Abstract
The functions of bovine respiratory syncytial virus (BRSV) nonstructural proteins NS1 and NS2 were studied by generation and analysis of recombinant BRSV carrying single and double gene deletions. Whereas in MDBK cells the lack of either or both NS genes resulted in a 5,000- to 10,000-fold reduction of virus titers, in Vero cells a moderate (10-fold) reduction was observed. Interestingly, cell culture supernatants from infected MDBK cells were able to restrain the growth of NS deletion mutants in Vero cells, suggesting the involvement of NS proteins in escape from cytokine-mediated host cell responses. The responsible factors in MDBK supernatants were identified as type I interferons by neutralization of the inhibitory effect with antibodies blocking the alpha interferon (IFN-α) receptor. Treatment of cells with recombinant universal IFN-α A/D or IFN-β revealed severe inhibition of single and double deletion mutants, whereas growth of full-length BRSV was not greatly affected. Surprisingly, all NS deletion mutants were equally repressed, indicating an obligatory cooperation of NS1 and NS2 in antagonizing IFN-mediated antiviral mechanisms. To verify this finding, we generated recombinant rabies virus (rRV) expressing either NS1 or NS2 and determined their IFN sensitivity. In cells coinfected with NS1- and NS2-expressing rRVs, virus replication was resistant to doses of IFN which caused a 1,000-fold reduction of replication in cells infected with wild-type RV or with each of the NS-expressing rRVs alone. Thus, BRSV NS proteins have the potential to cooperatively protect an unrelated virus from IFN-α/β mediated antiviral responses. Interestingly, BRSV NS proteins provided a more pronounced resistance to IFN in the bovine cell line MDBK than in cell lines of other origins, suggesting adaptation to host-specific antiviral responses. The findings described have a major impact on the design of live recombinant BRSV and HRSV vaccines.
Bovine respiratory syncytial virus (BRSV) is a major etiological agent of respiratory tract disease in calves and results in substantial economic loss (40, 45). The immune response and pathology in calves mimic symptoms caused by human respiratory syncytial virus (HRSV), which remains the leading cause of serious bronchiolitis and pneumonia in infants and young children throughout the world (9). Molecular cloning has confirmed a very close relationship between BRSV and HRSV and has revealed substantial differences from other members of the Paramyxoviridae family, leading to the establishment of the Pneumovirus genus within the Paramyxoviridae family (36, 37).
As with all members of the order Mononegavirales, the 15-kb genomic RNA of RSV is contained in a ribonucleoprotein (RNP) complex which serves as a template for sequential transcription of genes (25, 49). Eleven proteins are expressed from 10 transcription units, which are arranged in the order 3′-NS1-NS2-N-P-M-SH-G-F-M2-L-5′ (5, 9, 30, 31). The proteins encoded include five RNP-associated proteins, namely, the nucleoprotein N, the phosphoprotein P, the large catalytic subunit L of the RNA polymerase, and a transcription elongation factor (M2-1) encoded by the first of two overlapping open reading frames of the M2 gene (8, 17, 27, 38). The second open reading frame of the M2 transcription unit (M2-2) was reported to encode a nonessential protein (1) which is probably involved in the regulation of RNA synthesis (4, 28). Three viral proteins are associated with the viral envelope, namely, the fusion protein F, the putative attachment protein G, and a small hydrophobic protein SH.
The presence of two nonstructural protein genes, NS1 and NS2, at the 3′-terminal position of the genome distinguishes pneumoviruses from all other members of the Mononegavirales. Due to the 3′-proximal location, the NS genes are abundantly transcribed. The encoded proteins have been demonstrated in infected cells (10, 16). The BRSV NS1 and NS2 genes encode polypeptides of 136 and 124 amino acids, respectively. Comparison with NS proteins of HRSV subgroup A and B proteins revealed amino acid identities of 69 and 68% for NS1 proteins and 84 and 83%, for NS2 proteins, respectively (5, 34). The deduced sequences, however, did not provide obvious clues to the function of NS proteins in the virus life cycle. The HRSV NS1 protein was reported to be associated with the M protein, while the NS2 protein did not show any detectable association with RSV structural proteins, indicating distinct functions of NS1 and NS2 (16, 47). An inhibitory function of NS1 in virus RNA transcription and RNP replication was recently suggested by experiments in which artificial HRSV minigenomes were grown in the absence or presence of NS1. In the same study, an inhibitory but far less pronounced effect was also observed for NS2 (3).
Recently established protocols for recovery of infectious minus-strand RNA viruses from cDNA (11) have allowed the generation of recombinant HRSV (8, 29) and BRSV (5) and allowed researchers to address individual protein functions in the virus context. The successful recovery of viable NS2 gene deletion mutants has confirmed that NS2 is not essential for virus replication in cell culture (5, 43). However, the deletion mutants were attenuated, indicating that NS2 is an accessory factor able to substantially support virus growth, by a so far unknown mechanism.
To examine more closely the function of BRSV NS proteins, we generated additional BRSV deletion mutants lacking the NS1 gene or both NS1 and NS2 and analyzed their behavior in different cell lines. We have identified the BRSV NS proteins as potent antagonists of interferon (IFN)-mediated host cell responses. Most outstanding is the finding that the IFN antagonistic activity requires both NS1 and NS2 and that each protein alone does not show any activity. By using another minus-strand RNA virus, rabies virus (RV), as a vector for the expression of BRSV-derived NS genes, we could also show that the activity of the two NS proteins can enhance the IFN resistance of an unrelated virus.
MATERIALS AND METHODS
Cells and virus.
Recombinant BRSV (rBRSV) was derived from BRSV strain A51908 (American Type Culture Collection) (33) variant ATue51908 (GenBank accession no. AF092942) and grown in MDBK cells as described previously (5). For preparation of virus stocks, 80% confluent MDBK or Vero cell monolayers were infected at a multiplicity of infection (MOI) of 0.1 in serum-free Dulbecco's minimal essential medium (DMEM). After 1 h of adsorption, the inoculum was removed and the cells were incubated at 37°C in DMEM supplemented with 2.5% fetal calf serum (FCS) in a 5% CO2 atmosphere until an extensive cytopathic effect (CPE) was observed. Virus was released by freezing and thawing. Virus titers were determined on Vero cells by limiting dilution in microwell plates and counting of infected-cell foci after indirect staining with a fusion protein antibody (kindly provided by J. A. Melero, Madrid, Spain). Stocks of NS deletion mutants were prepared on Vero cell monolayers after infection at an MOI of 0.01. Bovine macrophages were isolated from the blood of a cow and a calf by Ficoll gradient centrifugation (Lymphoflot; Biotest, Dreieich, Germany) at 1,500 rpm in a Heraeus 8074 rotor and adsorption of the mononuclear cell fraction to the bottom of cell culture flasks. After overnight incubation, nonadherent cells were removed by washing three times with RPMI (Gibco) without FCS. The remaining adherent cells (90 to 95% positive for CD14) were incubated in RPMI with 10% FCS at 37°C and 5% CO2.
