Abstract
1. In the presence of a high concentration of p-nitrophenyl beta-D-glucopyranoside (donor) the rates of production of p-nitrophenol and a transglucosylation product (1-glyceryl beta-D-glucopyranoside) increased, whereas the rate of production of glucose decreased with increasing concentration of glycerol in reactions catalysed by the high-molecular-weight beta-glucosidase (beta-D-glucoside glucohydrolase, EC 3.2.1.21) obtained from culture filtrates of Botryodiplodia theobromae Pat. 2. When [donor] greater than Km the rate of production of p-nitrophenol was higher in the presence of glycerol than in its absence, whereas when [donor] less than Km the rate of production of p-nitrophenol was lower in the presence of glycerol than in its absence. 3. Glycerol increased both the Michaelis constant (Km) and maximum velocity (Vmax.), whereas dioxan increased Km but decreased Vmax. 4. Up to 1 mM-AgNO3 had no effect on enzyme activity. 5. A 2H-solvent-isotope-effect [Vmax. (H2O)/V max. (2H2O)] value of 1.40 +/- 0.05 was found at pH (or p2H) 5.8 6. alpha-2H-kinetic isotope-effect (kappa H/kappa 2H) values of 1.03 +/- 0.01 and 1.05 +/- 0.01 were found in the absence and presence of glycerol respectively. 