Abstract
Background
Muscle strength is essential for healthy ageing. Handgrip strength (HGS) has been recommended by expert bodies as the preferred measure of muscle strength, in addition to being considered a strong predictor of overall health. Cross‐sectional studies have shown several potential factors associated with HGS, but a systematic review of factors predicting HGS over time has not previously been conducted. The aim of this study is to systematically review the literature on the factors associated with adult HGS [at follow‐up(s) or its rate of change] across the life course.
Methods
Searches were performed in MEDLINE via Ebsco, Embase and SPORTDiscus databases. Longitudinal studies assessing potential factors impacting adult HGS over time were included in the analyses. Based on previously established definitions of consistency of results, a semiquantitative analysis was conducted using the proportions of studies supporting correlations with HGS.
Results
A total of 117 articles were included in this review. Factors associated with HGS were grouped into 11 domains: demographic, socioeconomic, genetic, early life, body composition, health markers/biomarkers, health conditions, psychosocial, lifestyle, reproductive and environmental determinants. Overall, 103 factors were identified, of which 10 showed consistent associations with HGS over time (i.e., in at least four studies with ≥60% agreement in the direction of association). Factors associated with greater declines in HGS included increasing age, male sex, higher levels of inflammatory markers and the presence of cardiovascular diseases. Education level, medication use, and self‐rated health were not associated with the rate of change in HGS. Increased birth weight was associated with a stronger HGS over time, whereas depressive symptoms were linked to a weaker HGS, and smoking habits showed null associations.
Conclusions
Comparison between studies and estimation of effect sizes were limited due to the heterogeneity in methods. Although sex and age may be the main drivers of HGS decline, it is crucial to prioritize modifiable factors such as inflammation and cardiovascular diseases in health interventions to prevent greater losses. Interventions to improve birth weight and mental health are also likely to produce positive effects on muscle strength. Our results point to the complexity of processes involving muscle strength and suggest that the need to better understand the determinants of HGS remains.
Keywords: Muscle strength, Life course perspective, Ageing, Risk factors, Muscle weakness
Introduction
The World Health Organization describes healthy ageing as ‘the process of developing and maintaining the functional ability that enables wellbeing in older age’. 1 Muscle strength is important for functional independence, and a predictor of healthy ageing. 2 , 3 Handgrip strength (HGS) is a frequently used measure of muscle strength, endorsed by expert bodies in sarcopenia and frailty, as it is a non‐invasive procedure, it is relatively low cost and correlates well with other physical functioning measurements. 3 , 4 , 5 Low HGS has been associated with an increased risk for a range of adverse health outcomes with ageing, including sarcopenia, frailty, cardiovascular diseases, type 2 diabetes, metabolic syndrome, cognitive decline and all‐cause mortality. 2 , 6
HGS peaks at around the age of 30 years, remains stable until about the ages of 40 to 50 years and starts to decline thereafter. 7 , 8 Nonetheless, advancing age alone cannot explain the decline in muscle strength after the fifth decade of life. 9 There have been multiple factors shown to be associated with HGS in cross‐sectional studies; however, findings are often mixed. 3 , 10 Moreover, the predictors of HGS over time remain largely unknown. To the best of our knowledge, there is no comprehensive review of the factors associated with HGS in adulthood.
Muscle strength and physical function in older life reflect not only the age‐related rate of decline rather the peak reached during the earlier stages of life. 11 , 12 A life course approach to physical functioning may provide a better understanding of the trajectories of functional ability and enable planning prevention strategies during people's lifetime. 11 Therefore, this review aims to understand the factors that determine adult muscle strength, measured by HGS, across the life course. We analysed all possible HGS outcomes, including measures of HGS in young to late adulthood, and the rate of decline in HGS over time.
Methods
This review is reported in line with the Preferred Reporting Items for Systematic‐Reviews and Meta‐Analyses (PRISMA). A protocol for the review was registered in the International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42022374356).
