Abstract
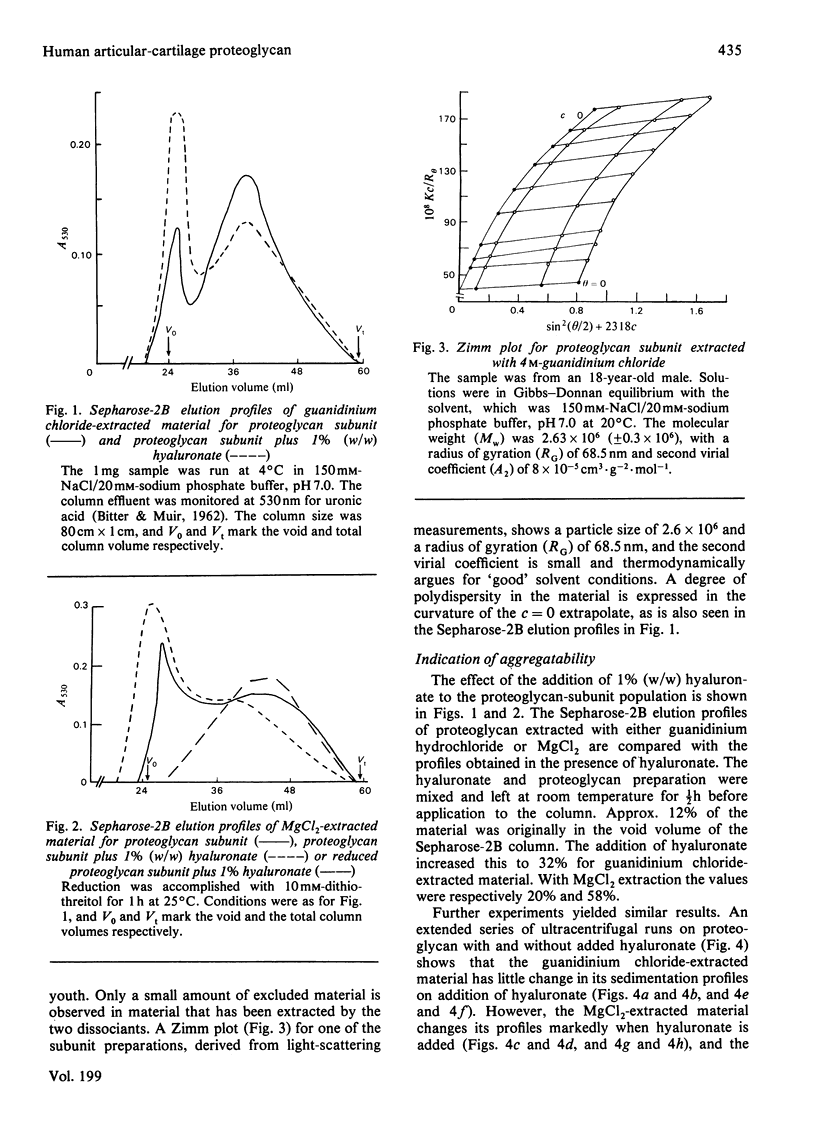
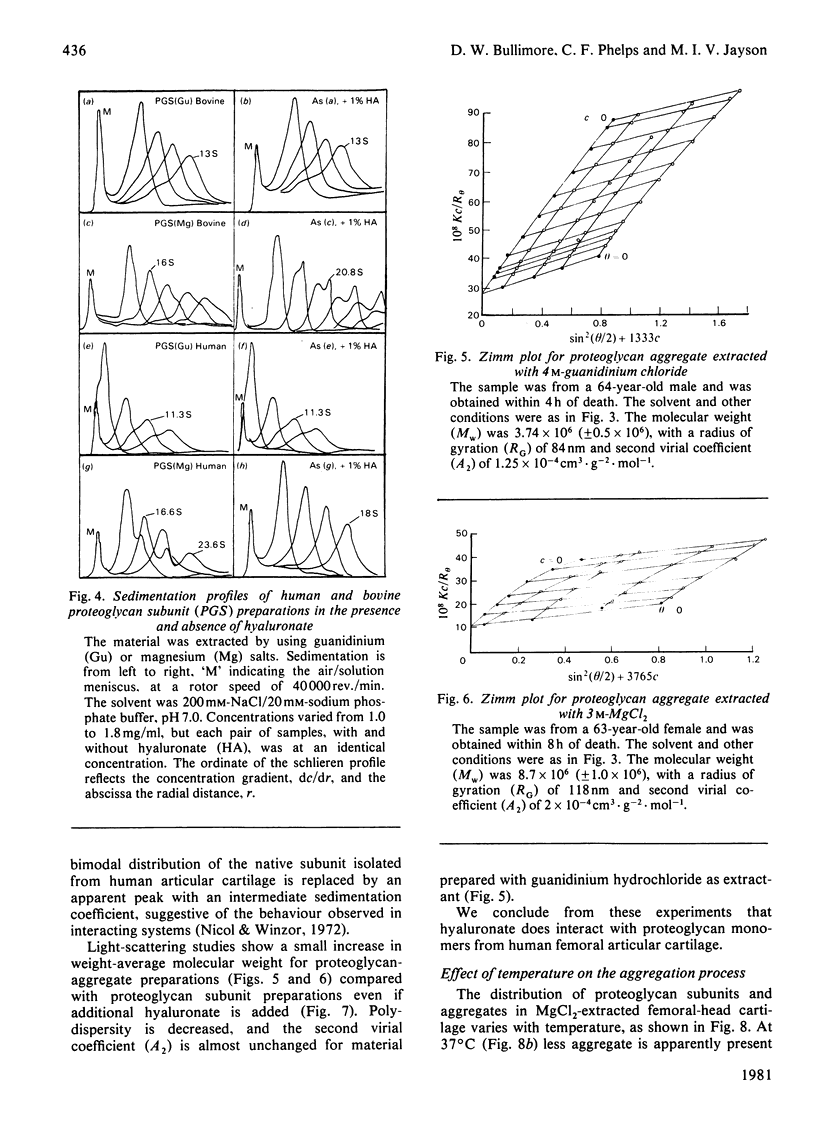
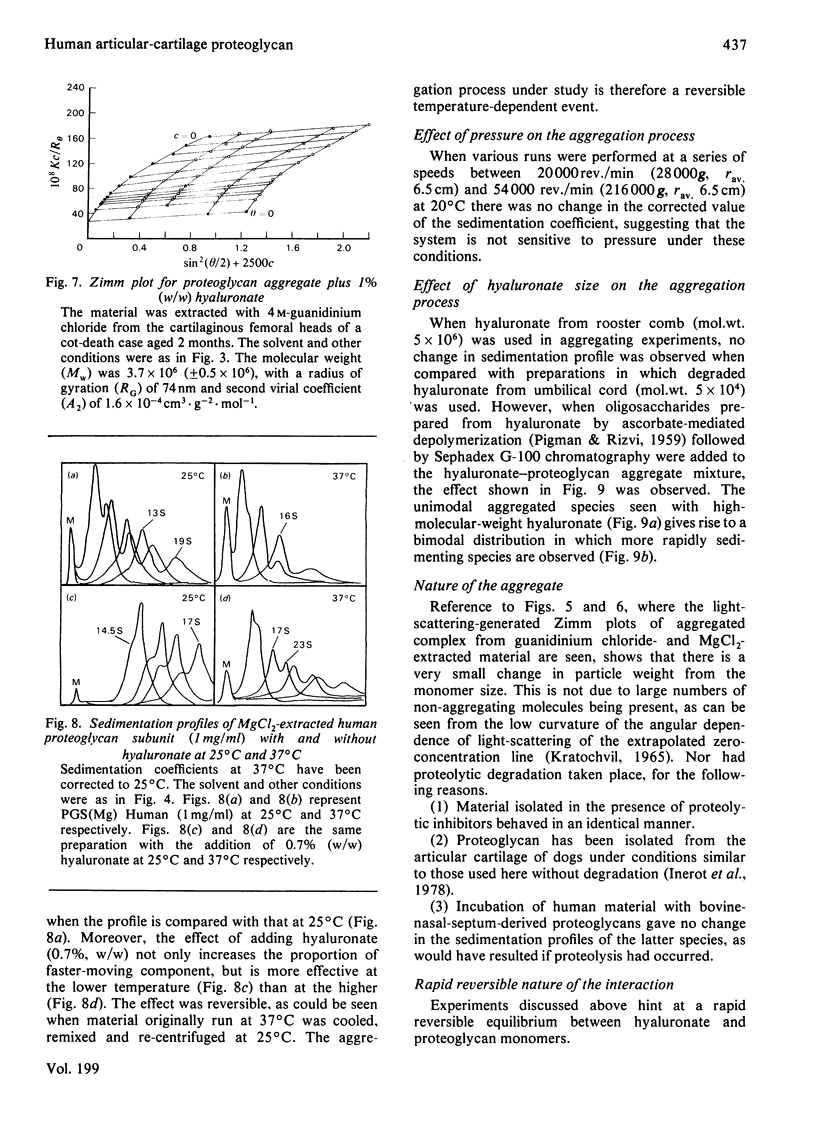
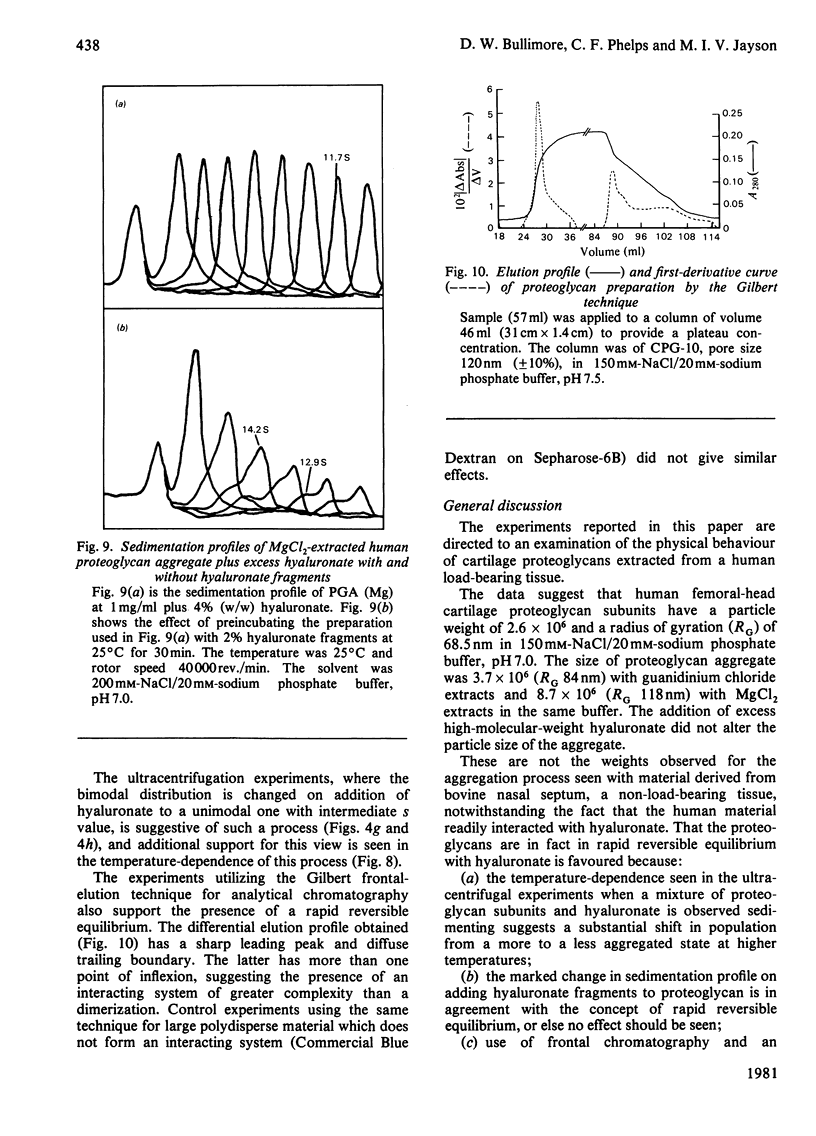
Proteoglycans were prepared from human femoral-head articular cartilage by using either guanidinium hydrochloride or MgCl2 as extractant, followed by density-gradient centrifugation. The proteoglycan subunit had a particle weight of 2.6 x 10(6), with a radius of gyration, RG, of 68.5 nm in 150 mM-NaCl/20 mM-sodium phosphate buffer, pH 7.0. The proteoglycan aggregate had a particle weight of 3.7 x 10(6) (RG 84 nm) for guanidinium hydrochloride extracts and 8.7 x 10(6) (RG 118 nm) for MgCl2 extracts in the same buffer. The addition of excess of high-molecular-weight hyaluronate did not significantly alter the particle size of the aggregate. The small increase in size probably reflects a rapid equilibrium between hyaluronate and proteoglycan monomers, and is not due to proteolytic