7. Although maltose was a non-competitive inhibitor of beta-glucosidase activity, the ratio of velocity in the presence of glycerol to that in its absence increased, after an initial decline, with increasing concentration of maltose. 8. These results are discussed in terms of a mechanism involving a solvent-separated glucosyl cation-carboxylate ion-pair, which has greater affinity for alcoholic glucosyl acceptors, and an intimate ion-pair, which has greater affinity for water as a glucosyl acceptor and which could collapse reversibly and rapidly into a preponderance of an unreactive covalent glucosyl-enzyme.
Full text
PDF
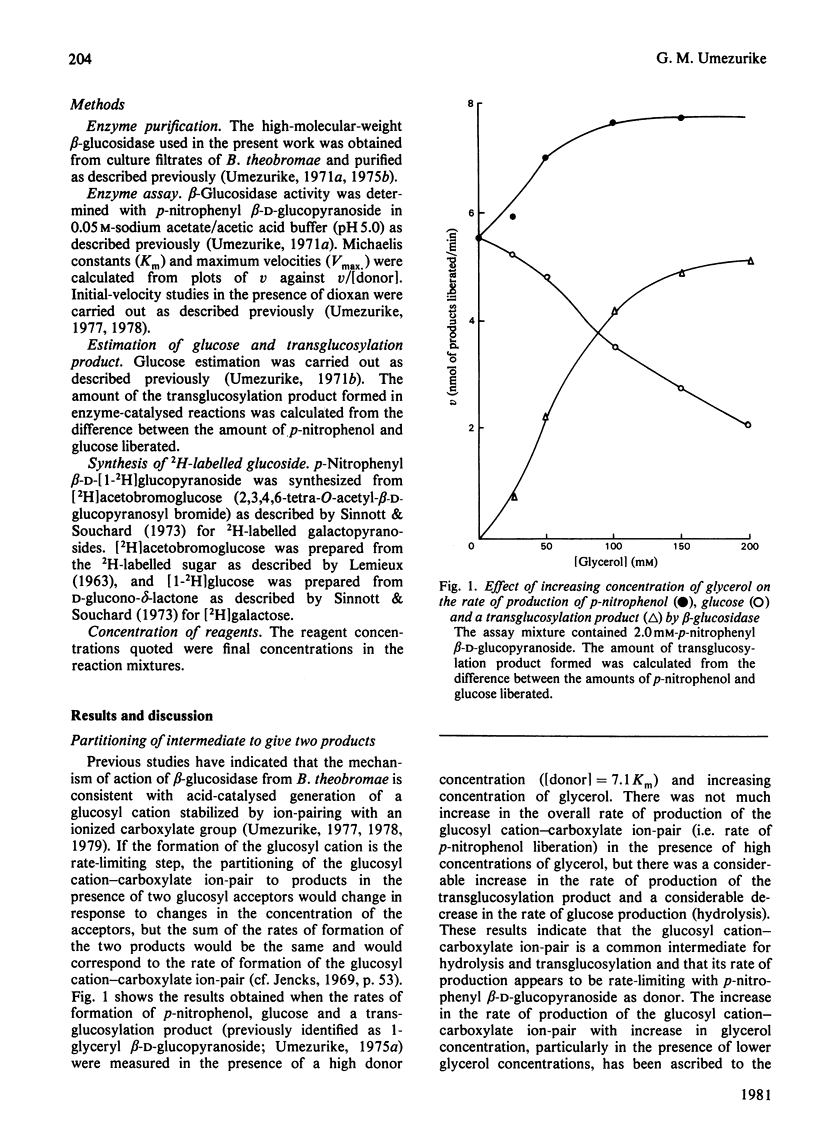
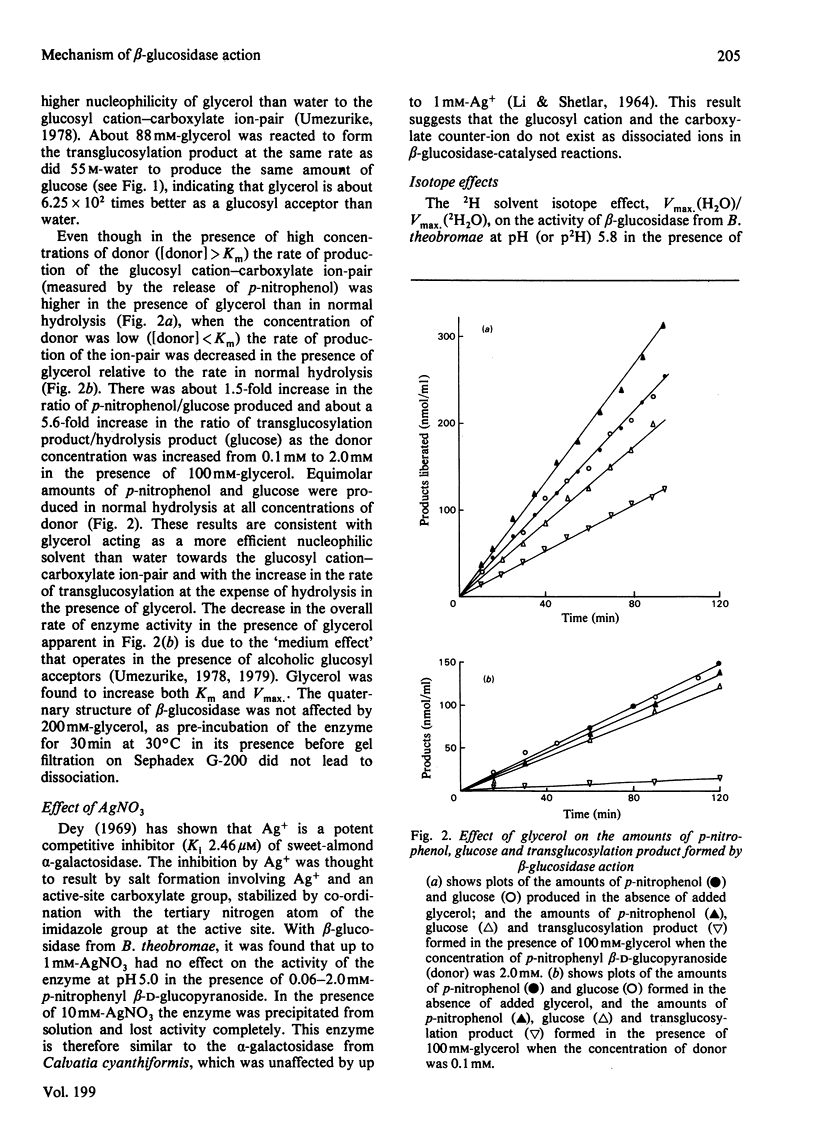

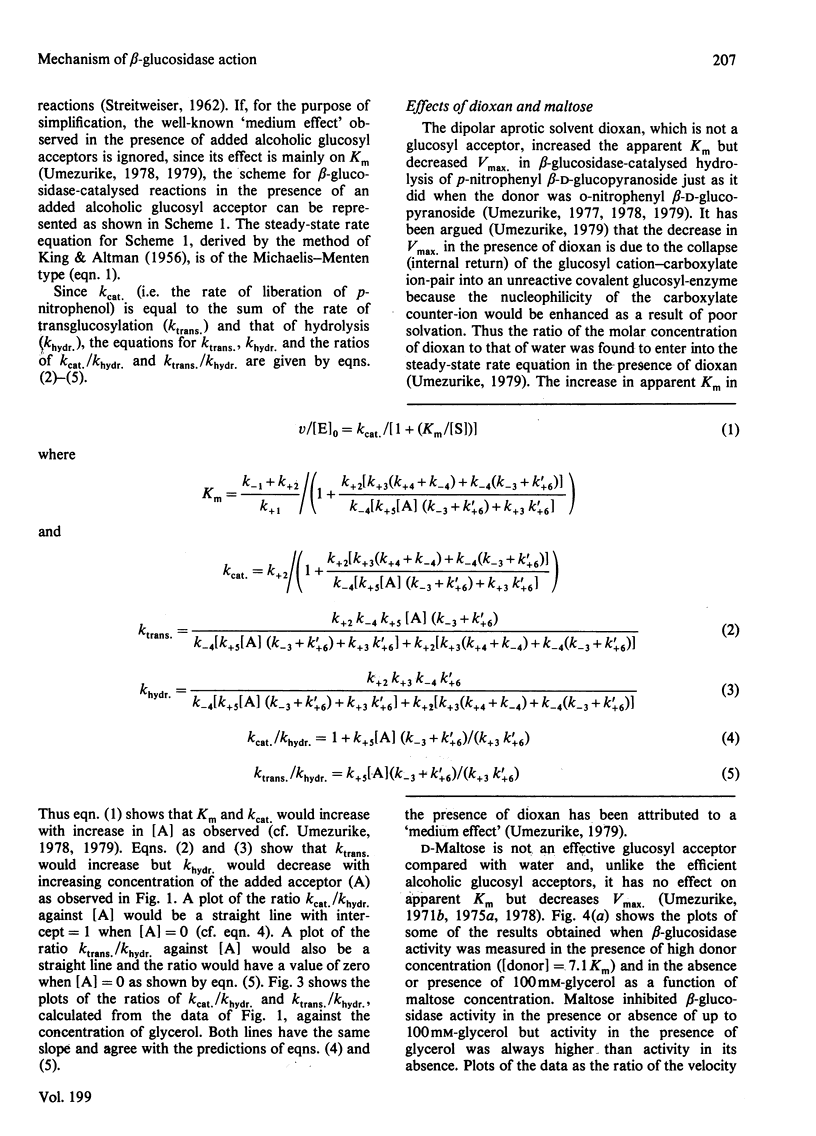
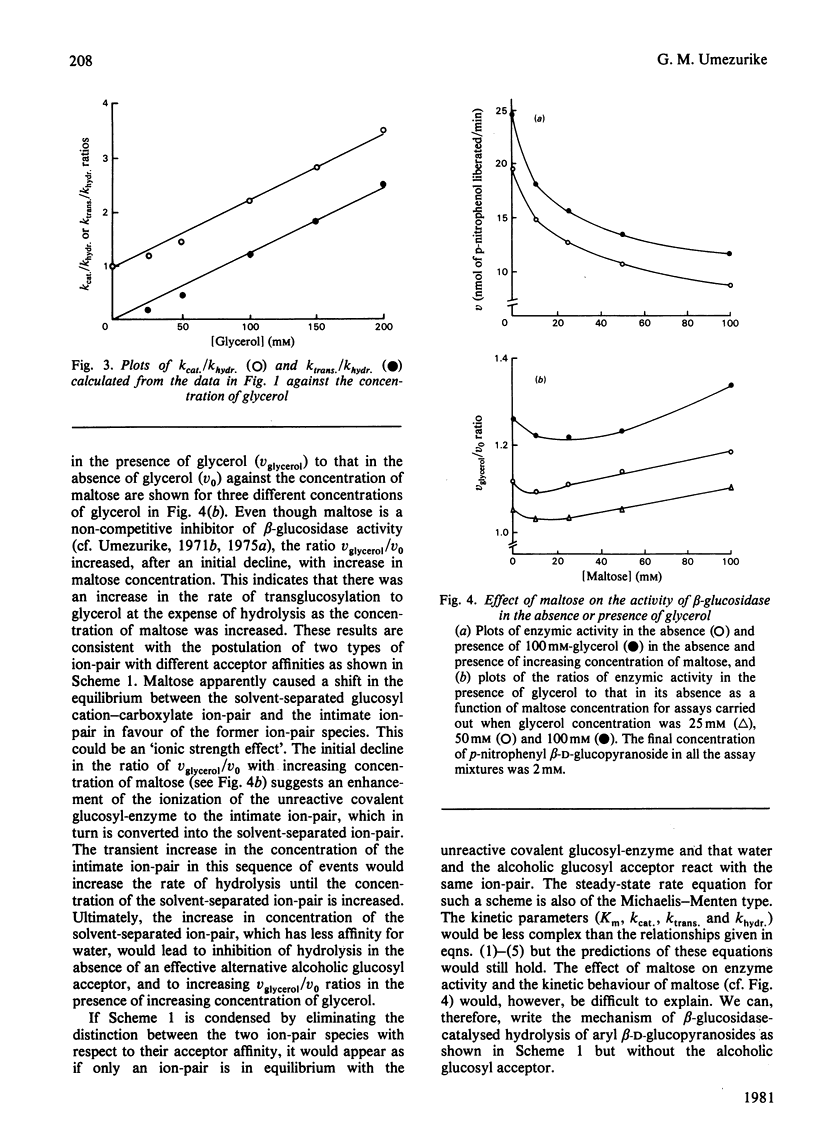

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dahlquist F. W., Rand-Meir T., Raftery M. A. Application of secondary alpha-deuterium kinetic isotope effects to studies of enzyme catalysis. Glycoside hydrolysis by lysozyme and beta-glucosidase. Biochemistry. 1969 Oct;8(10):4214–4221. doi: 10.1021/bi00838a045. [DOI] [PubMed] [Google Scholar]
- Dey P. M. Inhibition, transgalactosylation and mechanism of action of sweet almond alpha-galactosidase. Biochim Biophys Acta. 1969;191(3):644–652. doi: 10.1016/0005-2744(69)90357-x. [DOI] [PubMed] [Google Scholar]