Search strategy
We included MeSH or other subject terms and synonyms in the search string. The search was designed with the help of a librarian. The following automation tools were used in the design of the search: Polyglot Search Translator 13 and SearchRefinery. 14
Searches were run in the following databases: MEDLINE via Ebsco, Embase and SPORTDiscus. Searches were run from inception to 4th October 2022 (see Appendix S1A for the search strategies used in each database).
Restrictions were applied to the publication types. Conference abstracts, books, book chapters and thesis were purposefully removed from the search results. No language or publication date restrictions were applied to the search. However, reports in languages other than English, Japanese, Spanish or Portuguese were excluded.
Study selection and screening
We identified and included prospective studies that assessed factors associated with HGS in adulthood. Studies were included if the following criteria were met:
Epidemiological studies of non‐clinical populations conducted in the general community (e.g., people's homes, community facilities, sheltered housing complexes and retirement villages).
Assessed the prospective association of one or more exposure variables with HGS during adulthood (18+ years old).
Described either HGS at follow‐up(s) or the rate of change in HGS over the follow‐up period as the main outcome.
Studies that analysed grip strength as the exposure variable (e.g., that examined the association between changes in grip strength associated with reduced morbidity and mortality) or that were compared with any intervention (e.g., changes in grip strength after exercise training or dietary supplementation) were not included. The initial search returned 5044 studies and 155 were selected. Studies with sample sizes smaller than 560 (i.e., <25th percentile) were excluded because smaller samples can be less representative and yield less meaningful results.
Two authors (L. R. and S. B.) independently screened the titles and abstracts against the inclusion criteria. One review author (L. R.) retrieved the full text, and two authors (L. R. and S. B.) screened the full text for inclusion. Discrepancies were resolved by consensus or by referring to a third author. We used these automation tools to help with the screening of articles: Screenatron/Disputatron. 15
Data extraction
Data were extracted by two authors (L. R. and S. B.) using a standardized form based on the Critical Appraisal and Data Extraction for Systematic Review of Prediction Modelling Studies (CHARMS) checklist. 16 The following data for study characteristics were extracted from each included study: study design, participants (e.g., eligibility and recruitment method; age and gender distribution; duration of follow‐up), sample size, outcome (e.g., definition and method for HGS measurements), factors included in the analyses and statistical methods used (e.g., type of model, model evaluation and handling of missing data) and results.
Quality and risk of bias assessment
The Quality in Prognosis Studies (QUIPS) tool 17 was used for a systematic appraisal of bias in the studies. The justification for using the QUIPS tool lies in its detailed assessment of potential prognostic (or exposure) factors associated with an outcome of interest over time, of the outcome measurement itself, confounding factors and model analysis, offering a more comprehensive evaluation compared with other available tools. Two reviewers were involved in the quality assessment (L. R. and S. B.). Discrepancies were discussed between the two reviewers and/or solved by a third reviewer. Each study was assigned an overall risk of bias (high, moderate or low) based on the rating of the report of six areas, including study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding and statistical analysis and reporting.
Analysis of studies
We grouped potential factors into 11 domains: demographic, socioeconomic, genetic, early life, body composition, health markers/biomarkers, health conditions, psychosocial, lifestyle, reproductive and environmental determinants. Then, tables were created to provide a summary of the state of the literature for each factor associated with HGS, which allows for checking the consistency of the associations between studies. Conceptually similar factors were grouped, even if they were not measured in the same way. We separated variables with different exposure times, such as weight (single point in time) and weight history (multiple points in time).
