscle hypertrophy via multiple mechanisms. The present analysis suggests a model whereby a number of rare but important resident secretory cell types may regulate adaptation and release factors in a paracrine manner (e.g. a non-muscle source for various proposed myokines) to promote skeletal muscle remodelling (101). Collectively, our analyses reveal that low-abundance cell types can be directly studied in bulk transcriptomic profiles of human skeletal muscle tissue.
Limitations
The present human clinical studies are not without its limitations. Four out of the five exercise loading studies relied on DXA to quantify changes in LLM, and the hydration status of the participant can influence this measure and contribute to the clinical variation (126) and this may have reduced our ability to identify ncRNA hypertrophy candidates. With respect to the interpretation of our results, although ncRNAs are predicted to contain no protein-coding potential, some ncRNAs contain small open reading frames that encode peptides (6,12). Without additional experiments, some of the hypertrophy-related ncRNAs identified in the current study may also reflect the actions of novel bioactive peptides. Nonetheless, detailed mechanistic studies will be required to establish the direct involvement of each ncRNA in the hypertrophic response. Nevertheless, the few ncRNAs known to have causal links to cell growth and muscle tissue hypertrophy were identified by our methodologies.
Supplementary Material
Acknowledgements
J.C.M. was supported by a Queens University Vice Principal Research Postdoctoral Fund. This work was also supported by Medical Research Council, UK through grants G1100015 and MR/Y010329/1 (to J.A.T.) and by the National Institutes of Health, USA (1R56AG061911-01 to C.W. and J.T.). This work was also funded by a grant to S.M.P. from the National Science and Engineering Research Council (NSERC) of Canada. S.M.P. is supported by the Canada Research Chairs Program.
Contributor Information
Jonathan C Mcleod, Department of Kinesiology, McMaster University, Hamilton, Ontario, L8S 4L8, Canada.
Changhyun Lim, Department of Kinesiology, McMaster University, Hamilton, Ontario, L8S 4L8, Canada; Population Health Sciences Institute, Faculty of Medicial Sciences, Newcastle University, Newcastle upon Tyne, NE2 4AX, UK.
Tanner Stokes, Department of Kinesiology, McMaster University, Hamilton, Ontario, L8S 4L8, Canada.
Jalil-Ahmad Sharif, Faculty of Medicine and Dentistry, Queen Mary University London, London, E1 4NS, UK.
Vagif Zeynalli, Department of Kinesiology, McMaster University, Hamilton, Ontario, L8S 4L8, Canada.
Lucas Wiens, Department of Kinesiology, McMaster University, Hamilton, Ontario, L8S 4L8, Canada.
Alysha C D’Souza, Department of Kinesiology, McMaster University, Hamilton, Ontario, L8S 4L8, Canada.
Lauren Colenso-Semple, Department of Kinesiology, McMaster University, Hamilton, Ontario, L8S 4L8, Canada.
James McKendry, Department of Kinesiology, McMaster University, Hamilton, Ontario, L8S 4L8, Canada; Faculty of Land and Food Systems, Food, Nutrition & Health, University of British Columbia, BC, V6T 1Z4, Canada.
Robert W Morton, Department of Kinesiology, McMaster University, Hamilton, Ontario, L8S 4L8, Canada.
Cameron J Mitchell, School of Kinesiology, University of British Columbia, BC, V6T 1Z1, Canada.
Sara Y Oikawa, Department of Kinesiology, McMaster University, Hamilton, Ontario, L8S 4L8, Canada.
Claes Wahlestedt, University of Miami Miller School of Medicine, Miami, FL, 33136, USA.
J Paul Chapple, Faculty of Medicine and Dentistry, Queen Mary University London, London, E1 4NS, UK.
Chris McGlory, School of Kinesiology and Health Studies, Queens University, Kingston, ON, K7L 3N6, Canada.
James A Timmons, Faculty of Medicine and Dentistry, Queen Mary University London, London, E1 4NS, UK; University of Miami Miller School of Medicine, Miami, FL, 33136, USA.
Stuart M Phillips, Department of Kinesiology, McMaster University, Hamilton, Ontario, L8S 4L8, Canada.
Data availability
The accession number for the newly generated gene expression data reported in this paper is deposited at GSE154846, along with new raw data produced for this study (GSE270823). Code for the various informatics analyses can be readily obtained by contacting the authors.
Supplementary data
Supplementary Data are available at NARMME Online.
Funding
Medical Research Council [G1100015, MR/Y010329/1 to J.A.T.]; National Institutes of Health [1R56AG061911-01 to C.W., J.T.]; Natural Sciences and Engineering Research Council of Canada.
