Abstract
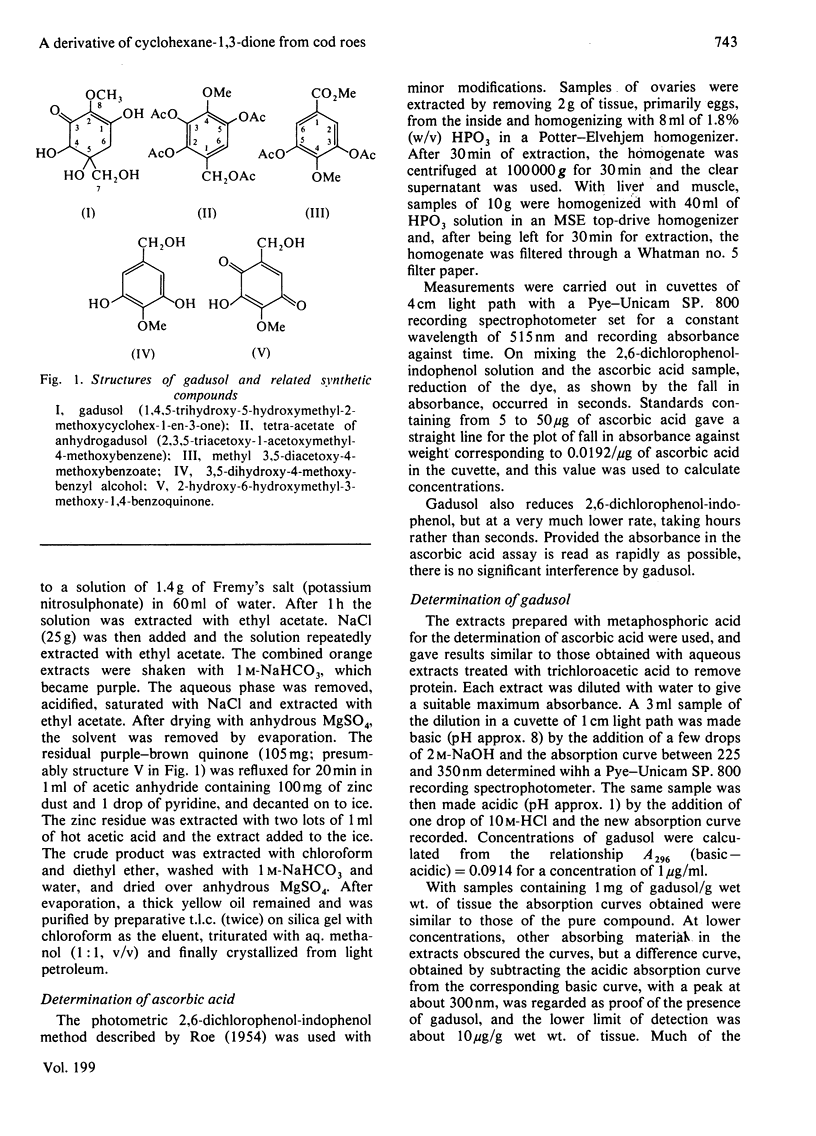
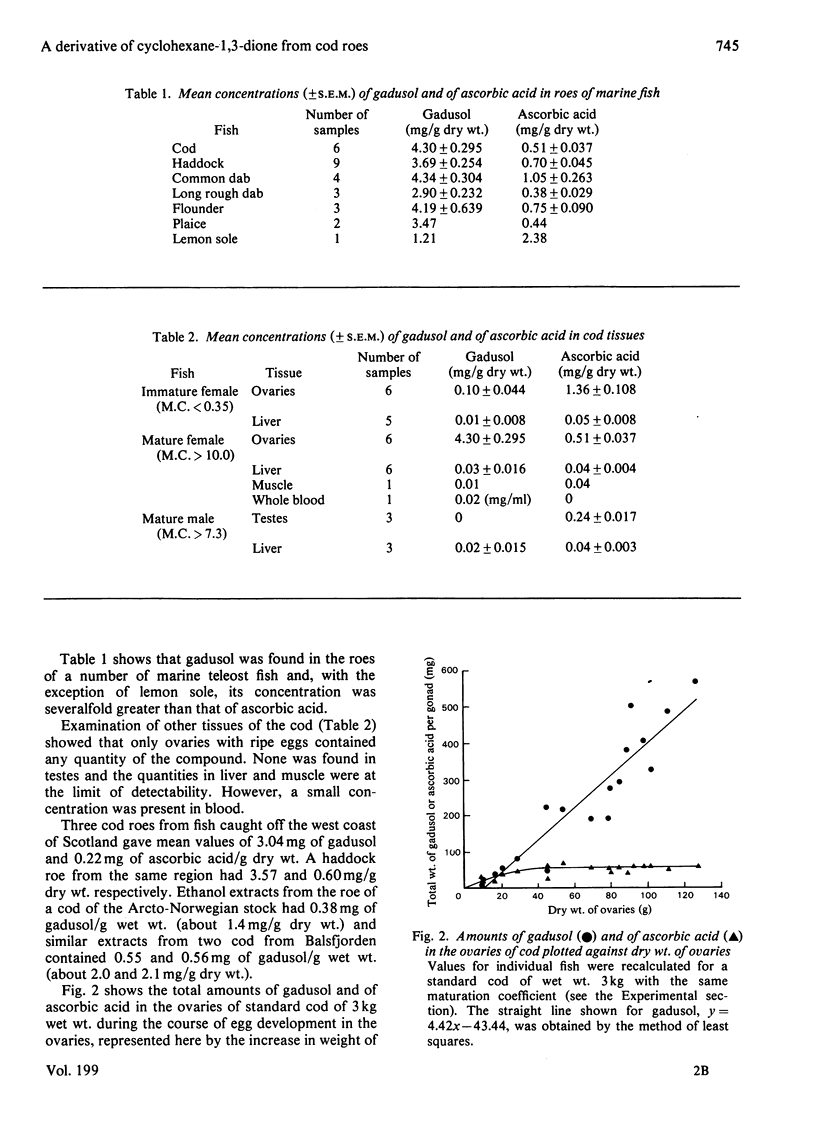
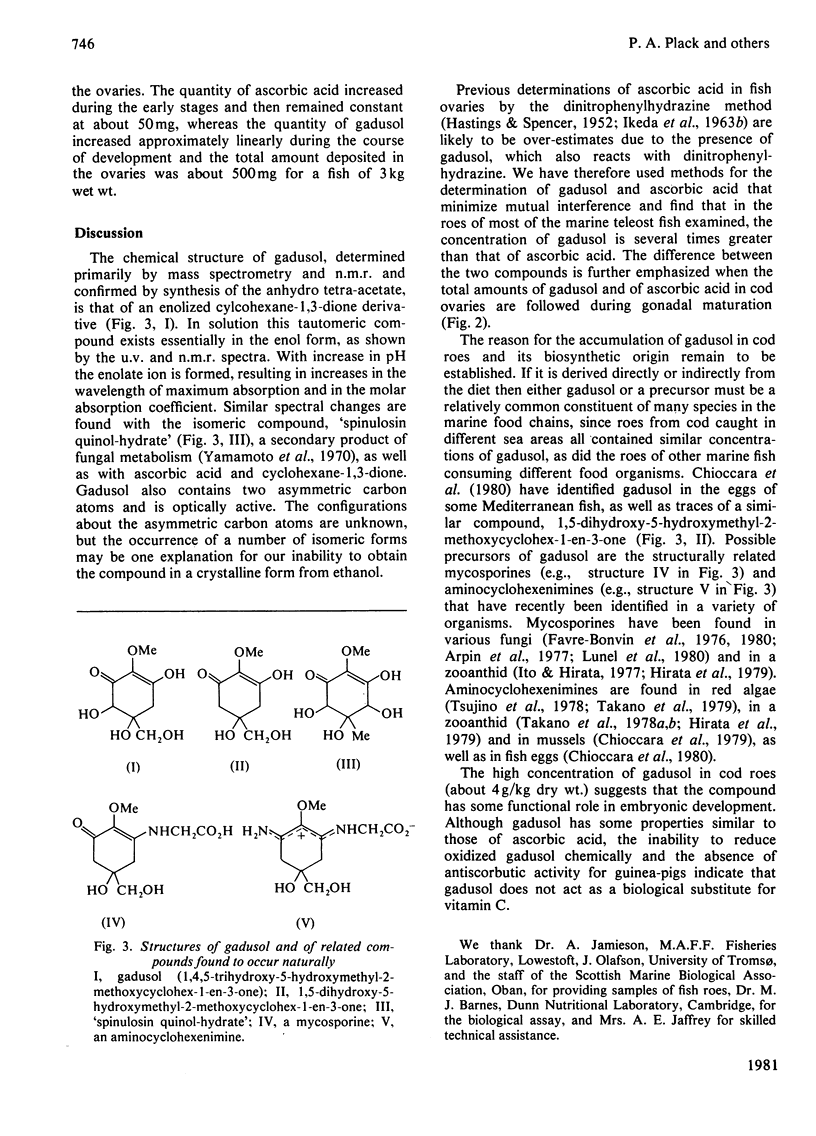
Gadusol, C8H12O6, has been isolated from roes of the cod (Gadus morhua L.), i.e., ovaries that contain ripe eggs just before spawning. The concentration is about 4 g/kg dry wt. It has been identified as 1,4,5-trihydroxy-5-hydroxymethyl-2-methoxycyclo-hex-1-en-3-one and this structure was confirmed by synthesis of the anhydro tetra-acetate derivative from methyl 3,5-diacetoxy-4-methoxybenzoate. Concentrations of gadusol in the roes of other marine teleost fish examined are of the same order as in cod roes. Gadusol has some properties similar to ascorbic acid and both compounds, after oxidation, react with 2,4-dinitrophenylhydrazine in the commonly-used assay procedure for ascorbic acid. Specific assays showed that the concentrations of gadusol in the roes of marine fish are severalfold greater than those of ascorbic acid. Gadusol is structurally related to the mycosporines previously reported from a number of different organisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Plack P. A., Fraser N. W. Incorporation of l-[C]leucine into egg proteins by liver slices from cod. Biochem J. 1971 Mar;121(5):857–862. doi: 10.1042/bj1210857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plack P. A., Pritchard D. J., Fraser N. W. Egg proteins in cod serum. Natural occurrence and induction by injections of oestradiol 3-benzoate. Biochem J. 1971 Mar;121(5):847–856. doi: 10.1042/bj1210847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROE J. H. Chemical determination of ascorbic, dehydroascorbic, and diketogulonic acids. Methods Biochem Anal. 1954;1:115–139. doi: 10.1002/9780470110171.ch5. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Shinya M., Ooata Y. Studies on the metabolic products of a strain of Aspergillus fumigatus (DH 413). IV. Biosynthesis of toluquinones and chemical structures of new metabolites. Chem Pharm Bull (Tokyo) 1970 Mar;18(3):561–569. doi: 10.1248/cpb.18.561. [DOI] [PubMed] [Google Scholar]


