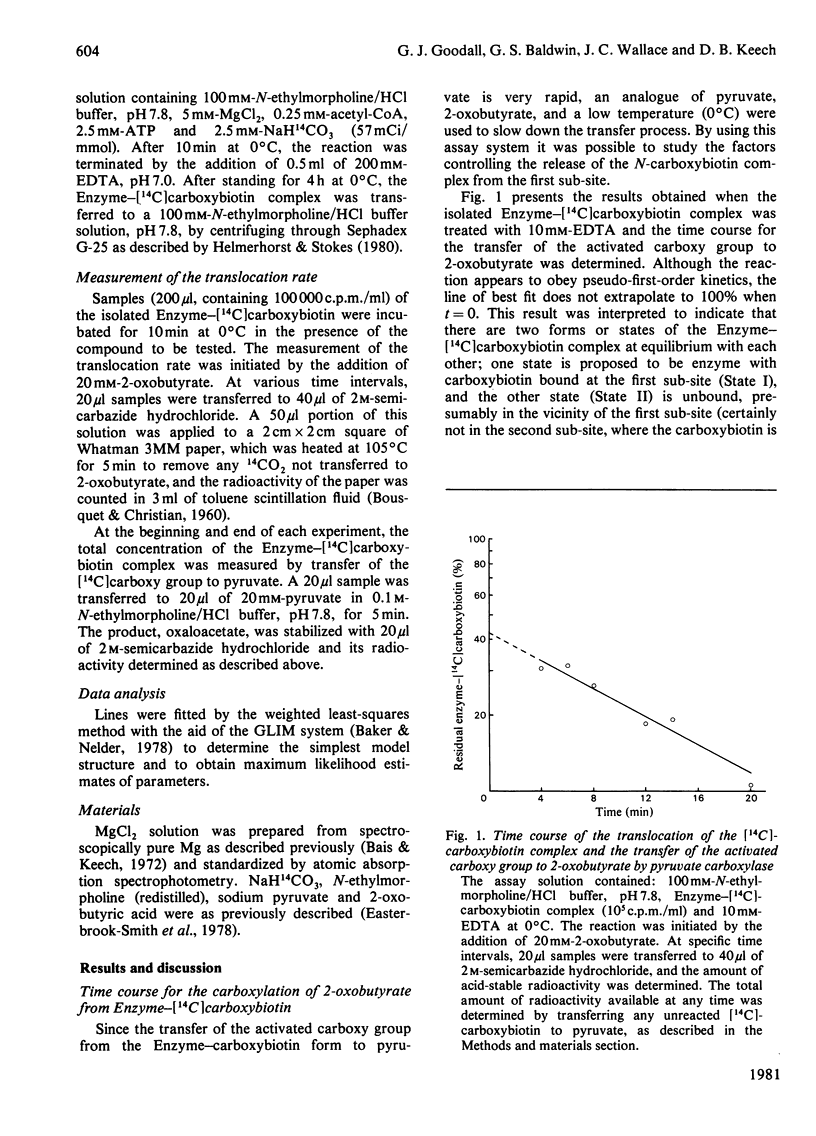
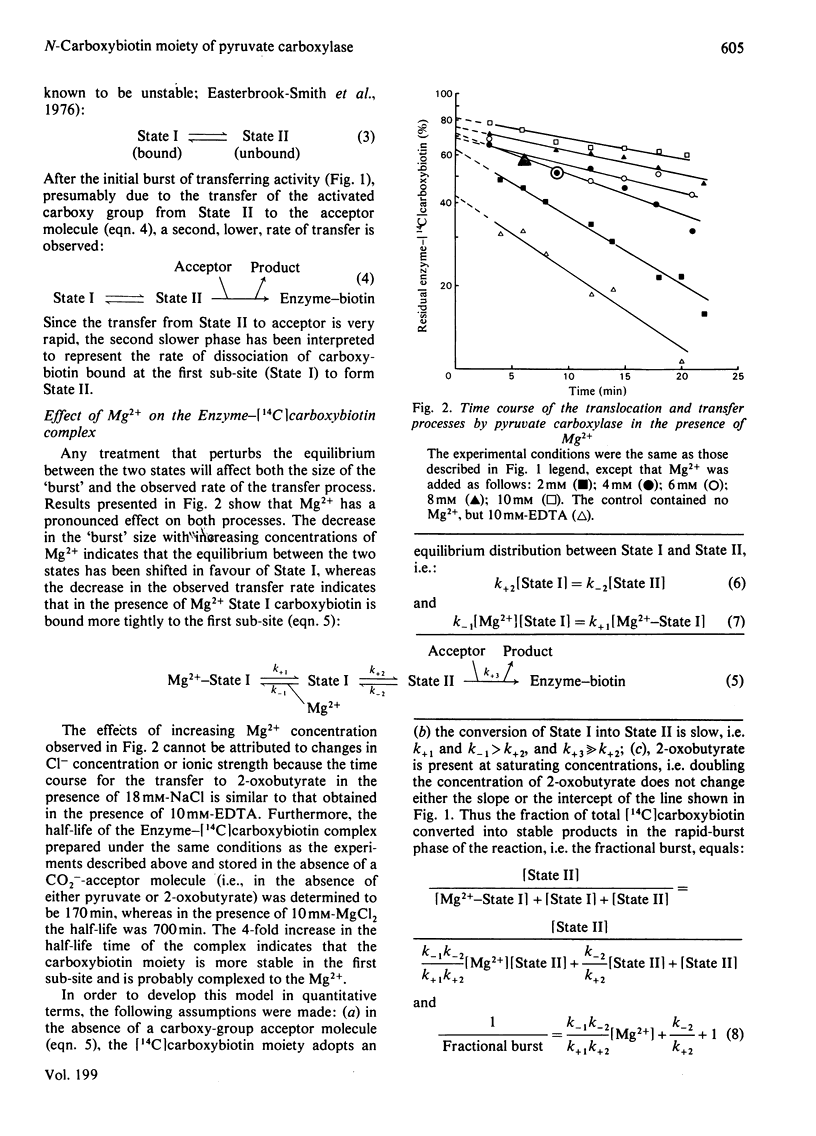
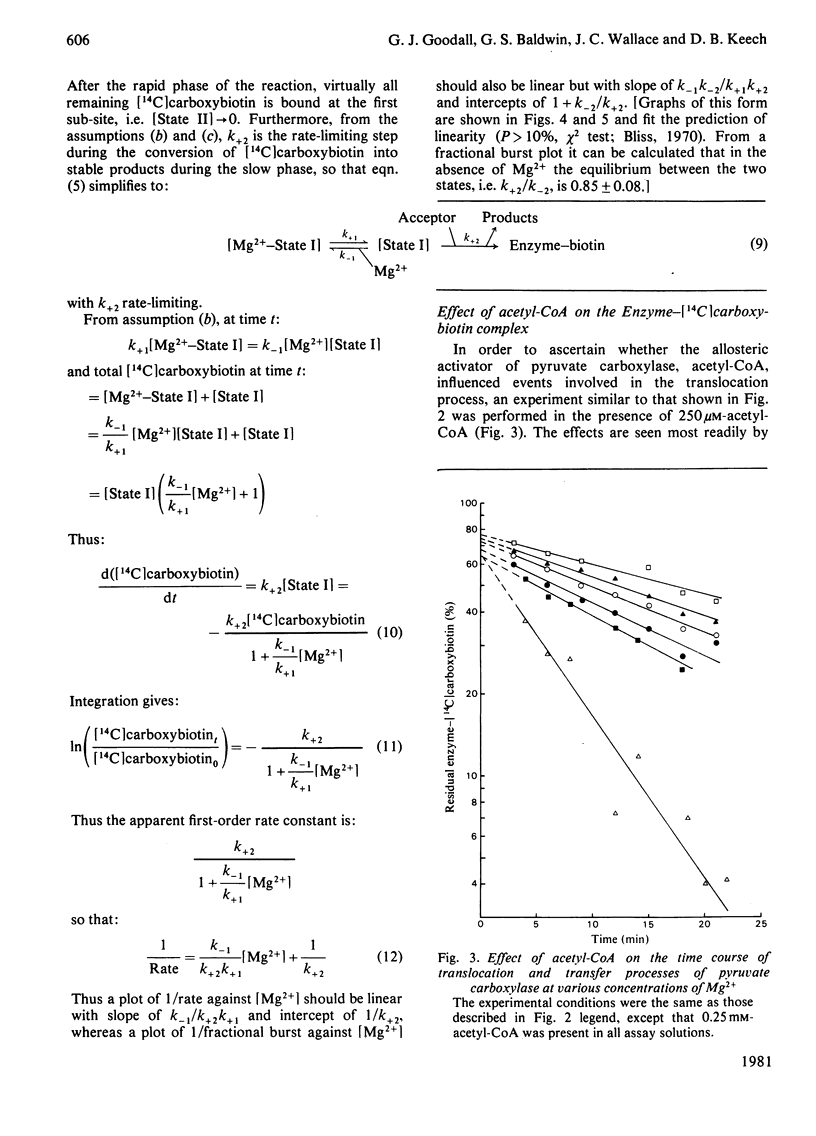
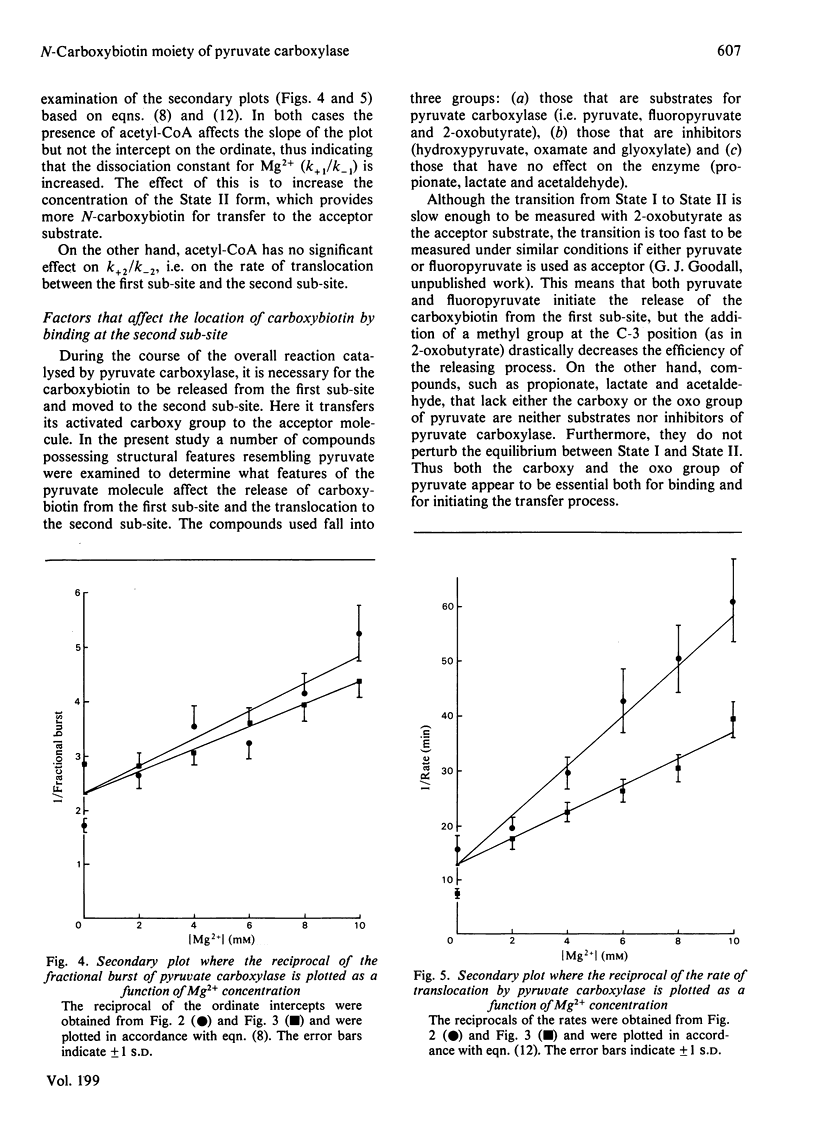
Abstract
The active site of pyruvate carboxylase, like those of all biotin-dependent carboxylases, is believed to consist of two spatially distinct sub-sites with biotin acting as a mobile carboxy-group carrier oscillating between the two sub-sites. Some of the factors that influence the location and rate of movement of the N-carboxybiotin were studied. The rate of carboxylation of the alternative substrate, 2-oxobutyrate, was measured at 0 degrees C in an assay system where the isolated enzyme--[14C]carboxybiotin was the carboxy-group donor. The results are consistent with the hypothesis that the location of the carboxybiotin in the active