- LI Y. T., SHETLAR M. R. OCCURRENCE OF ALPHA-GALACTOSIDASE IN HIGHER FUNGI: ISOLATION OF ALPHA-GALACTOSIDASE FROM CALVATIA CYATHIFORMIS. Arch Biochem Biophys. 1964 Dec;108:523–530. doi: 10.1016/0003-9861(64)90437-0. [DOI] [PubMed] [Google Scholar]
- Sinnott M. L., Souchard I. J. The mechanism of action of beta-galactosidase. Effect of aglycone nature and -deuterium substitution on the hydrolysis of aryl galactosides. Biochem J. 1973 May;133(1):89–98. doi: 10.1042/bj1330089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott M. L., Viratelle O. M. The effect of methanol and dioxan on the rates of the beta-galactosidase-catalysed hydrolyses of some beta-D-galactrophyranosides: rate-limiting degalactosylation. The ph-dependence of galactosylation and degalactosylation. Biochem J. 1973 May;133(1):81–87. doi: 10.1042/bj1330081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezurike G. M. Kinetic analysis of the mechanism of action of beta-glucosidase from Botryodiplodia theobromae Pat. Biochim Biophys Acta. 1975 Jul 27;397(1):164–178. doi: 10.1016/0005-2744(75)90190-4. [DOI] [PubMed] [Google Scholar]
- Umezurike G. M. Kinetic properties of -glucosidase from Botryodiplodia theobromae Pat. Biochim Biophys Acta. 1971 Oct;250(1):182–191. doi: 10.1016/0005-2744(71)90132-x. [DOI] [PubMed] [Google Scholar]