Conflict of interest statement
SMP reports grants or research contracts from the US National Dairy Council, Canadian Institutes for Health Research, Cargill, Friesland Campina, Dairy Farmers of Canada, Roquette Freres, Ontario Center of Innovation, Nestle Health Sciences, National Science and Engineering Research Council, and the US NIH during the conduct of the study; personal fees from Nestle Health Sciences; and nonfinancial support from Enhanced Recovery, outside the submitted work. SMP holds patents licensed to Exerkine but reports no financial gains from patents or related work. All other authors report no conflicts of interest.
References
- 1.Amaral P., Carbonell-Sala S., De L., Vega F.M., Faial T., Frankish A., Gingeras T., Guigo R., Harrow J.L., Hatzigeorgiou A.G.et al.. The status of the human gene catalogue. Nature. 2023; 622:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark M.B., Amaral P.P., Schlesinger F.J., Dinger M.E., Taft R.J., Rinn J.L., Ponting C.P., Stadler P.F., Morris K.V., Morillon A.et al.. The reality of pervasive transcription. PLoS Biol. 2011; 9:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu G., Mattick J., Taft R.J.. A meta-analysis of the genomic and transcriptomic composition of complex life. Cell Cycle. 2013; 12:2061–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattick J.S., Amaral P.P., Carninci P., Carpenter S., Chang H.Y., Chen L.L., Chen R., Dean C., Dinger M.E., Fitzgerald K.A.et al.. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023; 24:430–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ugalde A.P., Roiz-Valle D., Moledo-Nodar L., Caravia X.M., Freije J.M.P., López-Otín C.. Noncoding RNA contribution to aging and lifespan. J. Gerontol. A Biol. Sci. Med. Sci. 2024; 79:glae058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian S.H., Chen L., Xiong Y.-L., Chen Z.-X.. Evolution and function of developmentally dynamic pseudogenes in mammals. Genome Biol. 2022; 23:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kesner J.S., Chen Z., Shi P., Aparicio A.O., Murphy M.R., Guo Y., Trehan A., Lipponen J.E., Recinos Y., Myeku N.et al.. Noncoding translation mitigation. Nature. 2023; 617:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutbrod M.J., Martienssen R.A.. Conserved chromosomal functions of RNA interference. Nat. Rev. Genet. 2020; 21:311–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastori C., Kapranov P., Penas C., Peschansky V., Volmar C.H., Sarkaria J.N., Bregy A., Komotar R., Laurent G.S., Ayad N.G.et al.. The bromodomain protein BRD4 controls HOTAIR, a long noncoding RNA essential for glioblastoma proliferation. Proc. Natl Acad. Sci. U.S.A. 2015; 112:8326–8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang F., Lupski J.R.. Non-coding genetic variants in human disease: figure 1. Hum. Mol. Genet. 2015; 24:R102–R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler A.A., Johnston D.R., Kaur S., Lubin F.D.. Long noncoding RNA NEAT1 mediates neuronal histone methylation and age-related memory impairment. Sci. Signal. 2019; 12:eaaw9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee C., Zeng J., Drew B.G., Sallam T., Martin-Montalvo A., Wan J., Kim S.J., Mehta H., Hevener A.L., De Cabo R.et al.. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015; 21:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timmons J.A., Atherton P.J., Larsson O., Sood S., Blokhin I.O., Brogan R.J., Volmar C.H., Josse A.R., Slentz C., Wahlestedt C.et al.. A coding and non-coding transcriptomic perspective on the genomics of human metabolic disease. Nucleic Acids Res. 2018; 46:7772–7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeasmin F., Yada T., Akimitsu N.. Micropeptides encoded in transcripts previously identified as long noncoding RNAs: a new chapter in transcriptomics and proteomics. Front. Genet. 2018; 9:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stokes T., Cen H.H., Kapranov P., Gallagher I.J., Pitsillides A.A., Volmar C., Kraus W.E., Johnson J.D., Phillips S.M., Wahlestedt C.et al.. Transcriptomics for clinical and experimental biology research: hang on a Seq. Adv. Genet. 2023; 4:2200024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssen I., Heymsfield S.B., Wang Z., Ross R.. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000; 89:81–88. [DOI] [PubMed] [Google Scholar]
- 17.Timmons J.A. Molecular diagnostics of ageing and tackling age-related disease. Trends Pharmacol. Sci. 2017; 38:67–80. [DOI] [PubMed] [Google Scholar]