cleavage producing non-aggregating units. Experiments that support the rapid-interaction hypothesis include analytical ultracentrifugation and column chromatography. This interaction does not appear to be pressure-sensitive at 20 degrees C, but is sensitive to temperature variation near the physiological range.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Isolation of proteoglycans from human articular cartilage. Biochem J. 1978 Jan 1;169(1):123–132. doi: 10.1042/bj1690123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Binding of oligosaccharides of hyaluronic acid to proteoglycans. Biochem J. 1973 Dec;135(4):905–908. doi: 10.1042/bj1350905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Biosynthesis of proteoglycans in cartilage slices. Fractionation by gel chromatography and equilibrium density-gradient centrifugation. Biochem J. 1972 Feb;126(4):791–803. doi: 10.1042/bj1260791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D. Extraction, fractionation and characterization of proteoglycans from bovine tracheal cartilage. Biochim Biophys Acta. 1972 Nov 28;285(1):181–192. doi: 10.1016/0005-2795(72)90190-0. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Inerot S., Heinegård D., Audell L., Olsson S. E. Articular-cartilage proteoglycans in aging and osteoarthritis. Biochem J. 1978 Jan 1;169(1):143–156. doi: 10.1042/bj1690143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs R., Harrington W. F. An unusual pressure dependence for a reversibly associating protein system; sedimentation studies on myosin. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1587–1594. doi: 10.1073/pnas.58.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- WINZOR D. J., SCHERAGA H. A. STUDIES OF CHEMICALLY REACTING SYSTEMS ON SEPHADEX. I. CHROMATOGRAPHIC DEMONSTRATION OF THE GILBERT THEORY. Biochemistry. 1963 Nov-Dec;2:1263–1267. doi: 10.1021/bi00906a016. [DOI] [PubMed] [Google Scholar]