site is determined by the presence of Mg2+, acetyl-CoA and the oxo acid substrate. The presence of Mg2+ favours the holding of the complex at the first sub-site, whereas alpha-oxo acids induce the complex to move to the second sub-site. At low concentrations pyruvate induces this movement but does not efficiently act as a carboxy-group acceptor; hydroxypyruvate, glyoxylate and oxamate, though not carboxylated, still induce the movement. The allosteric activator acetyl-CoA exerts only a slight stimulation on the rate of translocation to the second sub-site, and this stimulation arises from an increase in the dissociation constant for Mg2+.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman L. K., Keech D. B. Sheep kidney pyruvate carboxylase. Studies on the coupling of adenosine triphosphate hydrolysis and CO2 fixation. J Biol Chem. 1975 Jan 10;250(1):14–21. [PubMed] [Google Scholar]
- Bais R., Keech B. The magnesium ion (Mg 2+ ) activation of sheep kidney pyruvate carboxylase. J Biol Chem. 1972 May 25;247(10):3255–3261. [PubMed] [Google Scholar]
- Barden R. E., Fung C. H., Utter M. F., Scrutton M. C. Pyruvate carboxylase from chicken liver. Steady state kinetic studies indicate a "two-site" ping-pong mechanism. J Biol Chem. 1972 Feb 25;247(4):1323–1333. [PubMed] [Google Scholar]
- Cheung Y. F., Walsh C. Studies on the intramolecular and intermolecular kinetic isotope effects in pyruvate carboxylase catalysis. Biochemistry. 1976 Aug 24;15(17):3749–3754. doi: 10.1021/bi00662a017. [DOI] [PubMed] [Google Scholar]
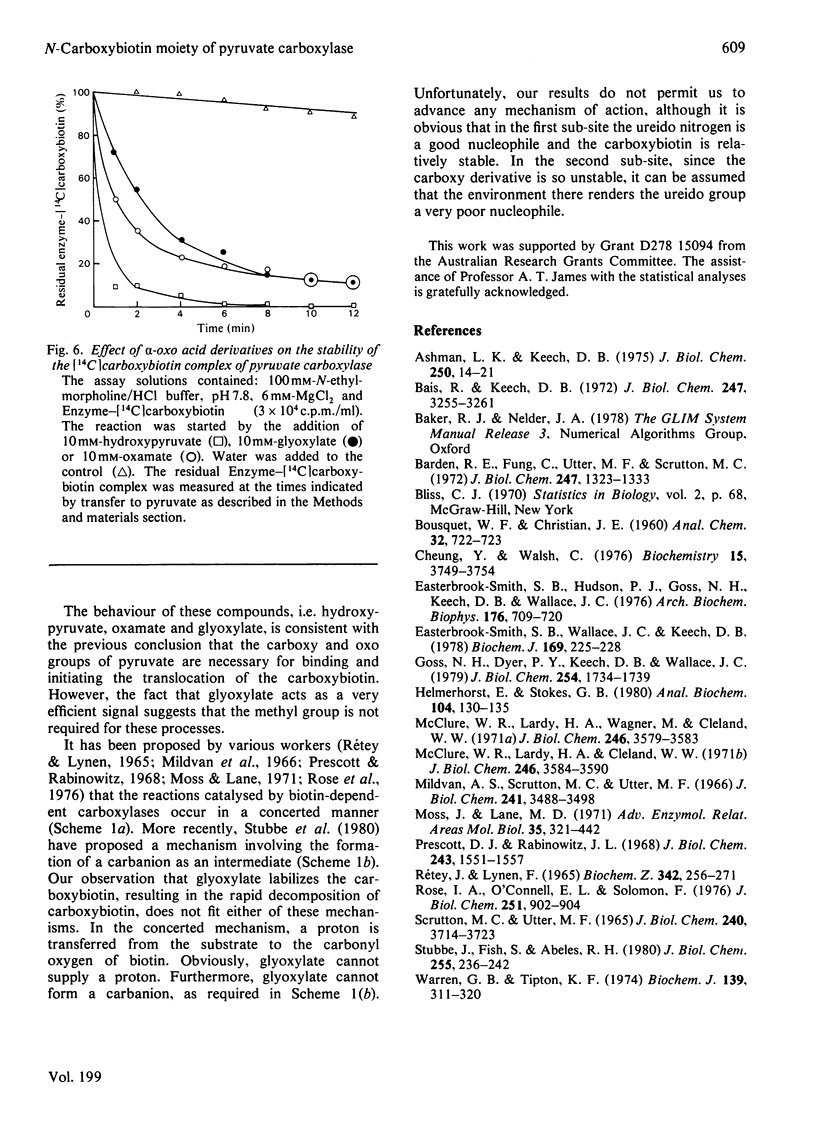