Construction of BRSV NS gene deletion mutants.
Construction of an expression plasmid containing full-length BRSV cDNA (pBRSV) and an NS2 gene deletion mutant (pBRSV ΔNS2) has been described previously (5). The NS1 gene was deleted from pBRSV by cutting with NotI and AseI, subsequently filling in with Klenow polymerase, and religation, giving rise to pBRSV ΔNS1. For generation of the double-deletion mutant pBRSV ΔNS1/2, a 0.6-kb PCR fragment spanning part of the N gene was amplified with primer NNot(+) (5′-TAGGCGGCCGCAAAAATGGCTCTTAGCAAGGTG-3′) containing a NotI recognition site (underlined) upstream of the N start codon (italics) and reverse primer NStu(−) (5′-TCCTTTGTATCGTTTCATTTC-3′) corresponding to nucleotides (nt) 1735 to 1715 of rBRSV located downstream of a unique StuI restriction site (rBRSV position 1671). After deletion of the NS21 and NS2 genes and part of the N gene from pBRSV by NotI (rBRSV position 72) and StuI (rBRSV position 1671) digestion, the NotI- and StuI-cut PCR fragment (543 nt) was used to replace the deleted sequence (Fig. 1).
FIG. 1.
(A) Diagram of the genomes of recombinant BRSV. The locations of transcripts (shaded bars) and protein-encoding frames (open bars) are shown relative to the viral genome (vRNA) (solid bars). In the enlargement, the organizations of full-length and NS deletion mutants are compared. Leader RNA is marked by vertical stripes, and the relative positions of the corresponding nucleotides and restriction sites used for cloning are indicated. (B) Organization of rRV containing tagged BRSV NS1 or BRSV NS2 open reading frames between the RV G and L genes.
rRVs carrying the NS1 or NS2 genes (Fig. 1) were constructed on the basis of a full-length RV cDNA (SAD L16) containing an extra transcriptional stop-restart sequence in the 3′ noncoding sequence of the G gene (SAD VB) (32). First, cDNAs encoding C-terminally tagged versions of BRSV NS1 or NS2 proteins were constructed. An additional 27 nt corresponding to an internal region of the influenza virus hemagglutinin (HA) protein was inserted right before the NS1 stop codon by PCR using a reverse primer, NS1HAr-EcoRI (5′-GCAATAGAATTCCTAAGCGTAATCTGGTACATCATAAGGATAAT TCAGACCAAGAAGAGT-3′), containing an EcoRI recognition sequence (underlined). For NS2, 24 nt encoding the synthetic FLAG peptide was inserted with reverse primer NS2FLr-EcoRI (5′-GCAATAGAATTCCTATTTATCGTCATCATCTTTATAATCTGGATTTAAATCATACTTATA-3′) (the EcoRI sequence is underlined). The PCR fragments were used to substitute corresponding sequences of a plasmid (pBSBRSVNS1NS2) containing nt 1 to 957 of the full-length BRSV cDNA (5). The NS1-HA gene was excised with NotI and EcoRI. After filling in with Klenow polymerase, the resulting 475-nt fragment was inserted into a unique SmaI site in pSAD VB immediately downstream of the extra transcription start signal, leading to pSAD VB-NS1HA. A 470-nt fragment containing NS2-FL was cloned accordingly, after excision with AseI and EcoRI and filling in with Klenow enzyme, resulting in SAD VB-NS2FL.
Transfection experiments and recovery of BRSVs and rRVs.
rBRSV lacking the NS1 or NS2 gene and rRV containing the NS1 or NS2 gene were rescued as described previously (5, 19), after transfection of CaPO4-precipitated (mammalian transfection kit [Stratagene]) T7 promoter-controlled plasmids containing the respective virus cDNA (10 μg). For BRSV, plasmids encoding BRSV proteins N and P (pTITB-N and pTITB-P, 4 μg each) and L and M2 (pTITB-L and pTITB-M2, 2 μg each), and for RV, plasmids encoding RV proteins N (pTIT-N, 5 μg) and P and L (pTIT-P and pTIT-L, 2.5 μg each), were cotransfected with the respective virus cDNA into approximately 106 BSR T7/5 cells stably expressing phage T7 RNA polymerase (5). The transfection medium was removed after 4 h, and the cells were further incubated in BHK-21 medium (Gibco) containing 5% FCS for BRSV and 10% calf serum for RV. Cells transfected with BRSV cDNA were split every 5 days at a ratio of 1:3 until a CPE was detectable. For RV recovery, cell culture supernatants were harvested 6 days posttransfection and were transferred onto fresh BSR cells. Infectious RV was detected by immunostaining with a fluorescein isothiocyanate conjugate (Centocor) recognizing RV N protein.
Northern hybridization.
Total RNA from Vero cells infected with the recombinant viruses rBRSV, rBRSV ΔNS1, rBRSV ΔNS2, and rBRSV ΔNS1/2 was isolated upon development of extensive CPE (RNeasy; Quiagen). RNA was separated by denaturing gel electrophoresis, blotted to nylon membranes (Duralon-UV; Stratagene), and crosslinked to membranes by UV irradiation. NS1, NS2, and N gene-specific DNA probes of approximately 500 nt were labeled with [α-32P]dCTP (3,000 Ci/mmol; Amersham) by nick translation (nick translation kit; Amersham). Hybridized filters were exposed to Kodak BioMax MS films at −70°C with intensifying screens or processed for phosphorimaging (Storm; Molecular Dynamics).
Cocultivation experiments and treatment with IFN.
For cocultivation experiments, Vero responder cells were mock infected or infected in suspension with rBRSV, rBRSV ΔNS1, rBRSV ΔNS2, or rBRSV ΔNS1/2 at an MOI of 0.1 for 1 h in DMEM without FCS. After being washed, 5 × 105 cells were seeded into six-well dishes in DMEM plus 2.5% FCS. MDBK effector cells or bovine macrophages activated by overnight incubation with 10 μg of lipopolysaccharide (LPS) (Sigma) per ml were infected in suspension for 1 h at a MOI of 1 with rBRSV. After being washed, 106 cells were seeded into 25-mm tissue culture inserts with 200-nm-pore-size Anopore membranes (Nunc) and placed into the wells containing the infected Vero responder cells. After cocultivation for 3 days, membrane inserts were removed and virus titers in Vero cells were determined as described above. Responder cells were treated with antibodies immediately after infection by incubation for 1 h with 5 μg of neutralizing mouse anti-human IFN-α/β receptor chain 2 (CD118) antibody (PBL Biomedical Laboratories) per ml or with 5 μg of a control antibody recognizing tumor necrosis factor receptor type I (TNFRI) or major histocompatibility complex (MHC) class I per ml. After being seeded into six-well plates, the cells were grown further in the presence of 1 μg of the respective antibody per ml.
