Abstract
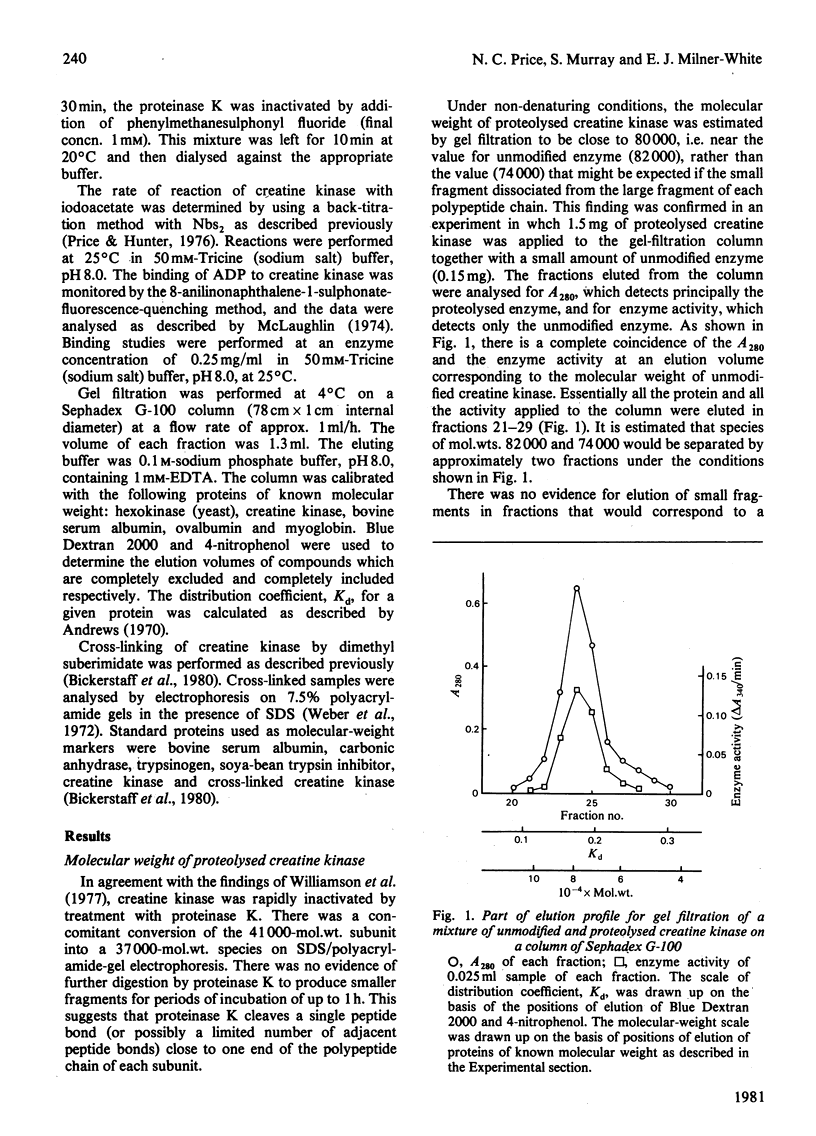
Creatine kinase from rabbit muscle is inactivated by limited proteolysis with proteinase K from Tritirachium album. Gel-filtration and cross-linking studies showed that the limited proteolysis did not affect the molecular weight of the enzyme under non-denaturing conditions, but did cause changes in the reactivity of the reactive thiol group on each subunit and in the ability of the enzyme to form a 'transition-state analogue' complex in the presence of magnesium acetate plus ADP plus creatinine plus NaNO3.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- Bickerstaff G. F., Paterson C., Price N. C. The refolding of denatured rabbit muscle creatine kinase. Biochim Biophys Acta. 1980 Feb 27;621(2):305–314. doi: 10.1016/0005-2795(80)90182-8. [DOI] [PubMed] [Google Scholar]
- Borders C. L., Jr, Riordan J. F. An essential arginyl residue at the nucleotide binding site of creatine kinase. Biochemistry. 1975 Oct 21;14(21):4699–4704. doi: 10.1021/bi00692a021. [DOI] [PubMed] [Google Scholar]
- Chan W. W., Enns C. A. Structure and function of aspartate transcarbamoylase studied using chymotrypsin as a probe. Can J Biochem. 1978 Jun;56(6):654–658. doi: 10.1139/o78-098. [DOI] [PubMed] [Google Scholar]
- Clarke D. E., Price N. C. The reaction of rabbit muscle creatine kinase with diethyl pyrocarbonate. Biochem J. 1979 Aug 1;181(2):467–475. doi: 10.1042/bj1810467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. F., Kenyon G. L., Cleland W. W. Use of pH studies to elucidate the catalytic mechanism of rabbit muscle creatine kinase. Biochemistry. 1981 Mar 3;20(5):1204–1210. doi: 10.1021/bi00508a023. [DOI] [PubMed] [Google Scholar]
- Dzugaj A., Chu D. K., El-Dorry H. A., Horecker B. L. Isolation of the S-peptide formed on digestion of fructose 1,6-bisphosphatase with subtilisin and its non-covalent association with the enzyme protein. Biochem Biophys Res Commun. 1976 May 17;70(2):638–646. doi: 10.1016/0006-291x(76)91095-0. [DOI] [PubMed] [Google Scholar]
- James T. L., Cohn M. The role of the lysyl residue at the active site of creatine kinase. Nuclear Overhauser effect studies. J Biol Chem. 1974 Apr 25;249(8):2599–2604. [PubMed] [Google Scholar]
- Keighren M. A., Price N. C. A study of the role of the reactive thiol group of rabbit muscle creatine kinase with a chromophoric reporter group. Biochem J. 1978 Apr 1;171(1):269–272. doi: 10.1042/bj1710269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner K., Bisswanger H. Multifunctional proteins. Annu Rev Biochem. 1976;45:143–166. doi: 10.1146/annurev.bi.45.070176.001043. [DOI] [PubMed] [Google Scholar]
