Abstract
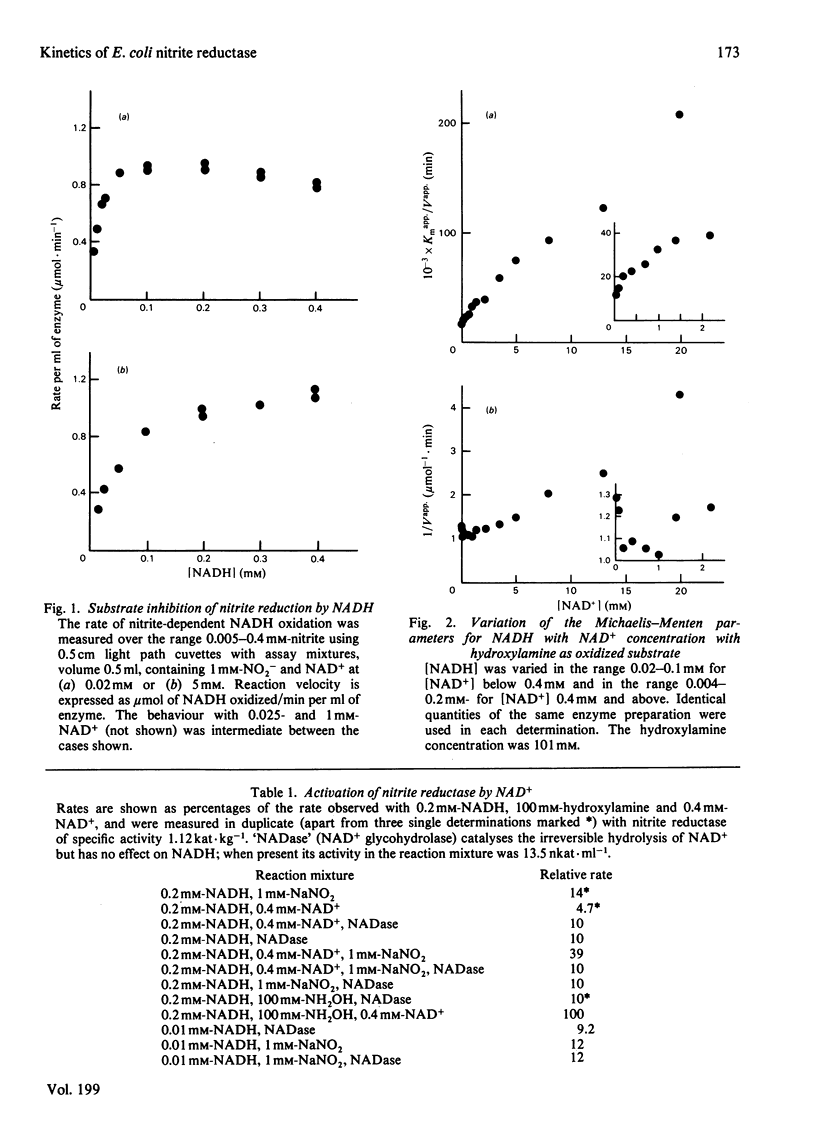
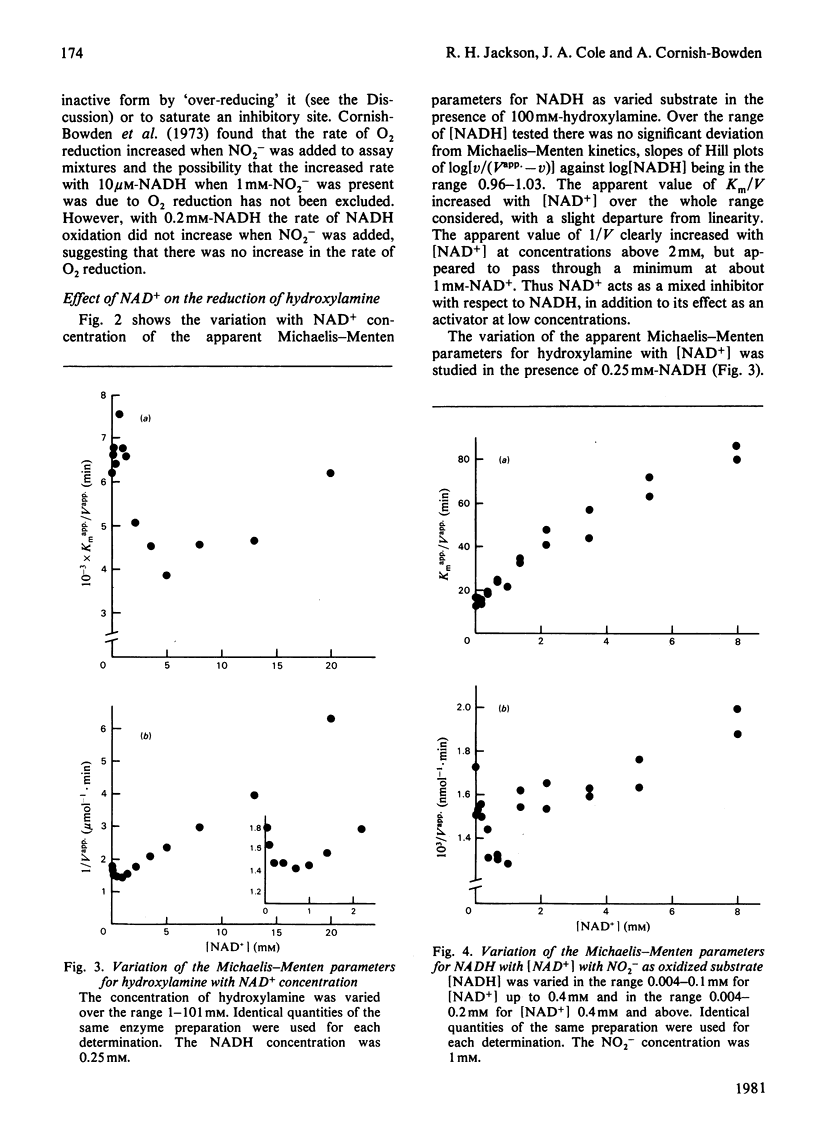
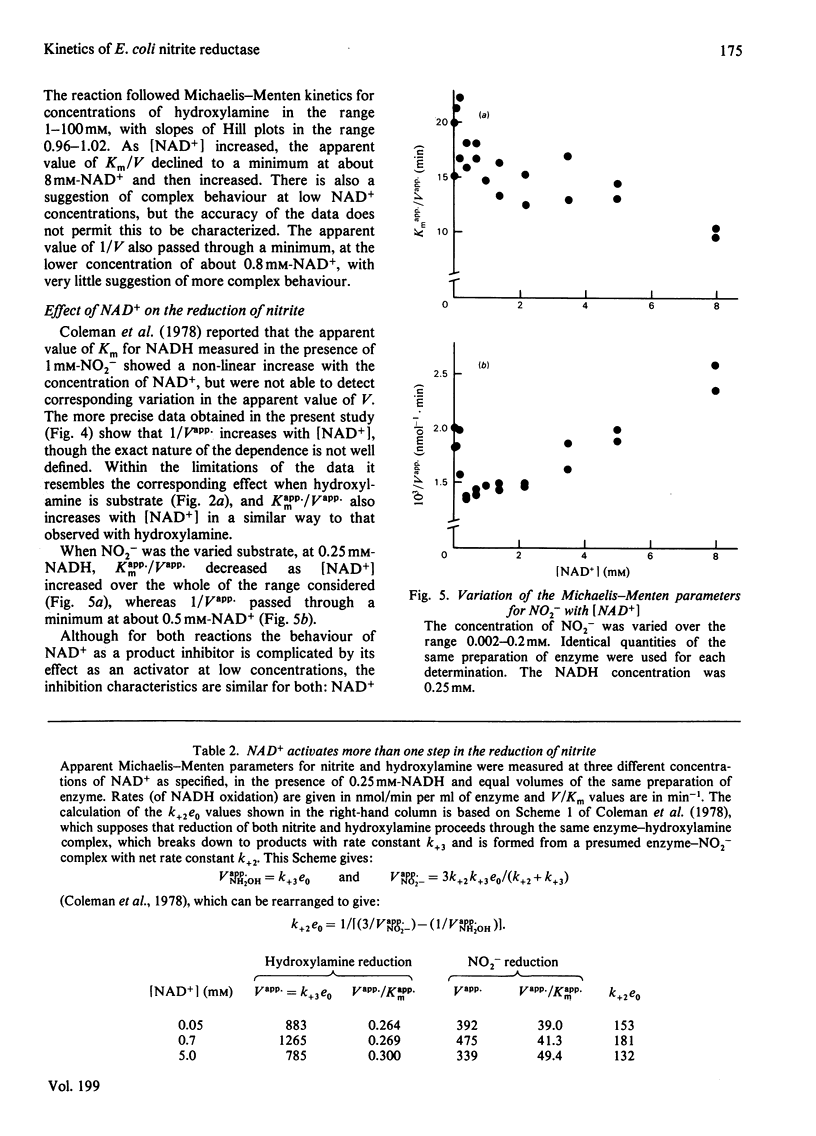
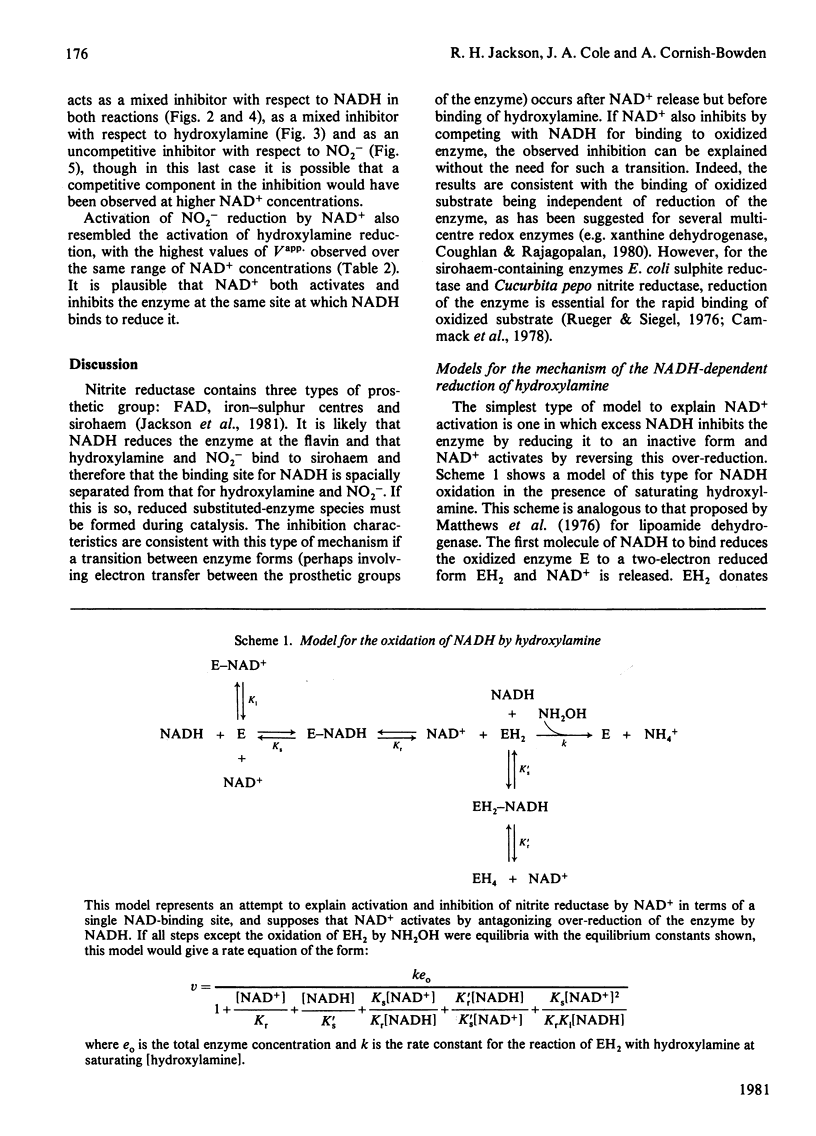
The reduction of both NO2- and hydroxylamine by the NADH-dependent nitrite reductase of Escherichia coli K 12 (EC 1.6.6.4) appears to follow Michaelis-Menten kinetics over a wide range of NADH concentrations. Substrate inhibition can, however, be detected at low concentrations of the product NAD+. In addition, NAD+ displays mixed product inhibition with respect to NADH and mixed or uncompetitive inhibition with respect to hydroxylamine. These inhibition characteristics are consistent with a mechanism in which hydroxylamine binds during catalysis to a different enzyme form from that generated when NAD+ is released. The apparent maximum velocity with NADH as varied substrate increases as the NAD+ concentration increases from 0.05 to 0.7 mM with 1 mM-NO2- or 100 mM-hydroxylamine as oxidized substrate. This