Based on previous reviews in physical activity and HGS, 18 , 19 , 20 an estimate was calculated of the proportion of studies indicating a correlation relative to the overall number of studies found within each variable. When 60–100% of studies supported a positive, negative or no association at all the symbols ‘+’, ‘‐’ and ‘0’ were used, respectively. Additionally, when there were four or more studies indicating such associations, it was coded as ‘++’, ‘−−’, or ‘00’, to indicate that the variable has been frequently studied and that there was consistency in the results. When there was a lack of consistency in the findings (i.e., 0–59% studies supporting the correlation), non‐linear associations or mainly sex‐specific associations the code ‘mixed’ was used. If the results were male or female‐specific, ‘M’ or ‘F’ was indicated next to the article's reference number. ‘I’ and ‘II’ were used to indicate studies that had an entire sample of males and females, respectively.
Given the substantial heterogeneity, a meta‐analysis was inappropriate as it would not provide meaningful or reliable results. The studies varied in key aspects such as the protocol for measuring HGS, the age and sex distribution of the sample and the covariates considered. Differences in HGS protocol include the type of measurement (average, maximal, summed or relative HGS), hands measured (both, dominant, non‐dominant and preferred), the test trials 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 and the unit of measurement (kg, N, psi, bar and kPa). Moreover, a variety of statistical methods was used in data analyses, such as correlation tests, analysis of variance (ANOVA) and covariance (ANCOVA), latent growth models, and most commonly, regression analysis, including linear, linear mixed‐effects, logistic, generalized estimating equation and hierarchical models. In addition, cut points for weakness and declines in HGS were frequently not described and derived from data of the studied samples and thus are not generalizable to other populations. Although we may show a combination of results across studies in terms of annualized change in HGS, for example, it is important to note that those results should be interpreted with caution.
Results
A total of 117 longitudinal studies were included in this review, with a median follow‐up time of 7 years (IQR: 4–12) (Figure 1 ). Fifty per cent of studies (n = 59) reported the associations between the exposure variables and the rate of change or the decline in HGS (e.g., in kg/year or absolute change), 39% (n = 46) examined the relationship with measurements of HGS in the follow‐up periods (e.g., in kg or weak/strong grip) and 10% (n = 12) reported both types of HGS outcome.
Figure 1.
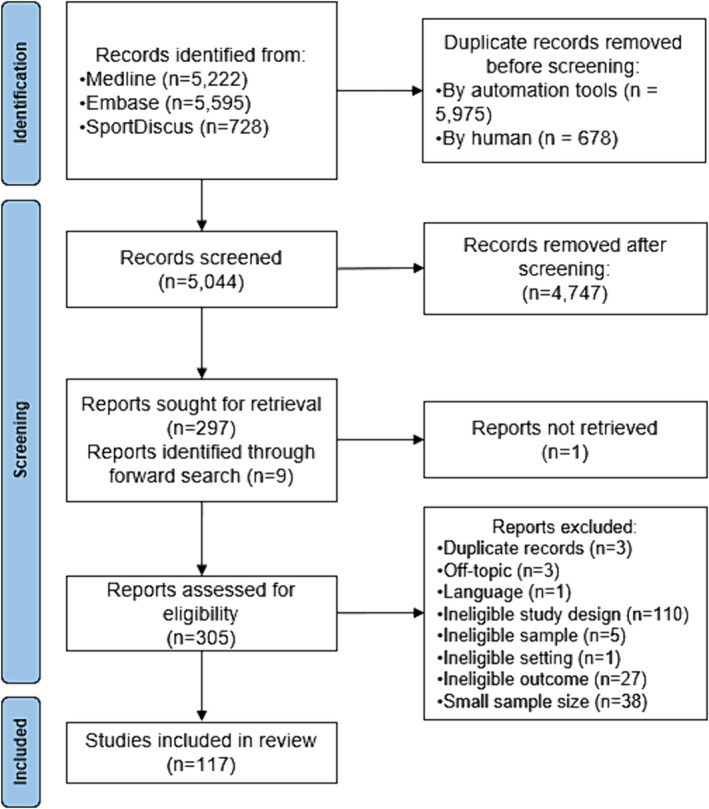
Flowchart of the study selection procedure.