- Easterbrook-Smith S. B., Hudson P. J., Goss N. H., Keech D. B., Wallace J. C. Pyruvate carboxylase: mechanism on the second partial reaction. Arch Biochem Biophys. 1976 Oct;176(2):709–720. doi: 10.1016/0003-9861(76)90215-0. [DOI] [PubMed] [Google Scholar]
- Easterbrook-Smith S. B., Wallace J. C., Keech D. B. A reappraisal of the reaction pathway of pyruvate carboxylase. Biochem J. 1978 Jan 1;169(1):225–228. doi: 10.1042/bj1690225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss N. H., Dyer P. Y., Keech D. B., Wallace J. C. An electron microscopic study of pyruvate carboxylase. J Biol Chem. 1979 Mar 10;254(5):1734–1739. [PubMed] [Google Scholar]
- Helmerhorst E., Stokes G. B. Microcentrifuge desalting: a rapid, quantitative method for desalting small amounts of protein. Anal Biochem. 1980 May 1;104(1):130–135. doi: 10.1016/0003-2697(80)90287-0. [DOI] [PubMed] [Google Scholar]
- Mildvan A. S., Scrutton M. C., Utter M. F. Pyruvate carboxylase. VII. A possible role for tightly bound manganese. J Biol Chem. 1966 Aug 10;241(15):3488–3498. [PubMed] [Google Scholar]
- Morell A. G., Irvine R. A., Sternlieb I., Scheinberg I. H., Ashwell G. Physical and chemical studies on ceruloplasmin. V. Metabolic studies on sialic acid-free ceruloplasmin in vivo. J Biol Chem. 1968 Jan 10;243(1):155–159. [PubMed] [Google Scholar]
- Moss J., Lane M. D. The biotin-dependent enzymes. Adv Enzymol Relat Areas Mol Biol. 1971;35:321–442. doi: 10.1002/9780470122808.ch7. [DOI] [PubMed] [Google Scholar]
- Rose I. A., O'Connell E. L., Solomon F. Intermolecular tritium transfer in the transcarboxylase reaction. J Biol Chem. 1976 Feb 10;251(3):902–904. [PubMed] [Google Scholar]