- Umezurike G. M. The active site of beta-glucosidase from Botryodiplodia theobromae. Effects of pH and dioxan on enzyme-catalysed reactions. Biochem J. 1977 Dec 1;167(3):831–833. doi: 10.1042/bj1670831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezurike G. M. The beta-glucosidase from Botryodiplodia theobromae Pat. Kinetics of enzyme-catalysed hydrolysis of o-nitrophenyl beta-D-glucopyranoside in dioxan/water. Biochem J. 1978 Nov 1;175(2):455–459. doi: 10.1042/bj1750455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezurike G. M. The beta-glucosidase from Botryodiplodia theobromae. Mechanism of enzyme-catalysed reactions. Biochem J. 1979 Jun 1;179(3):503–507. doi: 10.1042/bj1790503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezurike G. M. The purification and properties of extracellular beta-glucosidase from Botryodiplodia theobromae Pat. Biochim Biophys Acta. 1971 Feb 10;227(2):419–428. doi: 10.1016/0005-2744(71)90073-8. [DOI] [PubMed] [Google Scholar]
- Umezurike G. M. The subunit structure of beta-glucosidase from Botryodiplodia theobromae Pat. Biochem J. 1975 Feb;145(2):361–368. doi: 10.1042/bj1450361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLENFELS K., MALHOTRA O. P. Galactosidases. Adv Carbohydr Chem. 1961;16:239–298. doi: 10.1016/s0096-5332(08)60264-7. [DOI] [PubMed] [Google Scholar]