To determine the effect of IFN-α/β on the replication of BRSV and NS deletion mutants, Vero or MDBK cells were infected at a MOI of 0.1 with the different viruses as described above and incubated in six-well dishes in DMEM plus 2.5% FCS. Recombinant human IFN-α A/D or human IFN-β (PBL Biomedical Laboratories) was added to concentrations of up to 15,000 U/ml immediately after seeding. Virus titers were determined after 3 days of incubation by limiting dilution and indirect staining of infected cell foci with an F protein antibody as described above.
Infections with rRV SAD VB, SAD VB-NS1, and SAD VB-NS2, respectively, were done in suspension as described previously (18) with an MOI of 5. For coinfections with SAD VB-NS1 and SAD VB-NS2, an MOI of 2.5 was used for each recombinant. Recombinant IFN-α A/D was added to concentrations of up to 500 U/ml immediately after seeding. Virus titers were determined 2 days postinfection by limiting dilution and immunostaining with a fluorescein isothiocyanate conjugate against RV N protein (Centocor).
RESULTS
Construction and rescue of BRSV deletion mutants lacking NS genes.
We have previously described the cloning of full-length cDNA of BRSV strain ATue51908 and the construction of plasmids allowing T7 RNA polymerase-driven transcription of BRSV full-length antigenome RNA (pBRSV) or of RNA lacking the NS2 gene (pBRSV ΔNS2) (5). Constructs lacking the NS1 gene (pBRSV ΔNS1) or both NS1 and NS2 (pBRSV ΔNS1/2) were also made from pBRSV, as detailed in Materials and Methods. Compared to the sequence of the recombinant wild-type (wt) virus rBRSV, the NS1, NS2, and NS1/2 deletion mutants lack 496, 509, and 1,060 nt, respectively. In all constructs, transcription of the 3′ terminal gene is directed by the original leader/NS1 transcription start signal junction (Fig. 1).
Viable recombinant viruses rBRSV, rBRSV ΔNS1, rBRSV ΔNS2, and rBRSV ΔNS1/2 were recovered from the respective cDNA constructs in BSR T7/5 cells expressing T7 RNA polymerase as previously described (5). In all cases, including the NS1/2 double-deletion mutant, cotransfection with support plasmids encoding BRSV N, P, L, and M2 proteins resulted in the formation of syncytia. Viruses were isolated after a 1:3 splitting of transfected cells and the appearance of extensive CPE. For production of virus stocks, Vero cells were infected with the recombinant viruses at an MOI of 0.1 until pronounced CPE was visible, which took 3 days for rBRSV and 5 days for the NS deletion mutants.
After infection of cells with rBRSV or NS deletion mutants at a MOI of 0.1 and incubation for 3 and 5 days, respectively, total RNA was isolated and processed for Northern hybridization. Similar transcript pattern were observed, with the viruses differing only in the presence or absence of NS1- and NS2-specific RNAs (Fig. 2). As previously observed with BRSV and HRSV NS2 deletion mutants (5, 43), similar amounts of N mRNA and genome RNA (data not shown) were present in cells infected with all recombinants.
FIG. 2.
Lack of NS1 and NS2 transcripts in recombinant BRSVs. Total RNA from BSR cells infected with the indicated viruses was isolated 2 to 4 days postinfection and analyzed by Northern hybridization with probes spanning the NS1, NS2, NS1 to NS2, and N genes, respectively. NS1, NS2, and N mRNAs are indicated.
The growth of NS deletion mutants is cell type dependent.
Virus growth characteristics were first analyzed in the BHK-derived BSR T7/5 cells which had been used for recovery experiments. In comparison with the parental full-length virus, all three mutants were attenuated, suggesting a contribution of both NS proteins to virus replication. Interestingly, no marked differences in spread in infected cell cultures and final titers were noted between the two NS single-gene deletion mutants and the NS1/2 double-deletion mutant. All mutants reached infectious titers of 2 × 105 PFU after infection of BSR T7/5 cells at an MOI at 0.1 and incubation for 6 days, while the parental virus yielded up to 106 PFU (Fig. 3A). Similar results with slightly augmented titers were obtained after culturing the viruses in HEp-2 cells or in Vero cells, the preferred cell lines for cultivation of HRSV (data not shown).
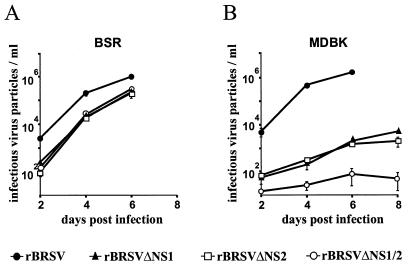
FIG. 3.
NS deletion mutants are more attenuated in MDBK cells than in BSR cells. Nearly confluent BSR T7-5 (A) and MDBK (B) cell monolayers were infected at an MOI of 0.1 with BRSV, BRSV ΔNS1, BRSV ΔNS2, or BRSV ΔNS1/2. Infectious virus titers were determined every 2 days as described in Materials and Methods. From day 6 onward, replication of all mutants in Vero cells and of wt BRSV in MDBK cells leads to massive cell destruction. Values are from two independent experiments, each performed in triplicate. Bars show standard deviations.
We then switched to a cell line of bovine origin, MDBK, which has been shown to optimally support the growth of wt BRSV (5). Indeed, MDBK yielded slightly increased wt BRSV titers of 2 × 106 PFU after 6 days of infection (Fig. 3B). Most unexpectedly, however, the growth of the deletion mutants was severely impeded in this cell line. The single-deletion mutants lacking NS1 or NS2 yielded titers of only 3 × 103 PFU after 6 days, which is 100-fold lower than in BSR cells. The NS1/2 double-deletion mutant was not able to grow significantly during the first 6 days. Only after splitting of cells and incubation for another 8 days could virus titers of 2 × 102 be obtained (data not shown). Obviously, MDBK cells represent the host of choice for full-length wt BRSV whereas they are nearly nonpermissive for all the NS deletion mutants. In striking contrast, BSR and Vero cells, which are suboptimal hosts for wt BRSV supported the NS deletion mutants rather well.
Soluble factors produced by MDBK cells and bovine macrophages affect the growth of NS deletion mutants in Vero cells.