- 18.McKendry J., Stokes T., McLeod J.C., Phillips S.M.. Resistance exercise, aging, disuse, and muscle protein metabolism. Compr. Physiol. 2021; 11:2249–2278. [DOI] [PubMed] [Google Scholar]
- 19.Davidsen P.K., Gallagher I.J., Hartman J.W., Tarnopolsky M.A., Dela F., Helge J.W., Timmons J.A., Phillips S.M.. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J. Appl. Physiol. 2011; 110:309–317. [DOI] [PubMed] [Google Scholar]
- 20.Lixandrão M.E., Bamman M., Vechin F.C., Conceicao M.S., Telles G., Longobardi I., Damas F., Lavin K.M., Drummer D.J., McAdam J.S.et al.. Higher resistance training volume offsets muscle hypertrophy nonresponsiveness in older individuals. J. Appl. Physiol. 2024; 136:421–429. [DOI] [PubMed] [Google Scholar]
- 21.Currier B.S., Mcleod J.C., Banfield L., Beyene J., Welton N.J., D’Souza A.C., Keogh J.A.J., Lin L., Coletta G., Yang A.et al.. Resistance training prescription for muscle strength and hypertrophy in healthy adults: a systematic review and Bayesian network meta-analysis. Br. J. Sports Med. 2023; 57:1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morton R.W., Murphy K.T., McKellar S.R., Schoenfeld B.J., Henselmans M., Helms E., Aragon A.A., Devries M.C., Banfield L., Krieger J.W.et al.. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018; 52:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts M.D., McCarthy J.J., Hornberger T.A., Phillips S.M., Mackey A.L., Nader G.A., Boppart M.D., Kavazis A.N., Reidy P.T., Ogasawara R.et al.. Mechanisms of mechanical overload-induced skeletal muscle hypertrophy: current understanding and future directions. Physiol. Rev. 2023; 103:2679–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips B.E., Williams J.P., Gustafsson T., Bouchard C., Rankinen T., Knudsen S., Smith K., Timmons J.A., Atherton P.J.. Molecular networks of human muscle adaptation to exercise and age. PLoS Genet. 2013; 9:e1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stokes T., Timmons J.A., Crossland H., Tripp T.R., Murphy K., McGlory C., Mitchell C.J., Oikawa S.Y., Morton R.W., Phillips B.E.et al.. Molecular transducers of human skeletal muscle remodeling under different loading states. Cell Rep. 2020; 32:107980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wohlwend M., Laurila P.-P., Williams K., Romani M., Lima T., Pattawaran P., Benegiamo G., Salonen M., Schneider B.L., Lahti J.et al.. The exercise-induced long noncoding RNA CYTOR promotes fast-twitch myogenesis in aging. Sci. Transl. Med. 2021; 13:7367. [DOI] [PubMed] [Google Scholar]
- 27.Timmons J.A., Volmar C.H., Crossland H., Phillips B.E., Sood S., Janczura K.J., Törmäkangas T., Kujala U.M., Kraus W.E., Atherton P.J.et al.. Longevity-related molecular pathways are subject to midlife “switch” in humans. Aging Cell. 2019; 18:e12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller M.J., Gries K.J., Marcotte G.R., Ryan Z., Strub M.D., Kunz H.E., Arendt B.K., Dasari S., Ebert S.M., Adams C.M.et al.. Human myofiber-enriched aging-induced lncRNAFRAIL1 promotes loss of skeletal muscle function. Aging Cell. 2024; 23:e14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neppl R.L., Wu C.-L., Walsh K.. lncRNA Chronos is an aging-induced inhibitor of muscle hypertrophy. J. Cell Biol. 2017; 216:3497–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biferali B., Mocciaro E., Runfola V., Gabellini D.. Long non-coding RNAs and their role in muscle regeneration. Cur. Top Dev. Biol. 2024; 158:433–465. [DOI] [PubMed] [Google Scholar]
- 31.Ballarino M., Morlando M., Fatica A., Bozzoni I.. Non-coding RNAs in muscle differentiation and musculoskeletal disease. J. Clin. Invest. 2016; 126:2021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sood S., Szkop K.J., Nakhuda A., Gallagher I.J., Murie C., Brogan R.J., Kaprio J., Kainulainen H., Atherton P.J., Kujala U.M.et al.. iGEMS: an integrated model for identification of alternative exon usage events. Nucleic Acids Res. 2016; 44:e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherman B.T., Hao M., Qiu J., Jiao X., Baseler M.W., Lane H.C., Imamichi T., Chang W.. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022; 50:W216–W221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Day A., Dong J., Funari V.A., Harry B., Strom S.P., Cohn D.H., Nelson S.F.. Disease gene characterization through large-scale co-expression analysis. PLoS One. 2009; 4:e8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song W.M., Zhang B.. Multiscale embedded gene co-expression network analysis. PLoS Comput. Biol. 2015; 11:e1004574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ponting C.P., Haerty W.. Genome-wide analysis of human long noncoding RNAs: a provocative review. Annu. Rev. Genomics. Hum. Genet. 2022; 31:153–172. [DOI] [PubMed] [Google Scholar]