Studies were published between 1990 and 2022, with over 65% of all studies published in the previous 10 years. More than half of the studies were from Europe, 26% from North America, 15% from Asia, 3% from Oceania and 3% from South America. The age distribution of study samples by groups is described in Figure 2 , and the median age was 66 (IQR: 58.4–74.8). Sample sizes ranged from 563 to 144 369 individuals, with a median of 1904 (IQR: 925–5,131). Eight had female‐only and another 10 studies had male‐only samples, totalling 15.4% of all studies.
Figure 2.
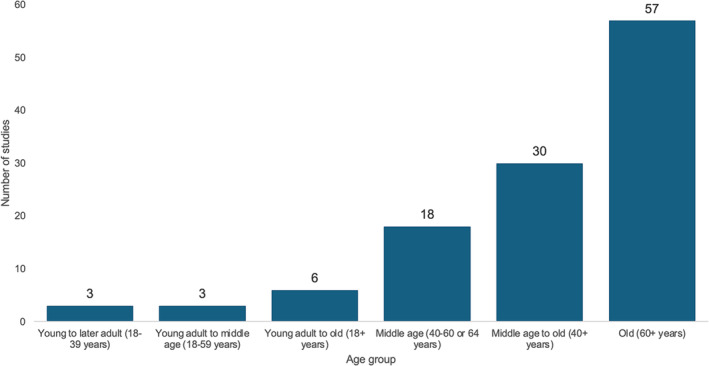
Number of studies by age group of the sample.
A total of 103 factors were identified and allocated to the 11 domains accordingly. Each study analysed from 1 up to 18 factors as predictors of HGS over time. The variables in each of the major predictor domains and the number of studies per variable (n) are outlined below. Figure 3 shows factors in which the same direction of association with HGS over time was found in at least four articles and 60% of all studies that looked at that variable and, thus, were deemed as consistently associated with HGS. To summarize the results, we will discuss in more detail the variables that had associations consistently documented in studies. A summary of the consistent associations with the rate of change in HGS and HGS at follow‐up(s) can be found in Tables 1 and 2 , respectively. The list of all identified factors divided by predictors domains, the number of studies in each arm and a summary of associations with the rate of change in HGS and HGS at follow‐up(s) can be found in Appendix S1B, Table S1 B.1 .
Figure 3.
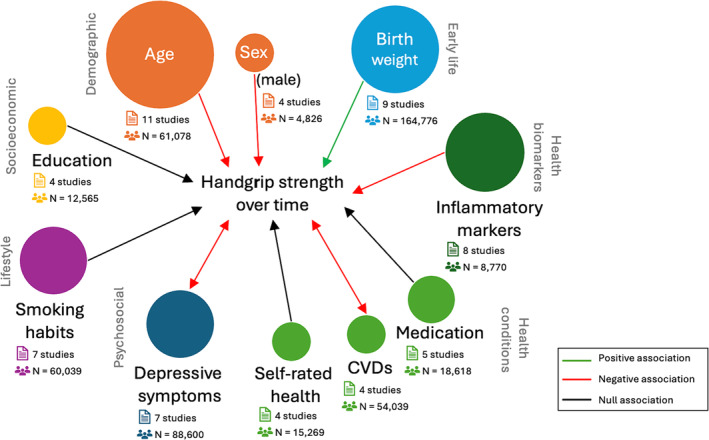
Network illustration indicating the number of studies in each arm (size of circles) and the direction of longitudinal associations with handgrip strength that were consistent (i.e., at least four studies with 60% agreement). Double‐ended arrows indicate findings of bidirectional associations. It is important to note that possible bidirectional associations are not excluded in other cases but were simply not discussed in the studies included.
Table 1.
