Abstract
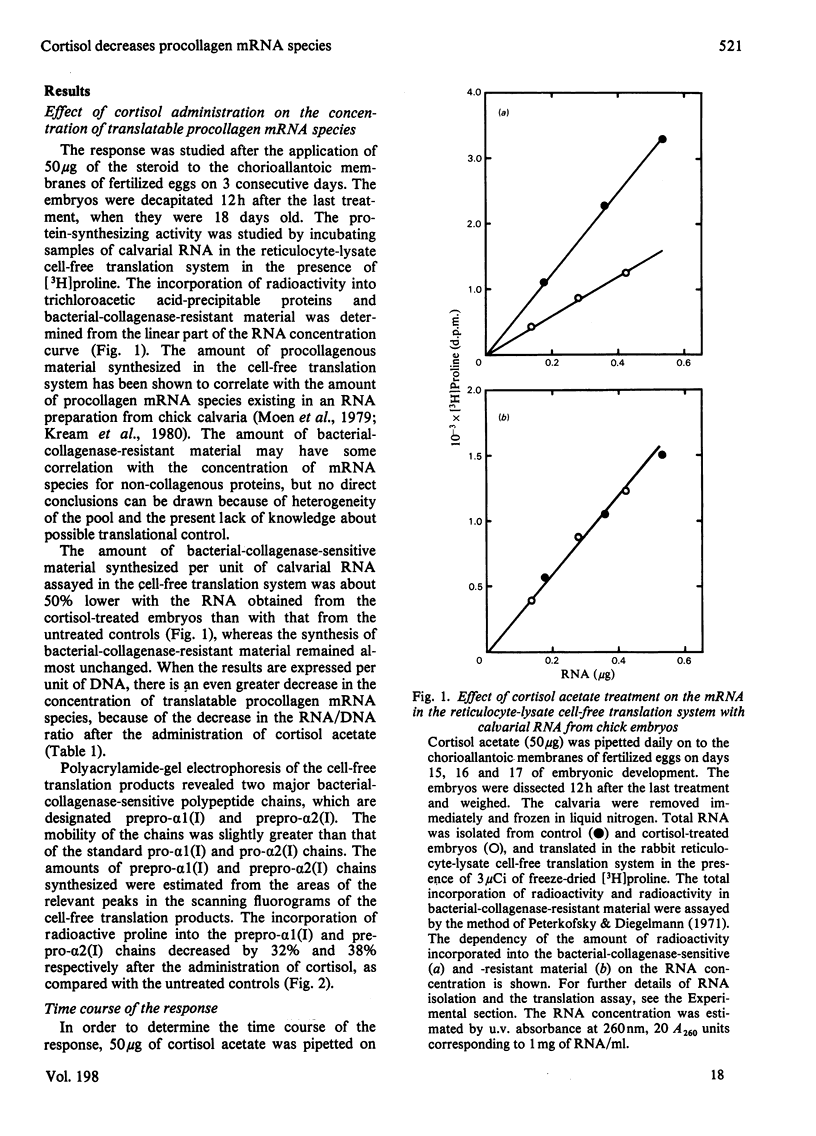
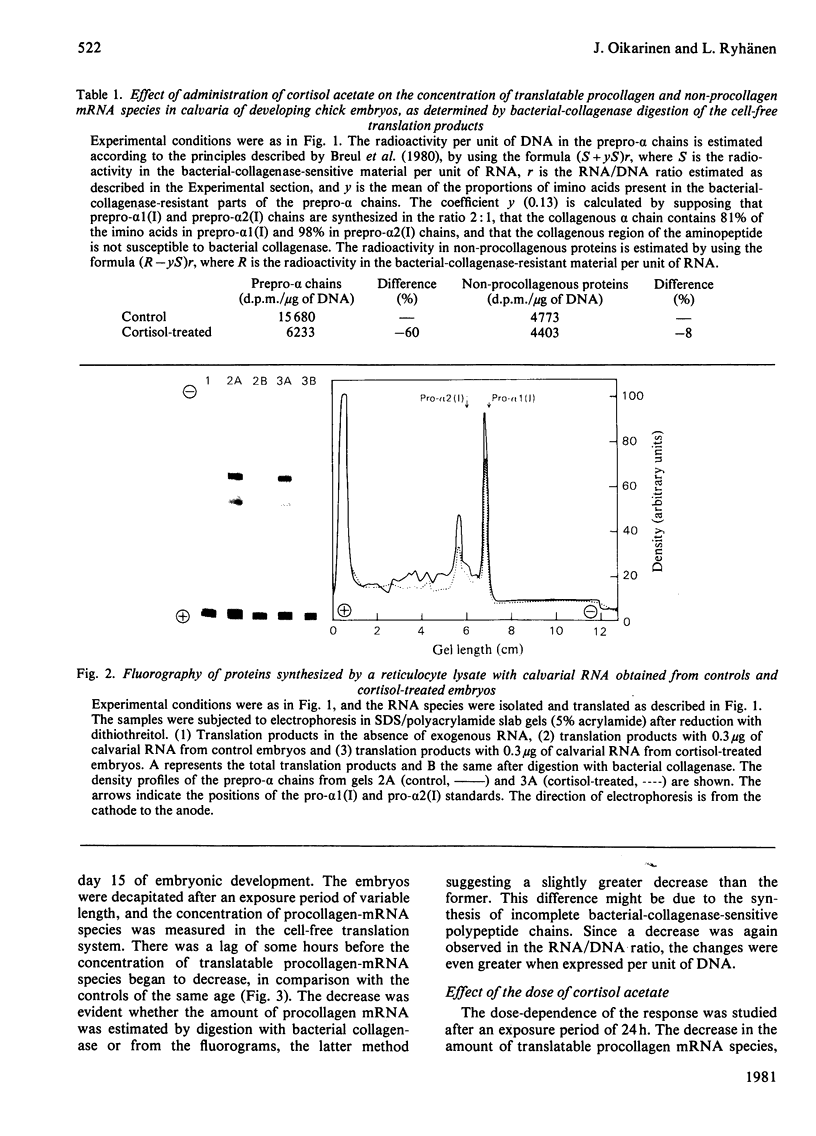
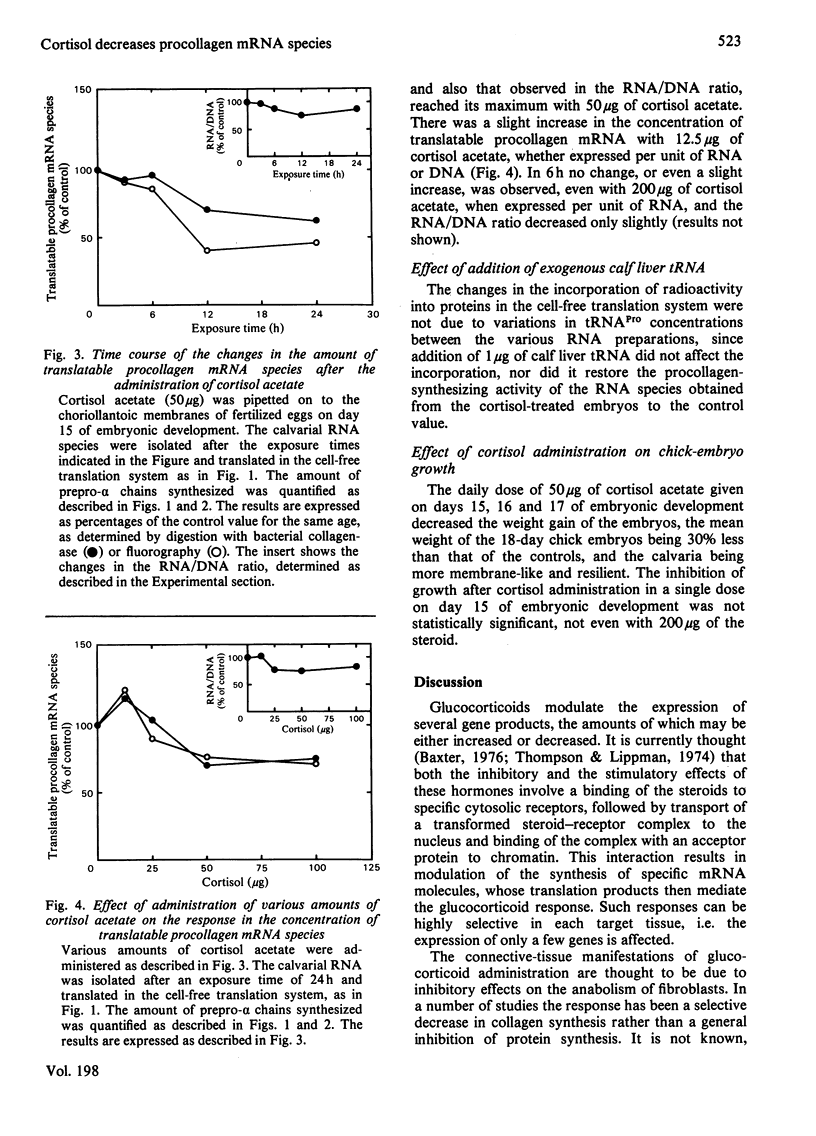
The calvarial mRNA species of chick embryos were translated in the rabbit reticulocyte-lysate cell-free translation system. The amount of procollagen type-I mRNA species was determined by digestion with bacterial collagenase and by fluorography of the cell-free translation products. Administration of cortisol resulted in a specific decrease in the cellular concentration of translatable procollagen type-I mRNA species in the calvaria of developing chick embryos. There was a lag period of up to 12 h before the response, which was dose-dependent. The data suggest that the decrease in amounts of procollagen mRNA species is the main reason for the lower amount of tissue collagen after topical or systemic administration of glucocorticoids, although other factors may contribute to the response.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Breul S. D., Bradley K. H., Hance A. J., Schafer M. P., Berg R. A., Crystal R. G. Control of collagen production by human diploid lung fibroblasts. J Biol Chem. 1980 Jun 10;255(11):5250–5260. [PubMed] [Google Scholar]
- Cheah K. S., Grant M. E., Jackson D. S. Translation of embryonic-chick tendon procollagen messenger ribonucleic acid in two cell-free protein-synthesizing systems. Biochem J. 1979 Jul 15;182(1):81–93. doi: 10.1042/bj1820081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutroneo K. R., Stassen F. L., Cardinale G. J. Anti-inflammatory steroids and collagen metabolism: glucocorticoid-mediated decrease of prolyl hydroxylase. Mol Pharmacol. 