- 37.Cabili M.N., Dunagin M.C., McClanahan P.D., Biaesch A., Padovan-Merhar O., Regev A., Rinn J.L., Raj A.. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 2015; 16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez P., Radaelli R., Taaffe D.R., Newton R.U., Galvão D.A., Trajano G.S., Teodoro J.L., Kraemer W.J., Häkkinen K., Pinto R.S. Resistance training load effects on muscle hypertrophy and strength gain: systematic review and network meta-analysis. Med. Sci. Sports Exerc. 2021; 53:1206–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morton R.W., Oikawa S.Y., Wavell C.G., Mazara N., McGlory C., Quadrilatero J., Baechler B.L., Baker S.K., Phillips S.M.. Neither load nor systemic hormones determine resistance training-mediated hypertrophy or strength gains in resistance-trained young men. J. Appl. Physiol. 2016; 121:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morton R.W. Resistance exercise-induced muscle hypertrophy. 2019; McMaster University; http://hdl.handle.net/11375/24767. [Google Scholar]
- 41.Phillips B.E., Kelly B.M., Lilja M., Ponce-González J.G., Brogan R.J., Morris D.L., Gustafsson T., Kraus W.E., Atherton P.J., Vollaard N.B.J.et al.. A practical and time-efficient high-intensity interval training program modifies cardio-metabolic risk factors in adults with risk factors for type II diabetes. Front. Endocrinol. (Lausanne). 2017; 8:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell C.J., Churchward-Venne T.A., Parise G., Bellamy L., Baker S.K., Smith K., Atherton P.J., Phillips S.M.. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PLoS One. 2014; 9:e89431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powers C., Fan B., Borrud L.G., Looker A.C., Shepherd J.A.. Long-term precision of dual-energy X-ray absorptiometry body composition measurements and association with their covariates. J. Clin. Densitom. 2015; 18:76–85. [DOI] [PubMed] [Google Scholar]
- 44.Barlow M.J., Oldroyd B., Smith D., Lees M.J., Brightmore A., Till K., Jones B., Hind K.. Precision error in dual-energy X-ray absorptiometry body composition measurements in elite male rugby league players. J. Clin. Densitom. 2015; 18:546–550. [DOI] [PubMed] [Google Scholar]
- 45.LeBlanc A.D., Schneider V.S., Evans H.J., Pientok C., Rowe R., Spector E.. Regional changes in muscle mass following 17 weeks of bed rest. J. Applied Physiol. 1992; 73:2172–2178. [DOI] [PubMed] [Google Scholar]
- 46.Bengtsson H., Simpson K., Bullard J., Hansen K.. aroma.affymetrix: a generic framework in R for analyzing small to very large Affymetrix data sets in bounded memory. Department of Statistics, University of California, Berkeley. 2008; 745:1–9. [Google Scholar]
- 47.Harrow J., Frankish A., Gonzalez J.M., Tapanari E., Diekhans M., Kokocinski F., Aken B.L., Barrell D., Zadissa A., Searle S.et al.. GENCODE: the reference human genome annotation for the ENCODE project. Genome Res. 2012; 22:1760–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frankish A., Carbonell-Sala S., Diekhans M., Jungreis I., Loveland J.E., Mudge J.M., Sisu C., Wright J.C., Arnan C., Barnes I.et al.. GENCODE: reference annotation for the human and mouse genomes in 2023. Nucleic Acids Res. 2023; 51:D942–D949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R.. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013; 29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gautier L., Cope L., Bolstad B.M., Irizarry R.A.. Affy - Analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004; 20:307–315. [DOI] [PubMed] [Google Scholar]
- 51.Welsh E.A., Eschrich S.A., Berglund A.E., Fenstermacher D.A.. Iterative rank-order normalization of gene expression microarray data. BMC Bioinform. 2013; 14:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tusher V.G., Tibshirani R., Chu G.. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl Acad. Sci. U.S.A. 2001; 98:5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shuken S.R., McNerney M.W.. Costs and benefits of popular P-value correction methods in three models of quantitative omic experiments. Anal. Chem. 2023; 95:2732–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cinar O., Viechtbauer W.. The poolr package for combining independent and dependent p values. J. Stat. Softw. 2022; 101:1–42. [Google Scholar]
- 55.Benjaminit Y., Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. Soc. B. 1995; 57:289–300. [Google Scholar]