To reveal the MDBK cell factors responsible for the apparently selective impediment of NS deletion mutants but not of wt BRSV, we first checked whether soluble molecules produced by MDBK cells are able to restrain the growth of NS deletion mutants in Vero cells. This was done by coculturing MBDK and Vero cells in devices in which the two cell cultures are separated by a virus-tight membrane filter allowing the passage of soluble factors (Fig. 4A). In the upper well, MDBK cells were used as effector cells. In the lower well, Vero cells infected at a MOI of 0.1 with wt BRSV or with each of the NS deletion mutants served as responder cells. At least five independent cocultivation experiments were performed. Noninfected MDBK effector cells, or BSR cells which were used as a negative control, did not show an inhibitory effect on the growth of either wt BRSV or the NS deletion mutants in the Vero responder cells. Cocultivation with MDBK cells infected with BRSV at an MOI of 1, however, resulted in a small but reproducible inhibition of the NS deletion mutants whereas the growth of wt BRSV was not affected (Fig. 4B). The most clear-cut effect was observed with the NS1/NS2 double-deletion mutant, whose titers were reduced approximately sevenfold in the presence of infected MDBK cells compared to noninfected MDBK cells. The titers of the single-deletion mutants rBRSV ΔNS1 and rBRSV ΔNS2 were reduced two- and threefold, respectively.
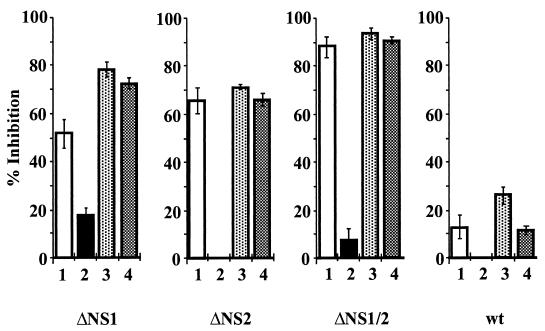
FIG. 4.
Supernatants from virus-infected MDBK cells or infected macrophages inhibit the growth of BRSV NS deletion mutants in cocultured Vero cells. (A) Schematic of the design of cocultivation experiments. (B) MDBK cells or LPS-stimulated bovine macrophage effector cells (EC) were infected with BRSV at a MOI of 1, seeded into Nunc Anopore membrane cell culture inlays, and cocultured with Vero responder cells (RC) infected with wt BRSV, BRSV ΔNS1, BRSV ΔNS2, or BRSV ΔNS1/2. After 3 days, the inlays were removed and infectious virus titers in Vero cultures were determined. The results are shown as percent inhibition, including standard deviation (+/−), and fold reduction (of the mean) relative to controls using noninfected MDBK or noninfected, nonstimulated macrophages. Values were obtained from six (MDBK) and four (macrophages) cocultivation experiments.
Since supernatants from noninfected MDBK cells were not able to suppress the growth of the NS deletion mutants in Vero responder cells, the effective MDBK factor(s) seemed to be induced by virus infection. Not only wt BRSV infection but also infection with a series of BRSV gene deletion mutants, including rBRSV ΔNS1/2 and a mutant lacking the SH and G-genes (rBRSV ΔSH/G; unpublished data), led to comparable secretion of the suppressive factors. In addition, infection with another RNA virus, RV, was found to induce the effective substance in MDBK cells to a similar degree (data not shown). These results strongly argued in favor of cytokine-mediated induction of an antiviral state in Vero responder cells, with the prime candidates being IFNs.
To further address the nature of cytokines involved, we used bovine macrophages as effector cells in cocultivation experiments. Bovine macrophages isolated from two animals were stimulated with LPS overnight, infected with rBRSV, and cocultivated with Vero cells. Yields of full-length rBRSV were similar after incubation with stimulated, virus-infected macrophages and with nonstimulated macrophages. However, compared to the nonstimulated, noninfected macrophage control, a prominent 30- to 50-fold reduction was noted for the NS deletion mutants (Fig. 4B). Also, stimulation of macrophages with LPS alone, without subsequent virus infection, was sufficient to cause approximately a 10-fold reduction in the yield of rBRSV ΔNS1/2 (data not shown). Since stimulated macrophages are known to be producers of IFN-α/β, these experiments pointed to an involvement of IFN-α and/or IFN-β in repressing the growth of the BRSV NS deletion mutants.
Deletion of NS genes renders BRSV IFN-α/β sensitive.
It was previously reported that Vero cells have a genetic defect in IFN production (15, 46). However, as determined by fluorescence-activated cell sorter analysis (data not shown), the Vero cells used as responder cells in cocultivation experiments expressed the alpha subunit of the IFN-α/β receptor (IFNAR2) (44). To determine whether IFN-α and/or IFN-β produced by infected MDBK cells or macrophages was mediating the inhibitory effects on NS deletion mutants, Vero responder cells were infected with rBRSV ΔNS1/2, rBRSV ΔNS2, or rBRSV ΔNS1/2 and were treated with a monoclonal antibody blocking IFNAR2 (PBL Laboratories). Control cells were treated in parallel with MHC class I- and TNFAR-specific monoclonal antibodies. The cells were then incubated in the presence of infected MDBK effector cells for 3 days. Whereas inhibition of NS deletion mutants was noted in cell cultures incubated with the control antibodies or in the absence of antibody, the inhibitory effect was almost completely neutralized in cells incubated with the IFNAR2 antibody (Fig. 5). Thus, the induction of an antiviral state in Vero responder cells leading to suppression of NS deletion mutants was exclusively due to IFN-α/β produced by MDBK cells or macrophages.
FIG. 5.
An IFNAR2 monoclonal antibody neutralizes the effect of the inhibitory factor produced by MDBK or macrophage supernatants. Vero responder cells infected at an MOI of 0.1 with rBRSV ΔNS1, rBRSV ΔNS2, rBRSV ΔNS1/2, or wt rBRSV were incubated for 3 h with 5 μg each of a monoclonal antibody against IFNAR2 (lanes 2), MHC class I (lanes 3), or TNFR1 (lanes 4) or in the absence of antibodies (lanes 1). Cocultivation with infected MDBK cells (see Fig. 4 for the design of the experiment) was done in the presence of 1 μg of the respective antibody per ml. Titers were determined in six (lanes 1 and 2) or two experiments (lanes 3 and 4). Bars show standard deviation.
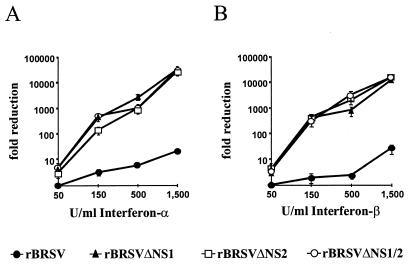
Recombinant human IFN-α/β were then used to directly analyze the behavior of wt and mutant BRSV in IFN-stimulated cells. Vero cells infected at an MOI of 0.1 with BRSV or NS deletion mutants were treated with increasing amounts of IFN-α A/D or IFN-β immediately after infection, and virus titers were determined 3 days after infection. All three NS deletion mutants showed a highly similar, severe, and dose-dependent sensitivity to the IFN-induced cellular response, with 1,500 U resulting in more than a 10,000-fold reduction of infectious titers (Fig. 6). In contrast, wt BRSV was significantly resistant to IFN treatment. However, protection was not complete, and an approximately 13-fold reduction in yield was caused by 1,500 U of IFN-α or IFN-β.
FIG. 6.