Summary of findings of factors that had consistent associations with the rate of change in handgrip strength
| Exposure | Positive association (+) | Negative association (−) | No association (0) | Summary b | |||
|---|---|---|---|---|---|---|---|
| Studies a | N (%) | Studies a | N (%) | Studies a | N (%) | ||
| Demographic factors | |||||||
| Age | (n/a) | 0 | 21 , 22 , 23 , 24 , 25 , 26 II 27 I 28 I 29 I | 9 (81.8%) | 30 II 31 | 2 (19.2%) | −− |
| Sex (male) | (n/a) | 0 | 21 , 25 , 32 , 33 | 4 (100%) | (n/a) | 0 | −− |
| Socioeconomic factors | |||||||
| Education | (n/a) | 0 | (n/a) | 0 | 21 , 22 , 26 , 34 II | 4 (100%) | 00 |
| Health marker/biomarker factors | |||||||
| Inflammatory markers | (n/a) | 0 | 35 I 36 , 37 , 38 , 39 F 40 | 6 (75%) | 41 , 42 I | 2 (25%) | −− |
| Health condition factors | |||||||
| Cardiovascular diseases | (n/a) | 0 | 21 , 25 , 29 , 43 I | 4 (100%) | (n/a) | 0 | −− |
| Medication use | (n/a) | 0 | 44 | 1 (20%) | 45 , 46 , 47 II 26 II | 4 (80%) | 00 |
| Self‐rated health | (n/a) | 0 | (n/a) | 0 | 23 , 26 , 32 , 34 II | 4 (100%) | 00 |
Numbers in the columns refer to numbers in the References. If the direction of the association was specific to male or female participants of the study, ‘M’ or ‘F’ is indicated. ‘I’ and ‘II’ indicate 100% male or female sample, respectively.
Summary of association with handgrip strength (HGS): ++, repeatedly documented positive association with HGS (≥60% and at least 4 studies); 00, repeatedly documented lack of association with HGS; −−, repeatedly documented negative association with HGS; mixed, repeatedly documented inconsistent or indeterminate direction of association (e.g., non‐linear relationships, sex or age‐specific relationships, or variety of exposure variables that do not allow one conclusion).
Table 2.
Summary of findings of factors that had consistent associations with handgrip strength at follow‐up(s)
| Exposure | Positive association (+) | Negative association (−) | No association (0) | Summary b | |||
|---|---|---|---|---|---|---|---|
| Studies a | N (%) | Studies a | N (%) | Studies a | N (%) | ||
| Early life factors | |||||||
| Birth weight | 48 , 49 , 50 , 51 , 52 , 53 , 54 I 55 M | 8 (88.9%) | (n/a) | 0 | 56 | 1 (11.1%) | ++ |
| Psychosocial factors | |||||||
| Depressive symptoms | (n/a) | 0 | 22 , 57 , 58 , 59 , 60 , 61 , 62 F | 7 (100%) | (n/a) | 0 | − |
| Lifestyle factors | |||||||
| Smoking habits (current smoker) | 43 | 1 (14.3%) | 49 M | 1 (14.3%) | 22 , 63 , 64 , 65 , 66 II | 5 (71.4%) | 00 |
Numbers in the columns refer to numbers in the References. If the direction of the association was specific to male or female participants of the study, ‘M’ or ‘F’ is indicated. ‘I’ and ‘II’ indicate 100% male or female sample, respectively.
Summary of association with handgrip strength (HGS): ++, repeatedly documented positive association with HGS (≥60% and at least 4 studies); 00, repeatedly documented lack of association with HGS; −−, repeatedly documented negative association with HGS; mixed, repeatedly documented inconsistent or indeterminate direction of association (e.g., non‐linear relationships, sex or age‐specific relationships, or variety of exposure variables that do not allow one conclusion).
Demographic variables included age (n = 13), sex (n = 5), birth cohort (n = 4), marital status (n = 3), marital status history (n = 1), race/ethnicity (n = 1) and years to death (n = 1). Age and sex were the most consistent demographic‐associated factors in this review.