- 56.Pollard K.S., Dudoit S., van der Laan M.J.. Multiple testing procedures: r multtest package and applications to genomics. Bioinformatics and Computational Biology Solutions Using R and Bioconductor Springer. 2005; California, US. [Google Scholar]
- 57.Lai Y., Ramírez-Pardo I., Isern J., An J., Perdiguero E., Serrano A.L., Li J., García-Domínguez E., Segalés J., Guo P.et al.. Multimodal cell atlas of the ageing human skeletal muscle. Nature. 2024; 2024:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schiaffino S., Rossi A.C., Smerdu V., Leinwand L.A., Reggiani C.. Developmental myosins: expression patterns and functional significance. Skelet Muscle. 2015; 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Talbot J., Maves L.. Skeletal muscle fiber type: using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. WIREs Development. Biol. 2016; 5:518–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snijders T., Nederveen J.P., McKay B.R., Joanisse S., Verdijk L.B., van Loon L.J.C., Parise G.. Satellite cells in human skeletal muscle plasticity. Front. Physiol. 2015; 6:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ritso M., Tung L.W., Rossi F.M.V.. Emerging skeletal muscle stromal cell diversity: functional divergence in fibro/adipogenic progenitor and mural cell populations. Exp. Cell Res. 2022; 410:112947. [DOI] [PubMed] [Google Scholar]
- 62.Malm C., Nyberg P., Engström M., Sjödin B., Lenkei R., Ekblom B., Lundberg I.. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J. Physiol. 2000; 529:243–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xia C., Babcock H.P., Moffitt J.R., Zhuang X.. Multiplexed detection of RNA using MERFISH and branched DNA amplification. Sci. Rep. 2019; 9:7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moffitt J.R., Bambah-Mukku D., Eichhorn S.W., Vaughn E., Shekhar K., Perez J.D., Rubinstein N.D., Hao J., Regev A., Dulac C.et al.. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science (1979). 2018; 362:eaau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Timmons J.A., Szkop K.J., Gallagher I.J.. Multiple sources of bias confound functional enrichment analysis of global -omics data. Genome Biol. 2015; 16:15–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merico D., Isserlin R., Stueker O., Emili A., Bader G.D.. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One. 2010; 5:e13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu S., Hu E., Cai Y., Xie Z., Luo X., Zhan L., Tang W., Wang Q., Liu B., Wang R.et al.. Using clusterProfiler to characterize multiomics data. Nat. Protoc. 2024; 19:3292–3320. [DOI] [PubMed] [Google Scholar]
- 68.Timmons J.A., Larsson O., Jansson E., Fischer H., Gustafsson T., Greenhaff P.L., Ridden J., Rachman J., Peyrard-Janvid M., Wahlestedt C.et al.. Human muscle gene expression responses to endurance training provide a novel perspective on Duchenne muscular dystrophy. FASEB J. 2005; 19:750–760. [DOI] [PubMed] [Google Scholar]
- 69.Timmons J.A., Jansson E., Fischer H., Gustafsson T., Greenhaff P.L., Ridden J., Rachman J., Sundberg C.J.. Modulation of extracellular matrix genes reflects the magnitude of physiological adaptation to aerobic exercise training in humans. BMC Biol. 2005; 3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scott L.J., Erdos M.R., Huyghe J.R., Welch R.P., Beck A.T., Wolford B.N., Chines P.S., Didion J.P., Narisu N., Stringham H.M.et al.. The genetic regulatory signature of type 2 diabetes in human skeletal muscle. Nat. Commun. 2016; 7:11764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kulkarni A.S., Peck B.D., Walton R.G., Kern P.A., Mar J.C., Windham S.T., Bamman M.M., Barzilai N., Peterson C.A.. Metformin alters skeletal muscle transcriptome adaptations to resistance training in older adults. Aging. 2020; 12:19852–19866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zollinger D.R., Lingle S.E., Sorg K., Beechem J.M., Merritt C.R.. GeoMxTM RNA assay: high multiplex, digital, spatial analysis of RNA in FFPE tissue. Methods Mol. Biol. 2020; 2148:331–345. [DOI] [PubMed] [Google Scholar]