- Lei M., Aebi U., Heidner E. G., Eisenberg D. Limited proteolysis of glutamine synthetase is inhibited by glutamate and by feedback inhibitors. J Biol Chem. 1979 Apr 25;254(8):3129–3134. [PubMed] [Google Scholar]
- Maggio E. T., Kenyon G. L. Properties of a CH3-blocked creatine kinase with altered catalytic activity. Kinetic consequences of the presence of the blocking group. J Biol Chem. 1977 Feb 25;252(4):1202–1207. [PubMed] [Google Scholar]
- McLaughlin A. C., Leigh J. S., Jr, Cohn M. Magnetic resonance study of the three-dimensional structure of creatine kinase-substrate complexes. Implications for substrate specificity and catalytic mechanism. J Biol Chem. 1976 May 10;251(9):2777–2787. [PubMed] [Google Scholar]
- McLaughlin A. C. The interaction of 8-anilino-1-naphthalenesulfonate with creatine kinase. Evidence for cooperativitiy of nucleotide binding. J Biol Chem. 1974 Mar 10;249(5):1445–1452. [PubMed] [Google Scholar]
- Milner-White E. J., Watts D. C. Inhibition of adenosine 5'-triphosphate-creatine phosphotransferase by substrate-anion complexes. Evidence for the transition-state organization of the catalytic site. Biochem J. 1971 May;122(5):727–740. doi: 10.1042/bj1220727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price N. C., Hunter M. G. Non-identical behaviour of the subunits of rabbit muscule creatine kinase. Biochim Biophys Acta. 1976 Sep 14;445(2):364–376. doi: 10.1016/0005-2744(76)90090-5. [DOI] [PubMed] [Google Scholar]
- Price N. C. The reaction of rabbit muscle creatine kinase with some derivatives of iodoacetamide. Biochem J. 1979 Feb 1;177(2):603–612. doi: 10.1042/bj1770603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDS F. M., VITHAYATHIL P. J. The preparation of subtilisn-modified ribonuclease and the separation of the peptide and protein components. J Biol Chem. 1959 Jun;234(6):1459–1465. [PubMed] [Google Scholar]
- Raibaud O., Goldberg M. E. Characterization of two complementary polypeptide chains obtained by proteolysis of rabbit muscle phosphorylase. Biochemistry. 1973 Dec 4;12(25):5154–5161. doi: 10.1021/bi00749a021. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Maggio E. T., Kenyon G. L. Simple alkanethiol groups for temporary blocking of sulfhydryl groups of enzymes. Biochemistry. 1975 Feb 25;14(4):766–771. doi: 10.1021/bi00675a019. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Williamson J., Greene J., Chérif S., Milner-White E. J. Heterogeneity of rabbit muscle creatine kinase and limited proteolysis by proteinase K. Biochem J. 1977 Dec 1;167(3):731–737. doi: 10.1042/bj1670731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- der Terrossian E., Kassab R. Preparation and properties of S-cyano derivatives of creatine kinase. Eur J Biochem. 1976 Nov 15;70(2):623–628. doi: 10.1111/j.1432-1033.1976.tb11053.x. [DOI] [PubMed] [Google Scholar]


