Abstract
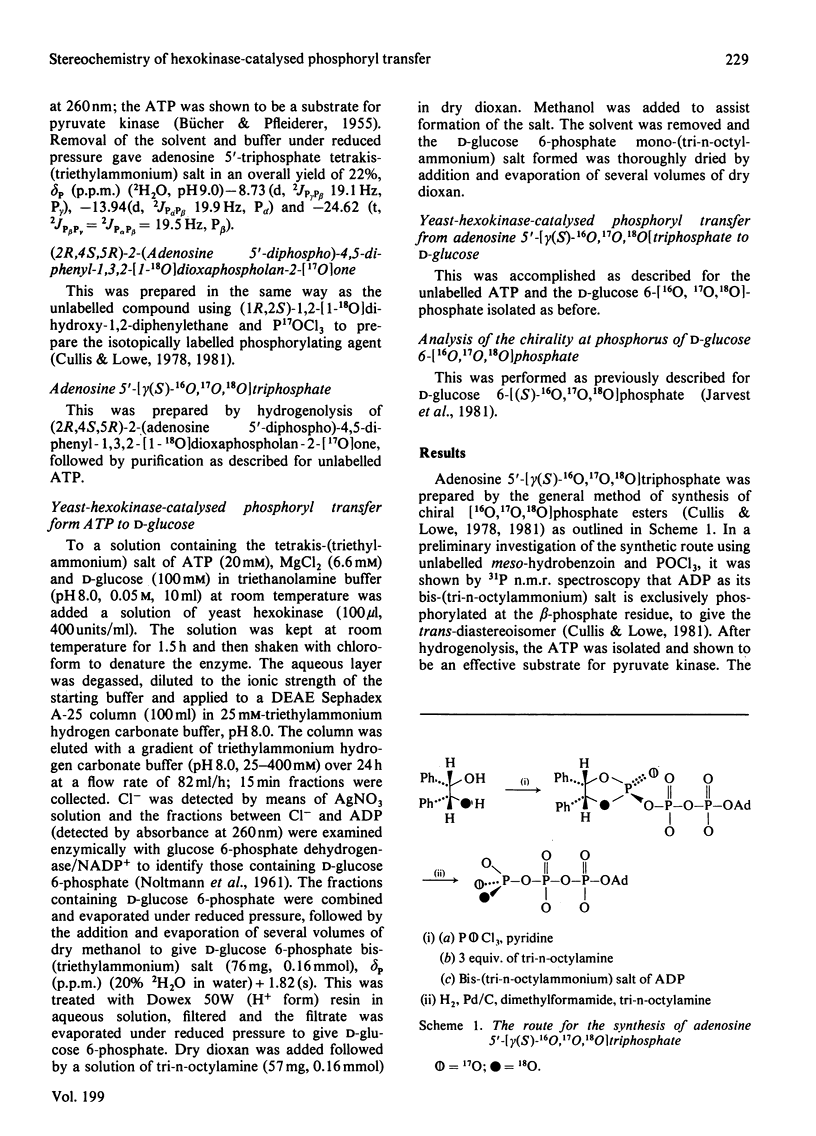
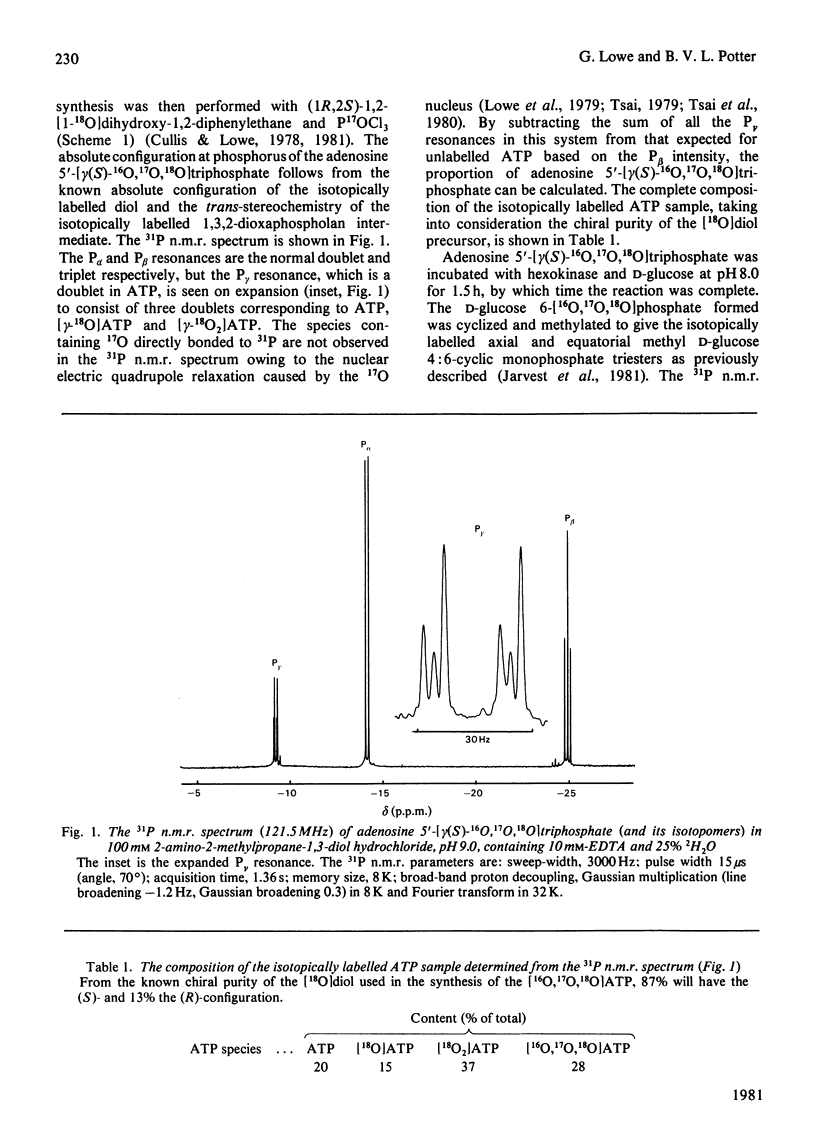
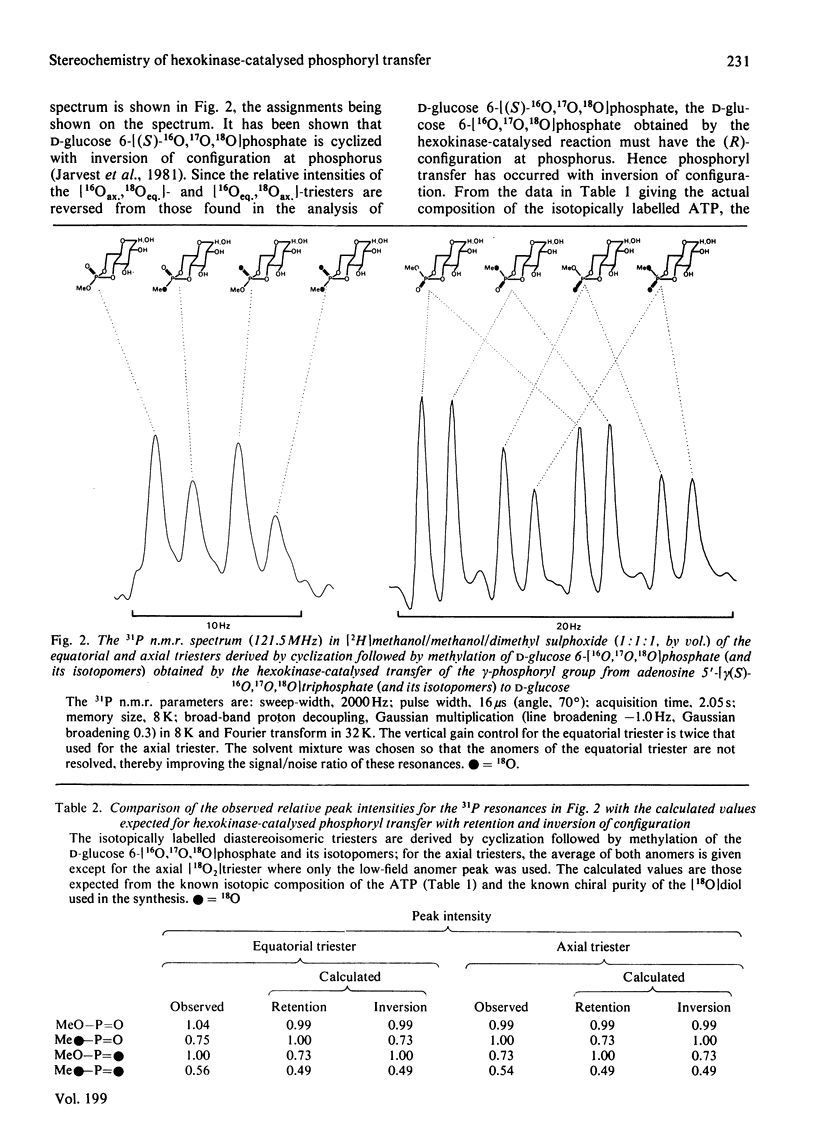
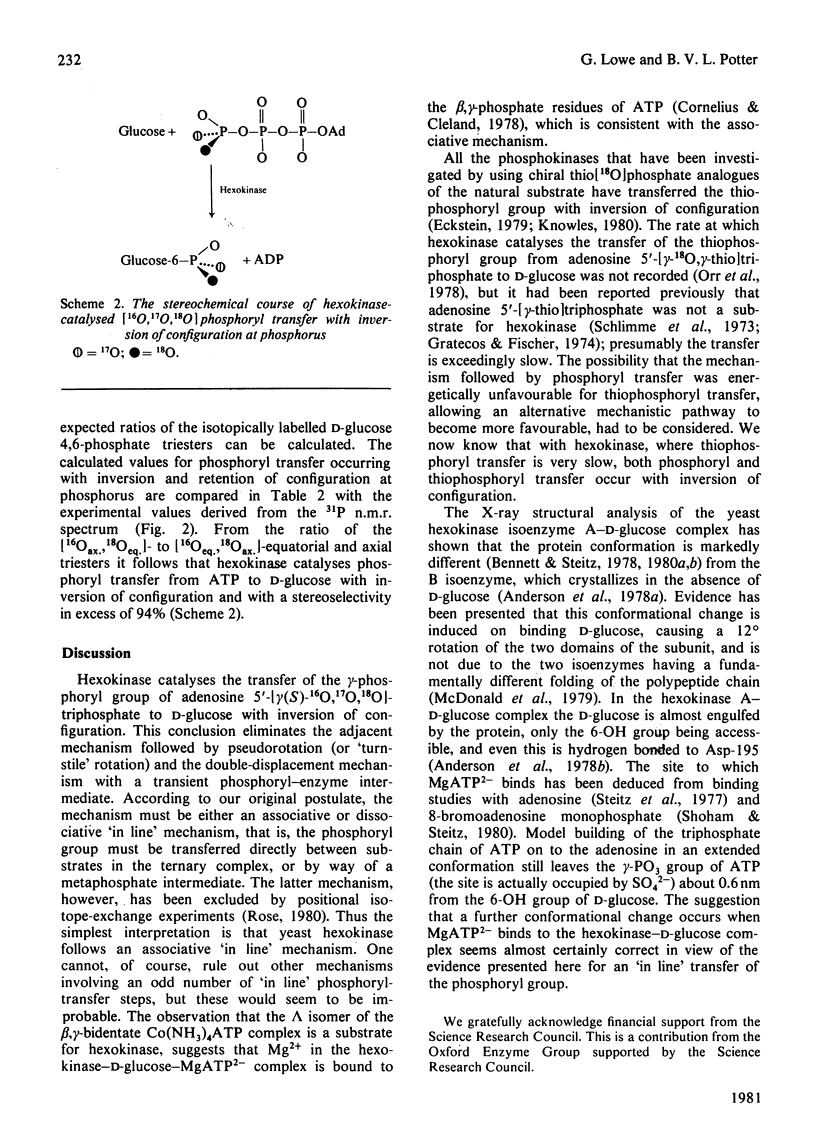
Adenosine 5'[gamma(S)-16O, 17O, 18O]triphosphate has been synthesized and used to determine the stereochemical course of phosphoryl transfer catalysed by yeast hexokinase. The chirality at phosphorus of the D-glucose 6-[16O,17O,18O]phosphate formed was analysed, after cyclization and methylation, by 31P n.m.r. spectroscopy. The phosphoryl transfer was found to occur with inversion of configuration, with a stereoselectivity in excess of 94%. The simplest interpretation of this result is that the phosphoryl group is transferred between substrates in the enzyme-substrate ternary complex by an 'in line' mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. M., Stenkamp R. E., McDonald R. C., Steitz T. A. A refined model of the sugar binding site of yeast hexokinase B. J Mol Biol. 1978 Aug 5;123(2):207–219. doi: 10.1016/0022-2836(78)90321-2. [DOI] [PubMed] [Google Scholar]
- Anderson C. M., Stenkamp R. E., Steitz T. A. Sequencing a protein by x-ray crystallography. II. Refinement of yeast hexokinase B co-ordinates and sequence at 2.1 A resolution. J Mol Biol. 1978 Jul 25;123(1):15–33. doi: 10.1016/0022-2836(78)90374-1. [DOI] [PubMed] [Google Scholar]