- 73.Chang M.-W., Yang J.-H., Tsitsipatis D., Yang X., Martindale J.L., Munk R., Pandey P.R., Banskota N., Romero B., Batish M.et al.. Enhanced myogenesis through lncFAM-mediated recruitment of HNRNPL to the MYBPC2 promoter. Nucleic Acids Res. 2022; 50:13026–13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li A., Nelson S.R., Rahmanseresht S., Braet F., Cornachione A.S., Previs S.B., O’Leary T.S., McNamara J.W., Rassier D.E., Sadayappan S.et al.. Skeletal MyBP-C isoforms tune the molecular contractility of divergent skeletal muscle systems. Proc. Natl Acad. Sci. U.S.A. 2019; 116:21882–21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gurnett C.A., Desruisseau D.M., McCall K., Choi R., Meyer Z.I., Talerico M., Miller S.E., Ju J.S., Pestronk A., Connolly A.M.et al.. Myosin binding protein C1: a novel gene for autosomal dominant distal arthrogryposis type 1. Hum. Mol. Genet. 2010; 19:1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Timmons J.A., Knudsen S., Rankinen T., Koch L.G., Sarzynski M., Jensen T., Keller P., Scheele C., Vollaard N.B.J., Nielsen S.et al.. Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. J. Appl. Physiol. 2010; 108:1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dill T.L., Carroll A., Pinheiro A., Gao J., Naya F.J.. The long noncoding RNA Meg3 regulates myoblast plasticity and muscle regeneration through epithelial-mesenchymal transition. Development (Cambridge). 2021; 148:dev194027. [DOI] [PubMed] [Google Scholar]
- 78.Dong K., Shen J., He X., Hu G., Wang L., Osman I., Bunting K.M., Dixon-Melvin R., Zheng Z., Xin H.et al.. CARMN is an evolutionarily conserved smooth muscle cell-specific LncRNA that maintains contractile phenotype by binding myocardin. Circulation. 2021; 144:1856–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shepro D., Morel N.M.L.. Pericyte physiology. FASEB J. 1993; 7:1031–1038. [DOI] [PubMed] [Google Scholar]
- 80.de Goede O.M., Nachun D.C., Ferraro N.M., Gloudemans M.J., Rao A.S., Smail C., Eulalio T.Y., Aguet F., Ng B., Xu J.et al.. Population-scale tissue transcriptomics maps long non-coding RNAs to complex disease. Cell. 2021; 184:2633–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lessard L., Liu M., Marzese D.M., Wang H., Chong K., Kawas N., Donovan N.C., Kiyohara E., Hsu S., Nelson N.et al.. The CASC15 long intergenic noncoding RNA locus is involved in melanoma progression and phenotype switching. J. Invest. Dermatol. 2015; 135:2464–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang C., Morley S.C., Donermeyer D., Peng I., Lee W.P., Devoss J., Danilenko D.M., Lin Z., Zhang J., Zhou J.et al.. Actin-bundling protein L-plastin regulates T cell activation. J. Immunol. 2010; 185:7487–7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wattez J.-S., Eury E., Hazen B.C., Wade A., Chau S., Ou S.-C., Russell A.P., Cho Y., Kralli A.. Loss of skeletal muscle estrogen-related receptors leads to severe exercise intolerance. Mol. Metab. 2023; 68:101670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Badin P.-M., Vila I.K., Sopariwala D.H., Yadav V., Lorca S., Louche K., Kim E.R., Tong Q., Song M.S., Moro C.et al.. Exercise-like effects by Estrogen-related receptor-gamma in muscle do not prevent insulin resistance in db/db mice. Sci. Rep. 2016; 6:26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Narkar V.A., Fan W., Downes M., Yu R.T., Jonker J.W., Alaynick W.A., Banayo E., Karunasiri M.S., Lorca S., Evans R.M.. Exercise and PGC-1α-independent synchronization of type I muscle metabolism and vasculature by ERRγ. Cell Metab. 2011; 13:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang L., Mascher H., Psilander N., Blomstrand E., Sahlin K.. Resistance exercise enhances the molecular signaling of mitochondrial biogenesis induced by endurance exercise in human skeletal muscle. J. Appl. Physiol. 2011; 111:1335–1344. [DOI] [PubMed] [Google Scholar]
- 87.Roberts M.D., Romero M.A., Mobley C.B., Mumford P.W., Roberson P.A., Haun C.T., Vann C.G., Osburn S.C., Holmes H.H., Greer R.A.et al.. Skeletal muscle mitochondrial volume and myozenin-1 protein differences exist between high versus low anabolic responders to resistance training. PeerJ. 2018; 6:e5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bryce S., Stolzer M., Crosby D., Yang R., Durand D., Lee T.H.. Human atlastin-3 is a constitutive ER membrane fusion catalyst. J. Cell Biol. 2023; 222:e202211021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mohammadi S., Jafari Khamirani H., Baneshi M., Kamal N., Manoocheri J., Saffar M., Dianatpour M., Tabei S.M.B., Dastgheib S.A.. A novel nonsense variant in the ATL3 gene is associated with disturbed pain sensitivity, numbness of distal limbs and muscle weakness. Ann. Hum. Genet. 2023; 87:147–157. [DOI] [PubMed] [Google Scholar]
- 90.Cheetham S.W., Faulkner G.J., Dinger M.E.. Overcoming challenges and dogmas to understand the functions of pseudogenes. Nat. Rev. Genet. 2020; 21:191–201. [DOI] [PubMed] [Google Scholar]