1975 Jan;11(1):44–51. [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J. S. Stimulation of division of sparse and confluent 3T3 cell populations by a fibroblast growth factor, dexamethasone, and insulin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4584–4588. doi: 10.1073/pnas.71.11.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan C., Young D. A., Munck A. Time course of early events in the action of glucocorticoids on rat thymus cells in vitro. Synthesis and turnover of a hypothetical cortisol-induced protein inhibition of glucose metabolism and of a presumed ribonucleic acid. J Biol Chem. 1973 Apr 25;248(8):2922–2927. [PubMed] [Google Scholar]
- Hubbard R. W., Matthew W. T., Dubowik D. A. Factors influencing the determination of DNA with indole. Anal Biochem. 1970 Nov;38(1):190–201. doi: 10.1016/0003-2697(70)90169-7. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Laitinen O., Aer J., Halme J. Studies with 14C-proline on the action of cortisone on the metabolism of collagen in the rat. Biochem Pharmacol. 1965 Oct;14(10):1445–1451. doi: 10.1016/0006-2952(65)90178-4. [DOI] [PubMed] [Google Scholar]
- Kream B. E., Rowe D. W., Gworek S. C., Raisz L. G. Parathyroid hormone alters collagen synthesis and procollagen mRNA levels in fetal rat calvaria. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5654–5658. doi: 10.1073/pnas.77.10.