In almost 82% of the studies, the HGS decline became progressively steeper with age, particularly after the fifth decade of life. 27 , 28 In young adulthood, the annual rate of change of maximal HGS was −0.4 to −0.2 kg/year, 21 compared with −0.6 to −0.2 kg/year in middle age and −1.0 to −0.4 kg/year in old age. 21 , 22 , 29 Overall, each 1 year increase in age after the fifth decade of life was associated with a 0.03 to 0.4 kg decrease in HGS. 23 , 24 , 67
In total, four studies (100%) agree with a negative association between male sex and decline in HGS. Men showed a steeper HGS decline than women in three studies, despite being around 28–47% stronger at baseline and still 21–49% stronger at follow‐up. 21 , 32 , 33 The average annual rate of change in maximal HGS varied between −1.2 and −0.4 kg/year among males against −0.4 to −0.2 kg/year among females. 21 , 33 Moreover, men from the African American Health project showed a 99% increased risk of losing 5 kg or more in HGS over a 3 year follow‐up compared with women. 25
The socioeconomic variables identified in studies were education (n = 6), occupation class (n = 3), income (n = 2), housing tenure (n = 1), cohabitation history (n = 1), employment status history (n = 1), country inequality history (n = 1) and health insurance/costs (n = 1).
Four studies examining the rate of decline in HGS did not find any differences based on education level, whether measured in continuous years 26 , 34 or categorized. 21 , 22 There was an indication of a cross‐sectional association between higher education and stronger grip, which may be more pronounced in women than in men. 22 , 34 In women from the British National Survey of Health and Development and the Swedish Adoption/Twin Study of Aging, higher education level was associated with 0.38 and a 2.20 kg stronger grip at baseline, respectively, but associations were not significant among men. 22 Additionally, there may be a mediating effect of body weight and percentage of body fat for both sexes. 34
Genetic factors were ageing markers (n = 5) and APOE alleles (n = 2). None of those factors had consistent associations with HGS at follow‐up or its decline.
Early life factors included birth weight (n = 9), growth trajectories (n = 5), pubertal timing (n = 1), childhood socioeconomic status (n = 3), childhood cognition (n = 2), motor development (n = 2), gestational age (n = 1), infant feeding (n = 1), adverse childhood events (n = 1) and childhood health biomarkers (n = 1).
Increased birth weight was associated with stronger grip strength at follow‐up in nine studies (81.8%), of which one study found it to be the case in males but not in females. 55 Estimates of the linear association between birth weight z‐score and HGS in adulthood varied from 0.68 [95% confidence interval (CI): 0.45, 0.92] in a sample of 4304 people from Finland aged 31 years 54 to 1.80 (95% CI: 1.75, 1.86) in 144 368 Swedish individuals with an average age of 18.3 years. 48
Body composition factors included body mass index (BMI, n = 11) and BMI history (n = 1), lean mass (n = 3) and fat mass parameters (n = 3), height (n = 3) and height history (n = 1), weight (n = 2) and weight history (n = 3), and waist circumference (n = 2). None of these factors were consistently associated with HGS over time. Despite the number of studies examining BMI, findings were mixed, and in some cases, the associations were restricted to males. 49 , 68 , 69
Health markers and biomarkers included inflammatory markers (n = 9), anti‐inflammatory markers (n = 9), blood pressure (n = 3), serum vitamin D (n = 3), metabolic syndrome components (n = 2), activities of daily living and instrumental activities of daily living disabilities (n = 1), fitness tests (n = 1), thyroid function (n = 1) and parathyroid function markers (n = 1) and plasma dp‐ucMGP (n = 1).