- Bennett W. S., Jr, Steitz T. A. Glucose-induced conformational change in yeast hexokinase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4848–4852. doi: 10.1073/pnas.75.10.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett W. S., Jr, Steitz T. A. Structure of a complex between yeast hexokinase A and glucose. I. Structure determination and refinement at 3.5 A resolution. J Mol Biol. 1980 Jun 25;140(2):183–209. doi: 10.1016/0022-2836(80)90102-3. [DOI] [PubMed] [Google Scholar]
- Bennett W. S., Jr, Steitz T. A. Structure of a complex between yeast hexokinase A and glucose. II. Detailed comparisons of conformation and active site configuration with the native hexokinase B monomer and dimer. J Mol Biol. 1980 Jun 25;140(2):211–230. doi: 10.1016/0022-2836(80)90103-5. [DOI] [PubMed] [Google Scholar]
- Cornelius R. D., Cleland W. W. Substrate activity of (adenosine triphosphato)tetraamminecobalt(III) with yeast hexokinase and separation of diastereomers using the enzyme. Biochemistry. 1978 Aug 8;17(16):3279–3286. doi: 10.1021/bi00609a016. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Investigation of enzyme mechanisms with nucleoside phosphorothioates. Angew Chem Int Ed Engl. 1975 Mar;14(3):160–166. doi: 10.1002/anie.197501601. [DOI] [PubMed] [Google Scholar]
- Gratecos D., Fischer E. H. Adenosine 5'-O(3-thiotriphosphate) in the control of phosphorylase activity. Biochem Biophys Res Commun. 1974 Jun 18;58(4):960–967. doi: 10.1016/s0006-291x(74)80237-8. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem. 1980;49:877–919. doi: 10.1146/annurev.bi.49.070180.004305. [DOI] [PubMed] [Google Scholar]