- 91.Jubran J., Hekselman I., Novack L., Yeger-Lotem E.. Dosage-sensitive molecular mechanisms are associated with the tissue-specificity of traits and diseases. Comput. Struct. Biotechnol. J. 2020; 18:4024–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sartori R., Romanello V., Sandri M.. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat. Commun. 2021; 12:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McIntosh M.C., Anglin D.A., Robinson A.T., Beck D.T., Roberts M.D.. Making the case for resistance training in improving vascular function and skeletal muscle capillarization. Front. Physiol. 2024; 15:1338507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wohlwend M., Laurila P.-P., Williams K., Romani M., Lima T., Pattawaran P., Benegiamo G., Salonen M., Schneider B.L., Lahti J.et al.. The exercise-induced long noncoding RNA CYTOR promotes fast-twitch myogenesis in aging. Sci. Transl. Med. 2021; 13:eabc7367. [DOI] [PubMed] [Google Scholar]
- 95.Tumasian R.A., Harish A., Kundu G., Yang J.H., Ubaida-Mohien C., Gonzalez-Freire M., Kaileh M., Zukley L.M., Chia C.W., Lyashkov A.et al.. Skeletal muscle transcriptome in healthy aging. Nat. Commun. 2021; 12:2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng Y., Liu T., Li Q., Li J.. Integrated analysis of long non-coding RNAs (lncRNAs) and mRNA expression profiles identifies lncRNA PRKG1-AS1 playing important roles in skeletal muscle aging. Aging. 2021; 13:15044–15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liang R., Shen X., Wang F., Wang X., DesJarlais A., Syed A., Saba R., Tan Z., Yu F., Ji X.et al.. H19X-encoded miR-322(424)/miR-503 regulates muscle mass by targeting translation initiation factors. J. Cachexia Sarcopenia Muscle. 2021; 12:2174–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Y., Li Y., Hu Q., Xi Y., Xing Z., Zhang Z., Huang L., Wu J., Liang K., Nguyen T.K.et al.. The lncRNA H19 alleviates muscular dystrophy by stabilizing dystrophin. Nat. Cell Biol. 2020; 22:1332–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ervasti J.M. Dystrophin, its interactions with other proteins, and implications for muscular dystrophy. Biochim. Biophys. Acta. 2007; 1772:108–117. [DOI] [PubMed] [Google Scholar]
- 100.Ziemkiewicz N., Hilliard G., Pullen N.A., Garg K.. The role of innate and adaptive immune cells in skeletal muscle regeneration. Int. J. Mol. Sci. 2021; 22:3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Langston P.K., Sun Y., Ryback B.A., Mueller A.L., Spiegelman B.M., Benoist C., Mathis D.. Regulatory T cells shield muscle mitochondria from interferon-γ-mediated damage to promote the beneficial effects of exercise. Sci. Immunol. 2023; 8:eadi5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dumont N.A., Bentzinger C.F., Sincennes M., Rudnicki M.A.. Satellite cells and skeletal muscle regeneration. Comprehensive Physiology. 2015; Wiley; 1027–1059. [DOI] [PubMed] [Google Scholar]
- 103.Sun Q., Hao Q., Prasanth K.V.. Nuclear long noncoding RNAs: key regulators of gene expression. Trends Genetics. 2018; 34:142–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ackermann M.A., Kontrogianni-Konstantopoulos A.. Myosin binding protein-C: a regulator of actomyosin interaction in striated muscle. J. Biomed. Biotechnol. 2011; 2011:636403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mayeur G.L., Fraser C.S., Peiretti F., Block K.L., Hershey J.W.B.. Characterization of eIF3k. Eur. J. Biochem. 2003; 270:4133–4139. [DOI] [PubMed] [Google Scholar]
- 106.Stouthamer A.H. A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Van Leeuwenhoek. 1973; 39:545–565. [DOI] [PubMed] [Google Scholar]
- 107.Zhou Y., Zhang X., Klibanski A.. MEG3 noncoding RNA: a tumor suppressor. J. Mol. Endocrinol. 2012; 48:R45–R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miyoshi N., Wagatsuma H., Wakana S., Shiroishi T., Nomura M., Aisaka K., Kohda T., Surani M.A., Kaneko-Ishino T., Ishino F.. Identification of an imprinted gene, Meg3 /Gtl2 and its human homologue MEG3, first mapped on mouse distal chromosome 12 and human chromosome 14q. Genes to Cells. 2000; 5:211–220. [DOI] [PubMed] [Google Scholar]
- 109.Zhao J., Ohsumi T.K., Kung J.T., Ogawa Y., Grau D.J., Sarma K., Song J.J., Kingston R.E., Borowsky M., Lee J.T.. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol. Cell. 2010; 40:939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Waddell J.N., Zhang P., Wen Y., Gupta S.K., Yevtodiyenko A., Schmidt J.V., Bidwell C.A., Kumar A., Kuang S.. Dlk1 is necessary for proper skeletal muscle development and regeneration. PLoS One. 2010; 5:e15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Andersen D.C., Petersson S.J., Jørgensen L.H., Bollen P., Jensen P.B., Teisner B., Schroeder H.D., Jensen C.H.. Characterization of DLK1+ cells emerging during skeletal muscle remodeling in response to myositis, myopathies, and acute injury. Stem Cells. 2009; 27:898–908. [DOI] [PubMed] [Google Scholar]