All BRSV NS deletion mutants are IFN-α/β sensitive. Vero cells infected at an MOI of 0.1 with BRSV, BRSV ΔNS1, BRSV ΔNS2, or BRSV ΔNS1/2 were incubated with the indicated amounts of recombinant IFN-α A/D (A) or IFN-β (B). Infectious virus titers from four independent experiments were determined 4 days postinfection. Bars show standard deviation.
To test the possibility that protection of wt BRSV is more pronounced in bovine cells than in Vero cells, we infected MDBK and Vero cells in parallel experiments at an MOI of 1 and added equal amounts of IFN. In untreated MDBK and Vero cells, BRSV grew to titers of 1.7 × 107 and 4 × 106 PFU/ml, respectively (Fig. 7). With IFN treatment, the titers in Vero cells declined more quickly than those in MDBK cells. After application of 10,000 U of IFN, infectious titers in Vero cells were 555-fold lower than were those in MDBK cells, although in the latter, substantial cell damage was already noted. As demonstrated by the vigorous inhibition of NS deletion mutants, the antiviral response of MDBK cells is at least as powerful as that of Vero cells. Thus, the enhanced protection of wt BRSV in MDBK cells indicates that BRSV may cope with bovine cell antiviral responses more efficiently than with primate cell responses.
FIG. 7.
IFN resistance of BRSV in MDBK and Vero cells. MDBK cells (solid columns) or Vero cells (open columns) were infected at an MOI of 1 with rBRSV and treated with the indicated amounts of recombinant IFN-α A/D. Infectious virus titers were determined 3 days postinfection. Bars represent standard deviations.
BRSV NS1 and NS2 cooperatively enhance the resistance of RV to IFN-mediated antiviral responses.
Deletion of each NS gene from BRSV leads to approximately equal degrees of sensitivity against IFN-mediated responses, suggesting that both NS proteins are required to counteract antiviral mechanisms. To verify the obligatory cooperative function of NS1 and NS2 and to determine whether the two NS proteins can be used to protect an unrelated virus, we generated rRV expressing either NS1 (SAD VB-NS1) or NS2 (SAD VB-NS2). The additional gene was introduced between the G and L genes of the attenuated RV SAD L16 (Fig. 1B), as previously described for successful expression of other genes (12, 39). Recombinants were rescued from cDNA in BSR T7/5 cells expressing the RV N, P, and L proteins from transfected plasmids. Expression of the NS proteins had no obvious adverse effect on the replication, growth characteristics, and infectious titers of the recombinants in BSR cells (data not shown).
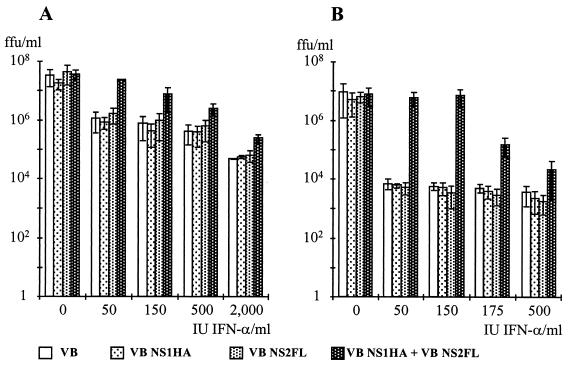
To study the activity of the expressed BRSV proteins, Vero cells were infected at an MOI of 5 with parental RV (SAD VB) or with each of the recombinants or were coinfected with SAD VB-NS1 and SAD VB-NS2 at an MOI of 2.5 each. Infected cultures were then treated with increasing amounts of IFN-α, and the production of infectious RV titers and the expression of RV proteins was analyzed 2 days postinfection in at least four independent experiments. Growth of the parental RV SAD VB and of NS1 or NS2 protein-expressing viruses from the single infections was similarly affected (Fig. 8A). On application of 50 IU of IFN-α, the titers dropped by approximately 1 log unit and then further decreased very slowly with increasing IFN amounts, indicating a weak IFN response of Vero cells or a high intrinsic resistance of RV to IFN-mediated responses in Vero cells. In cells coinfected with NS1- and NS2-expressing viruses, however, protection of virus replication could be shown. Virus titers remained significantly higher than in single infections and decreased slowly in a dose-dependent manner.
FIG. 8.
IFN resistance of RV in cells coinfected with RVs expressing NS1 and NS2. Vero cells (A) or MDBK cells (B) were infected with wt RV SAD VB, SAD VB-NS1, or SAD VB-NS2 or coinfected with SAD VB-NS1 and SAD VB-NS2. Immediately after infection, cultures were treated with the indicated amounts of IFN-α A/D. Infectious virus titers were determined 2 days postinfection. Results represent the mean values of at least four independent experiments, with error bars indicating standard deviation.
To re-examine the above observation that BRSV NS proteins are more efficacious in antagonizing antiviral responses in bovine cells than in Vero cells, parallel experiments were performed in MDBK cells (Fig. 8B). Standard RV SAD VB and the NS-expressing recombinants replicated to slightly lower titers in untreated MDBK cells than in Vero cells. In contrast to Vero cells, treatment with IFN dramatically reduced infectious titers of wt RV and of NS-expressing viruses from single infections. An immediate reduction in infectious titers by 3 log units indicated a highly effective IFN-mediated response. However, in cells coexpressing NS1 and NS2 proteins, virus replication was completely protected until more than 150 IU of IFN was applied (Fig. 8B). These results were reflected by the analysis of RV protein synthesis. In untreated cells, all recombinants produced substantial and comparable amounts of RV proteins, whereas in IFN-treated cells, only coinfections with SAD VB-NS1 and SAD VB-NS2 led to substantial protein synthesis, until more than 150 to 200 IU was applied (data not shown).
The above results showed that the two BRSV NS proteins are able to confer resistance to IFN-mediated antiviral response not only on BRSV but also on another, unrelated virus. In addition, they confirmed that both NS proteins are required, and sufficient, for exerting the IFN antagonist activity.
DISCUSSION
In this study, we could assign an important biological function to the NS proteins of BRSV, namely, in mediating virus escape from cellular antiviral mechanisms induced by IFN-α/β. Moreover, we found that both NS proteins are required for this function and that either one alone has no substantial activity. To our knowledge, this is the first example of two virus proteins that obligatorily cooperate to antagonize IFN.