- McDonald R. C., Steitz T. A., Engelman D. M. Yeast hexokinase in solution exhibits a large conformational change upon binding glucose or glucose 6-phosphate. Biochemistry. 1979 Jan 23;18(2):338–342. doi: 10.1021/bi00569a017. [DOI] [PubMed] [Google Scholar]
- NOLTMANN E. A., GUBLER C. J., KUBY S. A. Glucose 6-phosphate dehydrogenase (Zwischenferment). I. Isolation of the crystalline enzyme from yeast. J Biol Chem. 1961 May;236:1225–1230. [PubMed] [Google Scholar]
- Orr G. A., Simon J., Jones S. R., Chin G. J., Knowles J. R. Adenosine 5'-O-([gamma-18O]gamma-thio)triphosphate chiral at the gamma-phosphorus: stereochemical consequences of reactions catalyzed by pyruvate kinase, glycerol kinase, and hexokinase. Proc Natl Acad Sci U S A. 1978 May;75(5):2230–2233. doi: 10.1073/pnas.75.5.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliura D. H., Schomburg D., Richard J. P., Frey P. A., Knowles J. R. Stereochemical course of a phosphokinase using a chiral [18O]phosphorothioate. Comparison with the transfer of a chiral [16O,17O,18O]phosphoryl group. Biochemistry. 1980 Jan 22;19(2):325–329. doi: 10.1021/bi00543a012. [DOI] [PubMed] [Google Scholar]
- Purich D. L., Fromm H. J., Rudolph F. B. The hexokinases: kinetic, physical, and regulatory properties. Adv Enzymol Relat Areas Mol Biol. 1973;39:249–326. doi: 10.1002/9780470122846.ch4. [DOI] [PubMed] [Google Scholar]
- Rose I. A. Mechanism of phosphoryl transfer by hexokinase. Biochem Biophys Res Commun. 1980 May 30;94(2):573–578. doi: 10.1016/0006-291x(80)91270-x. [DOI] [PubMed] [Google Scholar]