- 112.Davis E., Jensen C.H., Schroder H.D., Farnir F., Shay-Hadfield T., Kliem A., Cockett N., Georges M., Charlier C.. Ectopic expression of DLK1 protein in skeletal muscle of padumnal heterozygotes causes the callipyge phenotype. Current Biology. 2004; 14:1858–1862. [DOI] [PubMed] [Google Scholar]
- 113.Cohen P. The structure and regulation of protein phosphatases. Annu. Rev. Biochem. 1989; 58:453–508. [DOI] [PubMed] [Google Scholar]
- 114.Seila A.C., Calabrese J.M., Levine S.S., Yeo G.W., Rahl P.B., Flynn R.A., Young R.A., Sharp P.A.. Divergent transcription from active promoters. Science. 2008; 322:1849–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luo S., Lu J.Y., Liu L., Yin Y., Chen C., Han X., Wu B., Xu R., Liu W., Yan P.et al.. Divergent lncRNAs regulate gene expression and lineage differentiation in pluripotent cells. Cell Stem Cell. 2016; 18:637–652. [DOI] [PubMed] [Google Scholar]
- 116.Subhash S., Mishra K., Akhade V.S., Kanduri M., Mondal T., Kanduri C.. H3K4me2 and WDR5 enriched chromatin interacting long non-coding RNAs maintain transcriptionally competent chromatin at divergent transcriptional units. Nucleic Acids Res. 2018; 46:9384–9400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Betz M.W., Aussieker T., Kruger C.Q., Gorissen S.H.M., van Loon L.J.C., Snijders T.. Muscle fiber capillarization is associated with various indices of skeletal muscle mass in healthy, older men. Exp. Gerontol. 2021; 143:111161. [DOI] [PubMed] [Google Scholar]
- 118.Thomas A.C.Q., Brown A., Hatt A.A., Manta K., Costa-Parke A., Kamal M., Joanisse S., McGlory C., Phillips S.M., Kumbhare D.et al.. Short-term aerobic conditioning prior to resistance training augments muscle hypertrophy and satellite cell content in healthy young men and women. FASEB J. 2022; 36:e22500. [DOI] [PubMed] [Google Scholar]
- 119.Smart N., Rossdeutsch A., Riley P.R.. Thymosin β4 and angiogenesis: modes of action and therapeutic potential. Angiogenesis. 2007; 10:229–241. [DOI] [PubMed] [Google Scholar]
- 120.Sasikumar A.N., Perez W.B., Kinzy T.G.. The many roles of the eukaryotic elongation factor 1 complex. WIREs RNA. 2012; 3:543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Glenfield C., McLysaght A.. Pseudogenes provide evolutionary evidence for the competitive endogenous RNA hypothesis. Mol. Biol. Evol. 2018; 35:2886–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hildebrandt W., Schwarzbach H., Pardun A., Hannemann L., Bogs B., König A.M., Mahnken A.H., Hildebrandt O., Koehler U., Kinscherf R.. Age-related differences in skeletal muscle microvascular response to exercise as detected by contrast-enhanced ultrasound (CEUS). PLoS One. 2017; 12:e0172771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Phillips B.E., Atherton P.J., Varadhan K., Limb M.C., Wilkinson D.J., Sjøberg K.A., Smith K., Williams J.P.. The effects of resistance exercise training on macro- and micro-circulatory responses to feeding and skeletal muscle protein anabolism in older men. J. Physiol. 2015; 593:2721–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kotzin J.J., Spencer S.P., McCright S.J., Kumar D.B.U., Collet M.A., Mowel W.K., Elliott E.N., Uyar A., Makiya M.A., Dunagin M.C.et al.. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature. 2016; 537:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee U., Stuelsatz P., Karaz S., McKellar D.W., Russeil J., Deak M., De Vlaminck I., Lepper C., Deplancke B., Cosgrove B.D.et al.. A Tead1-Apelin axis directs paracrine communication from myogenic to endothelial cells in skeletal muscle. Iscience. 2022; 25:104589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Coletta G., Phillips S.M. An elusive consensus definition of sarcopenia impedes research and clinical treatment: a narrative review. Ageing Res. Rev. 2023; 86:101883. [DOI] [PubMed] [Google Scholar]
- 127.Heberle H., Meirelles V.G., da Silva F.R., Telles G.P., Minghim R.. InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics. 2015; 16:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for the newly generated gene expression data reported in this paper is deposited at GSE154846, along with new raw data produced for this study (GSE270823). Code for the various informatics analyses can be readily obtained by contacting the authors.