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse N. J., Rowe D. W., Fujimoto W. Y., Bornstein P. Inhibitory effects of glucocorticoids on collagen synthesis by mouse sponge granulomas and granuloma fibroblasts in culture. Biochim Biophys Acta. 1978 Apr 19;540(1):101–116. doi: 10.1016/0304-4165(78)90439-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Manthorpe R., Helin G., Kofod B., Lorenzen I. Effects of glucocorticoid on connective tissue of aorta and skin in rabbits. Biochemical studies on collagen, glycosaminoglycans, DNA and RNA. Acta Endocrinol (Copenh) 1974 Oct;77(2):310–324. doi: 10.1530/acta.0.0770310. [DOI] [PubMed] [Google Scholar]
- Martial J. A., Seeburg P. H., Guenzi D., Goodman H. M., Baxter J. D. Regulation of growth hormone gene expression: synergistic effects of thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4293–4295. doi: 10.1073/pnas.74.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNelis B., Cutroneo K. R. A selective decrease of collagen peptide synthesis by dermal polysomes isolated from glucocorticoid-treated newborn rats. Mol Pharmacol. 1978 Nov;14(6):1167–1175. [PubMed] [Google Scholar]
- Moen R. C., Rowe D. W., Palmiter R. D. Regulation of procollagen synthesis during the development of chick embryo calvaria. Correlation with procollagen mRNA content. J Biol Chem. 1979 May 10;254(9):3526–3530. [PubMed] [Google Scholar]
- Monson J. M., Goodman H. M. Translation of chick calvarial procollagen messenger RNA'S by a messenger RNA dependent reticulocyte lysate. Biochemistry. 1978 Nov 28;17(24):5122–5128. doi: 10.1021/bi00617a008. [DOI] [PubMed] [Google Scholar]
- Murota S. I., Koshihara Y., Tsurufuji S. Effects of cortisol and tetrahydrocortisol on the cloned fibroblast derived from rat carrageenin granuloma. Biochem Pharmacol. 1976 May 1;25(9):1107–1113. doi: 10.1016/0006-2952(76)90505-0. [DOI] [PubMed] [Google Scholar]