The first indication of the increased sensitivity of NS deletion mutants to host cell factors was observed after infection of MDBK cells, which are fully permissive for wt BRSV infection and which yield higher titers of wt BRSV than do any other cell lines tested. In striking contrast, viruses lacking one or both NS genes grew worst in MDBK cells whereas in other cells, like Vero or BSR, the lack of the NS genes caused only a moderate (10-fold) decrease in infectious titers. Cocultivation experiments identified IFN-α/β as the critical host cell factors produced by BRSV-infected MDBK cells. Obviously, in infected MDBK cultures, an antiviral state is induced by autocrine and paracrine stimulation of cells. While wt BRSV is able to counteract this response, none of the NS deletion mutants can do so. Vero cells, in contrast, lack IFN-α/β genes (15, 46), and so virus infection does not result in the induction of an antiviral state, allowing the NS deletion mutants to grow. The observed 10-fold-slower growth in Vero cells of the NS deletion mutants compared to wt BRSV may be due to some intrinsic antiviral activity or, more likely, may reflect the contribution of NS gene products to virus replication. Although not able to produce IFN, Vero cells are able to respond to exogenous IFN stimulation through the IFN-α receptor complex (IFNAR) by JAK/STAT signaling. Bovine IFNs secreted from infected MDBK cells induced an antiviral response in Vero “responder” cells, suppressing the growth of the NS deletion mutants but not of wt BRSV. The antiviral effect caused by MDBK supernatants was abolished by incubation of Vero cells with an antibody blocking the binding of IFN to IFNAR. Therefore, IFN-α and/or IFN-β was the sole active component of supernatants from MDBK cells in inducing the antiviral response in Vero cells. The cocultivation experiments also showed that the IFN antagonistic activity of BRSV NS proteins is acting on the IFN response rather than on IFN induction, since similar IFN activities were induced in effector cells by wt BRSV, BRSV deletion mutants, and RV rhabdovirus.
The inhibitory effect on NS deletion mutants in Vero cells treated with supernatants from MDBK cells was weak, with a maximum sevenfold reduction in the yield of the double-deletion mutant, and was thus not comparable to the severe inhibition of NS deletion mutants in MDBK cells. This may be due to various factors. Stimulation of the primate Vero cells with the heterologous IFN of bovine origin is probably less efficient than stimulation of MDBK cells. Similar to the situation in humans, different types of bovine IFN-α (types 2 to 8) and bovine IFN-β (types 1 and 3) which exhibit different biological activity have been identified (7). In addition, the induced antiviral mechanisms of Vero cells seem to be less effective than those of MDBK. Supernatants from stimulated and infected macrophages, which are known to secrete larger amounts of IFN-α/β than other cells, caused an increased reduction of up to 50-fold in the yield of NS deletion mutants in Vero responder cells. Finally, by stimulation of Vero cells with recombinant human IFN-α A/D or IFN-β, replication of the NS deletion mutants could be inhibited in a dose-dependent manner, with 500 U nearly abolishing any replication activity.
A series of proteins from diverse DNA viruses (reviewed in reference 35) and RNA viruses (14, 20, 21, 23, 24, 41, 42) with the ability to antagonize the effects of IFN have been described. Some viruses, like hepatitis C virus, appear to have multiple proteins able to independently target antiviral mechanisms (20, 42). However, we were not prepared for the finding that both NS1 and NS2 are necessary to accomplish IFN antagonist activity whereas each protein on its own apparently does not possess any activity. The observation that the growth characteristics of deletion mutants lacking either NS1 or NS2 or both NS genes were very similar in all cell lines was appealing. Only in the experiment involving the lowest dose of active IFN, namely, the cocultivation using MDBK effector cells, could a differential behavior of NS1 and NS2 deletion mutants be suspected. In these experiments, the average reduction in the yield of the NS1 deletion mutant was slightly lower than that of the NS2 and the NS1/2 double deletion mutant; however, variability was high in these experiments and so their significance is questionable. A reproducible difference was observed only in MDBK cells, where the single-deletion mutants could be grown to somewhat higher titers than the double-deletion mutant after splitting of infected cell cultures. However, it is unclear whether this should be attributed to functions of NS proteins in IFN escape or in RNA synthesis.
Final evidence that both proteins are required and also sufficient to interfere with the establishment of an antiviral state was obtained by using rRV expressing individual NS proteins. The BRSV NS1 and NS2 genes are located side by side in the most upstream position of the genome (Fig. 1), which should result in a high and similar level of expression in BRSV-infected cells. Accordingly, to approximately reproduce the conditions in BRSV-infected cells, coinfections with rRV vectors were done at the same MOI for both recombinants. Comparable amounts of NS1 or NS2 protein, respectively, should be expressed in cells infected with the single recombinants and in cells coinfected with both recombinants. However, only in coinfected cells was a significantly enhanced resistance of the RV vector to IFN stimulation observed. Whereas standard RV titers dropped by 3 log units in MDBK cells after IFN treatment, complete protection was observed in coinfected cells. Depending on the experiment, the effects caused by IFN doses of up to approximately 200 U were completely neutralized. Since coinfections are prone to some variability, we suspect that a more regulated coexpression of NS proteins, at appropriate stoichiometric amounts, might bring about a much greater protective capacity.
Treatment of Vero cells with recombinant IFN showed that full-length BRSV was not completely protected against the IFN response of the primate cells. Although the sensitivity was lower than that of the NS deletion mutants by several orders of magnitude (Fig. 6), a dose-dependent reduction of wt BRSV titers was observed. However, parallel experiments with the bovine MDBK cells revealed almost perfect protection until very high doses of IFN were reached and cell damage was apparent (Fig. 7). As with BRSV, a more pronounced effect in MDBK cells was indicated by the rRV vectors. From the severe inhibition of BRSV NS deletion mutants and of wt RV in MDBK cells, it is obvious that the antiviral response mechanisms of MDBK cells are at least as efficient as those of Vero cells. This may indicate that the NS proteins of BRSV have evolved with the bovine host to optimally counteract bovine cell antiviral responses. The human counterpart of BRSV, HRSV, was recently reported to be highly resistant to IFN-induced antiviral activity in human cells (2), and it appears that HRSV NS proteins are optimized to antagonize IFN responses of human cells.
Adaptation of viral proteins to counteract innate responses in cells of their natural host is an important factor in determining the virus host range and may prevent viruses from crossing species barriers (13, 22, 26). The V protein of simian virus 5 (SV5) for example, is able to block the activation of IFN-responsive genes in primate cells but not in murine cells (14). This may be the major element preventing productive SV5 infection of mice, even SCID mice (13).
Indeed, the contribution of NS proteins to the permissivity of hosts to RSV infection might be crucial and might explain why the closely related viruses display a highly restricted host spectrum. BRSV and HRSV are able to enter human, bovine, and murine cells; however, the differential capability of NS proteins to more or less efficiently antagonize the host-specific innate responses might then determine whether the virus is eliminated. This is also supported by previous observations. Growth of HRSV in primary mouse embryo cells was markedly restricted; however, when anti-mouse IFN serum was added to the medium, virus yields were enhanced and the infection spread in the entire monolayer (26). In addition, recombinant BRSV in which the G and F surface protein genes were replaced by their HRSV counterparts was somewhat more competent than BRSV for replication in chimpanzees. However, the infection remained highly restricted and was not sufficient to induce any protection against homologous HRSV challenge (6). On the basis of our observations we conclude that the low efficiency of BRSV NS proteins in antagonizing primate IFN responses represents a major determinant for host range.