- Schlimme E., Lamprecht W., Eckstein F., Goody R. S. Thiophosphate-analogues and 1-N-oxides of ATP and ADP in mitochondrial translocation and phosphoryl-transfer reactions. Eur J Biochem. 1973 Dec 17;40(2):485–491. doi: 10.1111/j.1432-1033.1973.tb03217.x. [DOI] [PubMed] [Google Scholar]
- Shoham M., Steitz T. A. Crystallographic studies and model building of ATP at the active site of hexokinase. J Mol Biol. 1980 Jun 15;140(1):1–14. doi: 10.1016/0022-2836(80)90353-8. [DOI] [PubMed] [Google Scholar]
- Steitz T. A., Anderson W. F., Fletterick R. J., Anderson C. M. High resolution crystal structures of yeast hexokinase complexes with substrates, activators, and inhibitors. Evidence for an allosteric control site. J Biol Chem. 1977 Jul 10;252(13):4494–4500. [PubMed] [Google Scholar]
- Tsai M. D., Huang S. L., Kozlowski J. F., Chang C. C. Applicability of the phosphorus-31 (oxygen-17) nuclear magnetic resonance method in the study of enzyme mechanism involving phosphorus. Biochemistry. 1980 Jul 22;19(15):3531–3536. doi: 10.1021/bi00556a018. [DOI] [PubMed] [Google Scholar]
- Tsai M. D. Use of phosphorus-31 nuclear magnetic resonance to distinguish bridge and nonbridge oxygens of oxygen-17-enriched nucleoside triphosphates. Stereochemistry of acetate activation by acetyl coenzyme A synthetase. Biochemistry. 1979 Apr 17;18(8):1468–1472. doi: 10.1021/bi00575a013. [DOI] [PubMed] [Google Scholar]


