Abstract
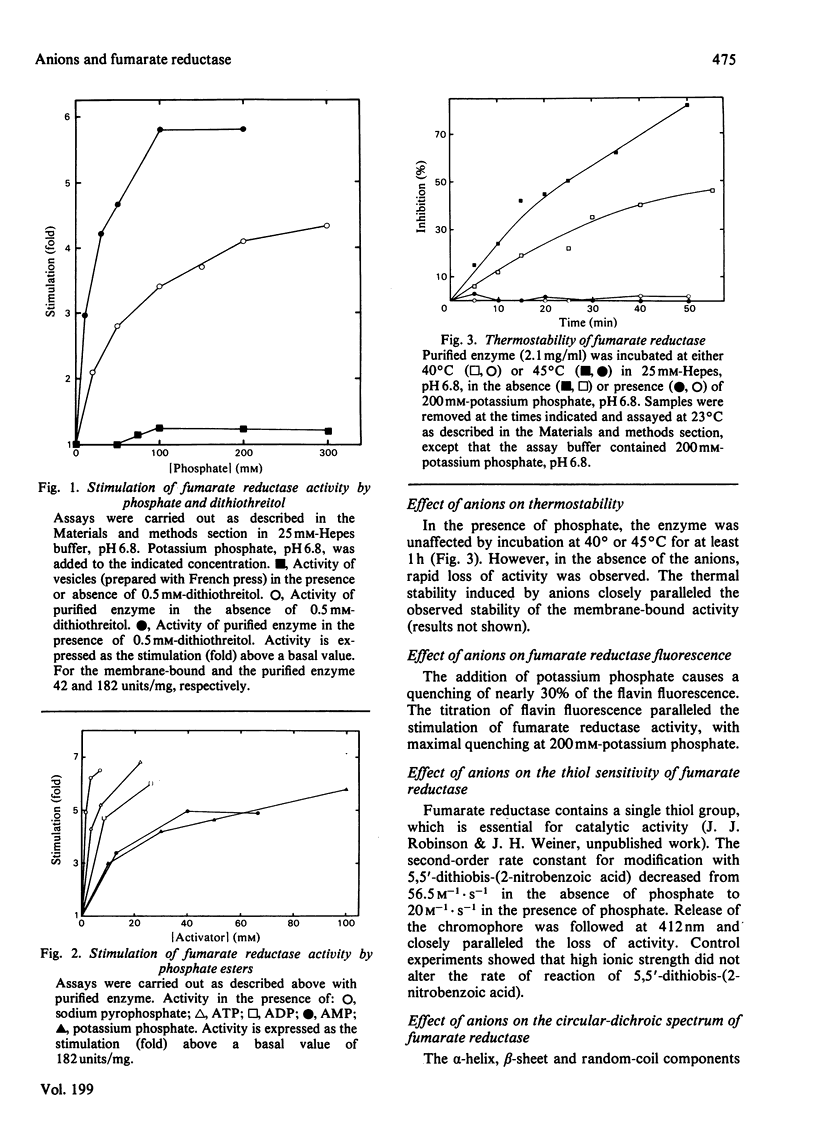
A broad range of anions was shown to stimulate the maximal velocity of purified fumarate reductase isolated from the cytoplasmic membrane of Escherichia coli, while leaving the Km for fumarate unaffected. Reducing agents potentiate the effects of anions on the activity, but have no effect by themselves. Thermal stability, conformation as monitored by circular dichroism and susceptibility to the thiol reagent 5,5'-dithiobis-(2-nitrobenzoic acid) are also altered by anions. The apparent Km for succinate in the reverse reaction (succinate dehydrogenase activity) varies as a function of anion concentration, but the maximal velocity is not affected. The membrane-bound activity is not stimulated by anions and its properties closely resemble those of the purified enzyme in the presence of anions. Thus it appears that anions alter the physical and chemical properties of fumarate reductase, so that it more closely resembles the membrane-bound form.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Dickie P., Weiner J. H. Purification and characterization of membrane-bound fumarate reductase from anaerobically grown Escherichia coli. Can J Biochem. 1979 Jun;57(6):813–821. doi: 10.1139/o79-101. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre J. O., Holladay L. A., Smigel M., Puett D., Fleischer S. Hydrodynamic properties of D-beta-hydroxybutyrate dehydrogenase, a lipid-requiring enzyme. Biochemistry. 1978 Oct 3;17(20):4169–4177. doi: 10.1021/bi00613a010. [DOI] [PubMed] [Google Scholar]
- Oikawa K., Kay C. M., McCubbin W. D. The ultraviolet circular dichroism of muscle proteins. Biochim Biophys Acta. 1968 Sep 10;168(1):164–167. doi: 10.1016/0005-2795(68)90248-1. [DOI] [PubMed] [Google Scholar]
- Robinson J. J., Weiner J. H. The effect of amphipaths on the flavin-linked aerobic glycerol-3-phosphate dehydrogenase from Escherichia coli. Can J Biochem. 1980 Oct;58(10):1172–1178. doi: 10.1139/o80-157. [DOI] [PubMed] [Google Scholar]
- Rosen B. P., Tsuchiya T. Preparation of everted membrane vesicles from Escherichia coli for the measurement of calcium transport. Methods Enzymol. 1979;56:233–241. doi: 10.1016/0076-6879(79)56026-1. [DOI] [PubMed] [Google Scholar]
- Stevens D. J., Gennis R. B. Studies on the quaternary structure of Escherichia coli pyruvate oxidase. J Biol Chem. 1980 Jan 25;255(2):379–383. [PubMed] [Google Scholar]