Our findings have important implications for the design of efficacious live attenuated RSV vaccines. It is suggested that deletion of either NS1 or NS2, as proposed previously (5, 43, 48), results in overattenuated viruses that are not able to escape from any IFN response. Promising approaches would be, for example, the reciprocal exchange of BRSV and HRSV NS proteins. This could generate BRSV and HRSV vaccines with intermediate ability to escape from the innate bovine and human response, respectively. In addition, mutations in NS proteins could be identified that only partially knock out the IFN antagonistic activity.
Further studies will reveal how NS proteins cooperate and how they interfere with antiviral responses. RV vectors as described here should prove especially helpful, since they provide the possibility to investigate NS protein functions independent of the so far unidentified functions they might have in BRSV replication.
ACKNOWLEDGMENTS
We thank Stefan Finke for critical reading of the manuscript and J. A. Melero, Madrid, Spain, and G. Taylor, Compton, United Kingdom, for providing RSV antibodies.
This work was supported in part by the Deutsche Forschungsgemeinschaft (SFB 455-A3) and the European Commission (EC 5th FP-RSV Vac, QLK2-CT-1999-00443).
REFERENCES
- 1.Ahmadian G, Chambers P, Easton A J. Detection and characterization of proteins encoded by the second ORF of the M2 gene of pneumoviruses. J Gen Virol. 1999;80:2011–2016. doi: 10.1099/0022-1317-80-8-2011. [DOI] [PubMed] [Google Scholar]
- 2.Atreya P L, Kulkarni S. Respiratory syncytial virus strain A2 is resistant to the antiviral effects of type I interferons and human MxA. Virology. 1999;261:227–241. doi: 10.1006/viro.1999.9835. [DOI] [PubMed] [Google Scholar]
- 3.Atreya P L, Peeples M E, Collins P L. The NS1 protein of human respiratory syncytial virus is a potent inhibitor of minigenome transcription and RNA replication. J Virol. 1998;72:1452–1461. doi: 10.1128/jvi.72.2.1452-1461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermingham A, Collins P L. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc Natl Acad Sci USA. 1999;96:11259–11264. doi: 10.1073/pnas.96.20.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchholz U J, Finke S, Conzelmann K K. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J Virol. 1999;73:251–259. doi: 10.1128/jvi.73.1.251-259.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchholz U J, Granzow H, Schuldt K, Whitehead S S, Murphy B R, Collins P L. Chimeric bovine respiratory syncytial virus with glycoprotein gene substitutions from human respiratory syncytial virus (HRSV): effects on host range and evaluation as a live-attenuated HRSV vaccine. J Virol. 2000;74:1187–1199. doi: 10.1128/jvi.74.3.1187-1199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaplin P J, Parsons K R, Collins R A. The cloning of cattle interferon-A subtypes isolated from the gut epithelium of rotavirus-infected calves. Immunogenetics. 1996;44:143–145. doi: 10.1007/BF02660063. [DOI] [PubMed] [Google Scholar]
- 8.Collins P L, Hill M G, Camargo E, Grosfeld H, Chanock R M, Murphy B R. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci USA. 1995;92:11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins P L, McIntosh K, Chanock R M. Respiratory syncytial virus. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnik J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1313–1352. [Google Scholar]
- 10.Collins P L, Wertz G W. Nucleotide sequences of the 1B and 1C nonstructural protein mRNAs of human respiratory syncytial virus. Virology. 1985;143:442–451. doi: 10.1016/0042-6822(85)90384-8. [DOI] [PubMed] [Google Scholar]
- 11.Conzelmann K K. Nonsegmented negative-strand RNA viruses: genetics and manipulation of viral genomes. Annu Rev Genet. 1998;32:123–162. doi: 10.1146/annurev.genet.32.1.123. [DOI] [PubMed] [Google Scholar]
- 12.Conzelmann K K, Cox J H, Schneider L G, Thiel H J. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology. 1990;175:485–499. doi: 10.1016/0042-6822(90)90433-r. [DOI] [PubMed] [Google Scholar]
- 13.Didcock L, Young D F, Goodbourn S, Randall R E. Sendai virus and simian virus 5 block activation of interferon-responsive genes: importance for virus pathogenesis. J Virol. 1999;73:3125–3133. doi: 10.1128/jvi.73.4.3125-3133.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Didcock L, Young D F, Goodbourn S, Randall R E. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J Virol. 1999;73:9928–9933. doi: 10.1128/jvi.73.12.9928-9933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emeny J M, Morgan M J. Regulation of the interferon system: evidence that Vero cells have a genetic defect in interferon production. J Gen Virol. 1979;43:247–252. doi: 10.1099/0022-1317-43-1-247. [DOI] [PubMed] [Google Scholar]
- 16.Evans J E, Cane P A, Pringle C R. Expression and characterisation of the NS1 and NS2 proteins of respiratory syncytial virus. Virus Res. 1996;43:155–161. doi: 10.1016/0168-1702(96)01327-5. [DOI] [PubMed] [Google Scholar]
- 17.Fearns R, Collins P L. Role of the M2-1 transcription antitermination protein of respiratory syncytial virus in sequential transcription. J Virol. 1999;73:5852–5864. doi: 10.1128/jvi.73.7.5852-5864.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finke S, Conzelmann K K. Ambisense gene expression from recombinant rabies virus: random packaging of positive- and negative-strand ribonucleoprotein complexes into rabies virions. J Virol. 1997;71:7281–7288. doi: 10.1128/jvi.71.10.7281-7288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finke S, Conzelmann K K. Virus promoters determine interference by defective RNAs: selective amplification of mini-RNA vectors and rescue from cDNA by a 3′ copy-back ambisense rabies virus. J Virol. 1999;73:3818–3825. doi: 10.1128/jvi.73.5.3818-3825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gale M J, Blakely C M, Kwieciszewski B, Tan S L, Dossett M, Tang N M, Korth M J, Polyak S J, Gretch D R, Katze M G. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol Cell Biol. 1998;18:5208–5218. doi: 10.1128/mcb.18.9.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gale M J J, Korth M J, Tang N M, Tan S L, Hopkins D A, Dever T E, Polyak S J, Gretch D R, Katze M G. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology. 1997;230:217–227. doi: 10.1006/viro.1997.8493. [DOI] [PubMed] [Google Scholar]