increase is more marked for hydroxylamine reduction than for NO2- reduction. Models incorporating only one binding site for NAD can account for the variation in the Michaelis-Menten parameters for both NADH and hydroxylamine with [NAD+] for hydroxylamine reduction. According to these models, activation of the reaction occurs by reversal of an over-reduction of the enzyme by NADH. If the observed activation of the enzyme by NAD+ derives both from activation of the generation of the enzyme-hydroxylamine complex from the enzyme-NO2- complex during NO2- reduction and from activation of the reduction of the enzyme-hydroxylamine complex to form NH4+, then the variation of Vapp. for NO2- or hydroxylamine with [NAD+] is consistent with the occurrence of the same enzyme-hydroxylamine complex as an intermediate in both reactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cammack R., Hucklesby D. P., Hewitt E. J. Electron-paramagnetic-resonance studies of the mechanism of leaf nitrite reductase. Signals from the iron-sulphur centre and haem under turnover conditions. Biochem J. 1978 Jun 1;171(3):519–526. doi: 10.1042/bj1710519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K. J., Cornish-Bowden A., Cole J. A. Activation of nitrite reductase from Escherichia coli K12 by oxidized nicotinamide-adenine dinucleotide. Biochem J. 1978 Nov 1;175(2):495–499. doi: 10.1042/bj1750495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A. An automatic method for deriving steady-state rate equations. Biochem J. 1977 Jul 1;165(1):55–59. doi: 10.1042/bj1650055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A., Eisenthal R. Estimation of Michaelis constant and maximum velocity from the direct linear plot. Biochim Biophys Acta. 1978 Mar 14;523(1):268–272. doi: 10.1016/0005-2744(78)90030-x. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A., Porter W. R., Trager W. F. Evaluation of distribution-free confidence limits for enzyme kinetic parameters. J Theor Biol. 1978 Sep 21;74(2):163–175. doi: 10.1016/0022-5193(78)90069-3. [DOI] [PubMed] [Google Scholar]
- Coughlan M. P., Rajagopalan K. V. The kinetic mechanism of xanthine dehydrogenase and related enzymes. Eur J Biochem. 1980 Mar;105(1):81–84. doi: 10.1111/j.1432-1033.1980.tb04476.x. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. H., Cornish-Bowden A., Cole J. A. Prosthetic groups of the NADH-dependent nitrite reductase from Escherichia coli K12. Biochem J. 1981 Mar 1;193(3):861–867. doi: 10.1042/bj1930861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R. G., Williams C. H., Jr Measurement of the oxidation-reduction potentials for two-electron and four-electron reduction of lipoamide dehydrogenase from pig heart. J Biol Chem. 1976 Jul 10;251(13):3956–3964. [PubMed] [Google Scholar]
- WONG J. T., HANES C. S. Kinetic formulations for enzymic reactions involving two substrates. Can J Biochem Physiol. 1962 Jun;40:763–804. [PubMed] [Google Scholar]