- Oikarinen A. Effect of betamethasone-17-valerate on synthesis of collagen and prolyl hydroxylase activity in chick embryo tendon cells. Biochem Pharmacol. 1977 May 1;26(9):875–879. doi: 10.1016/0006-2952(77)90403-8. [DOI] [PubMed] [Google Scholar]
- Oikarinen A. Effect of cortisol acetate on collagen biosynthesis and on the activities of prolyl hydroxylase, lysyl hydroxylase, collagen galactosyltransferase and collagen glucosyltransferase in chick-embryo tendon cells. Biochem J. 1977 Jun 15;164(3):533–539. doi: 10.1042/bj1640533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Ponec M., Kempenaar J. A., Van Der Meulen-Van Harskamp G. A., Bachra B. N. Effects of glucocorticosteroids on cultured human skin fibroblasts--IV. Specific decrease in the synthesis of collagen but no effect on its hydroxylation. Biochem Pharmacol. 1979 Sep 15;28(18):2777–2783. doi: 10.1016/0006-2952(79)90562-8. [DOI] [PubMed] [Google Scholar]
- Rowe D. W., Moen R. C., Davidson J. M., Byers P. H., Bornstein P., Palmiter R. D. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry. 1978 May 2;17(9):1581–1590. doi: 10.1021/bi00602a001. [DOI] [PubMed] [Google Scholar]
- Saarni H., Tammi M. Time and concentration dependence of the action of cortisol on fibroblasts in vitro. Biochim Biophys Acta. 1978 Apr 19;540(1):117–126. doi: 10.1016/0304-4165(78)90440-3. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Lippman M. E. Mechanism of action of glucocorticoids. Metabolism. 1974 Feb;23(2):159–202. doi: 10.1016/0026-0495(74)90113-9. [DOI] [PubMed] [Google Scholar]