- 22.García-Sastre A, Durbin R K, Zheng H, Palese P, Gertner R, Levy D E, Durbin J E. The role of interferon in influenza virus tissue tropism. J Virol. 1998;72:8550–8558. doi: 10.1128/jvi.72.11.8550-8558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.García-Sastre A, Egorov A, Matassov D, Brandt S, Levy D E, Durbin J E, Palese P, Muster T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252:324–330. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 24.Garcin D, Latorre P, Kolakofsky D. Sendai virus C proteins counteract the interferon-mediated induction of an antiviral state. J Virol. 1999;73:6559–6565. doi: 10.1128/jvi.73.8.6559-6565.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grosfeld H, Hill M G, Collins P L. RNA replication by respiratory syncytial virus (RSV) is directed by the N, P, and L proteins: transcription also occurs under these conditions but requires RSV superinfection for efficient synthesis of full-length mRNA. J Virol. 1995;69:5677–5686. doi: 10.1128/jvi.69.9.5677-5686.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanada N, Morishima T, Nishikawa K, Isomura S, Nagai Y. Interferon-mediated self-limiting growth of respiratory syncytial virus in mouse embryo cells. J Med Virol. 1986;20:363–370. doi: 10.1002/jmv.1890200409. [DOI] [PubMed] [Google Scholar]
- 27.Hardy R W, Wertz G W. The product of the respiratory syncytial virus M2 gene ORF1 enhances readthrough of intergenic junctions during viral transcription. J Virol. 1998;72:520–526. doi: 10.1128/jvi.72.1.520-526.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin H, Cheng X, Zhou H Z, Li S, Seid R. Respiratory syncytial virus that lacks open reading frame 2 of the M2 gene (M2-2) has altered growth characteristics and is attenuated in rodents. J Virol. 2000;74:74–82. doi: 10.1128/jvi.74.1.74-82.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin H, Clarke D, Zhou H Z, Cheng X, Coelingh K, Bryant M, Li S. Recombinant human respiratory syncytial virus (RSV) from cDNA and construction of subgroup A and B chimeric RSV. Virology. 1998;251:206–214. doi: 10.1006/viro.1998.9414. [DOI] [PubMed] [Google Scholar]
- 30.Lerch R A, Stott E J, Wertz G W. Characterization of bovine respiratory syncytial virus proteins and mRNAs and generation of cDNA clones to the viral mRNAs. J Virol. 1989;63:833–840. doi: 10.1128/jvi.63.2.833-840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mallipeddi S K, Samal S K, Mohanty S B. Analysis of polypeptides synthesized in bovine respiratory syncytial virus-infected cells. Arch Virol. 1990;115:23–36. doi: 10.1007/BF01310620. [DOI] [PubMed] [Google Scholar]
- 32.Mebatsion T, Schnell M J, Cox J H, Finke S, Conzelmann K K. Highly stable expression of a foreign gene from rabies virus vectors. Proc Natl Acad Sci USA. 1996;93:7310–7314. doi: 10.1073/pnas.93.14.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohanty S B, Ingling A L, Lillie M G. Experimentally induced respiratory syncytial viral infection in calves. Am J Vet Res. 1975;36:417–419. [PubMed] [Google Scholar]
- 34.Pastey M K, Samal S K. Nucleotide sequence analysis of the non-structural NS1 (1C) and NS2 (1B) protein genes of bovine respiratory syncytial virus. J Gen Virol. 1995;76:193–197. doi: 10.1099/0022-1317-76-1-193. [DOI] [PubMed] [Google Scholar]
- 35.Ploegh H L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 36.Pringle C R. Virus taxonomy 1996—a bulletin from the Xth International Congress of Virology in Jerusalem. Arch Virol. 1996;141:2251–2256. doi: 10.1007/BF01718231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pringle C R. The universal system of virus taxonomy of the International Committee on Virus Taxonomy (ICTV), including new proposals ratified since publication of the Sixth ICTV Report in 1995. Arch Virol. 1998;143:203–210. doi: 10.1007/s007050050280. . (Erratum, 143:630.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samal S K, Pastey M K, McPhillips T H, Mohanty S B. Bovine respiratory syncytial virus nucleocapsid protein expressed in insect cells specifically interacts with the phosphoprotein and the M2 protein. Virology. 1993;193:470–473. doi: 10.1006/viro.1993.1148. [DOI] [PubMed] [Google Scholar]
- 39.Schnell M J, Mebatsion T, Conzelmann K K. Infectious rabies viruses from cloned cDNA. EMBO J. 1994;13:4195–4203. doi: 10.1002/j.1460-2075.1994.tb06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stott E J, Thomas L H, Taylor G, Collins A P, Jebbett J, Crouch S. A comparison of three vaccines against respiratory syncytial virus in calves. J Hyg. 1984;93:251–261. doi: 10.1017/s0022172400064779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan S L, Katze M G. Biochemical and genetic evidence for complex formation between the influenza A virus NS1 protein and the interferon-induced PKR protein kinase. J Interferon Cytokine Res. 1998;18:757–766. doi: 10.1089/jir.1998.18.757. [DOI] [PubMed] [Google Scholar]
- 42.Taylor D R, Shi S T, Romano P R, Barber G N, Lai M M. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science. 1999;285:107–110. doi: 10.1126/science.285.5424.107. [DOI] [PubMed] [Google Scholar]
- 43.Teng M N, Collins P L. Altered growth characteristics of recombinant respiratory syncytial viruses which do not produce NS2 protein. J Virol. 1999;73:466–473. doi: 10.1128/jvi.73.1.466-473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uzé G, Lutfalla G, Mogensen K E. Alpha and beta interferons and their receptor and their friends and relations. J Interferon Cytokine Res. 1995;15:3–26. doi: 10.1089/jir.1995.15.3. [DOI] [PubMed] [Google Scholar]
- 45.van der Poel W H, Brand A, Kramps J A, van Oirschot J T. Respiratory syncytial virus infections in human beings and in cattle. J Infect. 1994;29:215–228. doi: 10.1016/s0163-4453(94)90866-4. [DOI] [PubMed] [Google Scholar]
- 46.Wathelet M G, Berr P M, Huez G A. Regulation of gene expression by cytokines and virus in human cells lacking the type-I interferon locus. Eur J Biochem. 1992;206:901–910. doi: 10.1111/j.1432-1033.1992.tb16999.x. [DOI] [PubMed] [Google Scholar]
- 47.Weber E, Humbert B, Streckert H J, Werchau H. Nonstructural protein 2 (NS2) of respiratory syncytial virus (RSV) detected by an antipeptide serum. Respiration. 1995;62:27–33. doi: 10.1159/000196385. [DOI] [PubMed] [Google Scholar]
- 48.Whitehead S S, Bukreyev A, Teng M N, Firestone C Y, St Claire M, Elkins W R, Collins P L, Murphy B R. Recombinant respiratory syncytial virus bearing a deletion of either the NS2 or SH gene is attenuated in chimpanzees. J Virol. 1999;73:3438–3442. doi: 10.1128/jvi.73.4.3438-3442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu Q, Hardy R W, Wertz G W. Functional cDNA clones of the human respiratory syncytial (RS) virus N, P, and L proteins support replication of RS virus genomic RNA analogs and define minimal trans-acting requirements for RNA replication. J Virol. 1995;69:2412–2419. doi: 10.1128/jvi.69.4.2412-2419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]