- Rétey J., Lynen F. Zur biochemischen Funktion des Biotins. IX. Der sterische Verlauf der Carboxylierung von Propionyl-CoA. Biochem Z. 1965 Aug 6;342(3):256–271. [PubMed] [Google Scholar]
- Scrutton M. C., Utter M. F. Pyruvate carboxylase. V. Interaction of the enzyme with adenosine triphosphate. J Biol Chem. 1965 Oct;240(10):3714–3723. [PubMed] [Google Scholar]
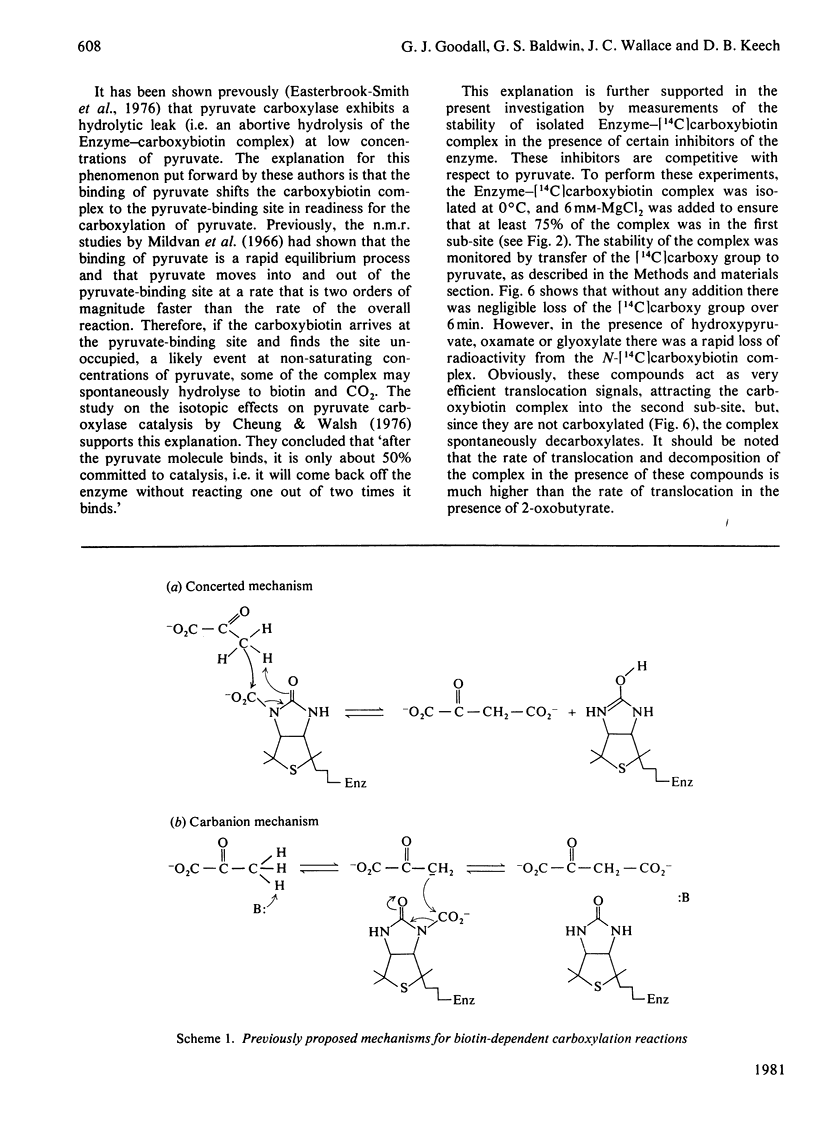
- Stubbe J., Fish S., Abeles R. H. Are carboxylations involving biotin concerted or nonconcerted? J Biol Chem. 1980 Jan 10;255(1):236–242. [PubMed] [Google Scholar]
- Warren G. B., Tipton K. F. Pig liver pyruvate carboxylase. The reaction pathway for the carboxylation of pyruvate. Biochem J. 1974 May;139(2):311–320. doi: 10.1042/bj1390311. [DOI] [PMC free article] [PubMed] [Google Scholar]