Higher concentrations of inflammatory markers were associated with a steeper decline in strength in 75% of studies. However, results must be taken with caution due to the heterogeneity of factors analysed, including interleukins (IL‐1β, IL‐1RA, IL‐6, IL‐8 and IL‐10), cytokines [tumour necrosis factor‐alpha (TNF‐α) and its receptor 1 (TNF‐αR1), C‐reactive protein (CRP) and high‐sensitivity CRP (hsCRP)], cortisol, homocysteine, serum albumin, serum haemoglobin, cholesterol, antichymotrypsin and adiponectin to leptin ratio. Among 716 older adults from the InCHIANTI study, a progressively greater rate of decline in HGS was observed with the increasing number of catabolic biomarkers, including hs‐CRP, IL‐6, IL‐1RA and TNF‐αR1. The average change was almost 6 times higher among those with four catabolic biomarkers in the highest tertile compared with those with none (−0.41 kg/year against −0.07 kg/year, respectively). 36
Health condition factors included comorbidities (n = 6), diabetes mellitus (n = 6), cardiovascular diseases (n = 5), medication use (n = 5), self‐rated health (n = 4), arthritis (n = 3), hypertension (n = 3), cancer (n = 3), respiratory conditions (n = 3), pain (n = 2), fatigue (n = 2), eye conditions (n = 2), kyphosis (n = 1), metabolic syndrome (n = 1), hepatic conditions (n = 1) and other health condition categories (n = 1).
Four studies (100%) reported an increased risk of decline in HGS among individuals with cardiovascular diseases (CVDs). Findings also suggest a two‐way relationship between poor HGS and CVDs. 21 , 43 However, there were slightly different definitions of CVDs, which included one or more of the following conditions: coronary heart disease, cerebrovascular disease, peripheral vascular (or arterial) disease, angina, arrhythmia, valvular heart disease and congenital heart disease, limiting the analysis of effect sizes. As an example, findings from the 44 315 UK Biobank participants suggest a 7% increased risk of decline and a 22% increased risk of stable low HGS (< −1 SD of the age and sex‐specific mean) over time. 43
Four out of five studies (80%) found no associations between HGS decline and medication use. Nevertheless, the medications varied between studies, including statin and ACE inhibitors (n = 2), insulin or oral anti‐diabetic medication (n = 1), calcium supplement and calcium channel blockers (n = 1), drugs with anticholinergic and sedative effects (n = 1) and a combination of oestrogen, thyroid hormone and corticosteroids (n = 1).
Even though two studies found a 0.6 to 0.9 kg stronger grip at baseline associated with better self‐rated health in older adults, 23 , 32 none of the four studies found longitudinal associations between those variables.
Psychosocial factors included depressive symptoms (n = 9), cognitive function (n = 5), psychiatric disorders (n = 2), stress (n = 1), life satisfaction (n = 1), purpose of life (n = 1) and partner's beliefs about ageing (n = 1).
There was a consistent association between depressive symptoms and weaker grip strength at follow‐up, supported by all seven studies examining this outcome (although one was a female‐specific association). The inverse association was also true, where those with weakness had an increased risk of depression than non‐weak participants. 57 , 58 The bidirectional relationship strongly supports the need for early detection and intervention strategies for both health outcomes to achieve reciprocal benefits. 57 , 58 Another interesting finding was an interplay between BMI, depressive symptoms and decreased grip strength shown by Luo et al. 57 and Rantanen et al. 69
Lifestyle factors were the most extensively studied associated factors in this review. Exposure variables analysed were physical activity (n = 14), smoking habits (n = 12), alcohol consumption (n = 7), protein intake (n = 7), physical inactivity or sedentarism (n = 6), fruit and vegetable intake (n = 5), physical activity history (n = 3), occupational physical activity (n = 3), healthy habits (n = 3), fat intake (n = 2), red and processed meat intake (n = 2), Mediterranean diet (n = 2), energy intake (n = 1), carbohydrate intake (n = 1), oily fish intake (n = 1), ultra‐processed food intake (n = 1), cereals, low‐fat milk and fish intake (n = 1), selenium intake (n = 1), antioxidants intake (n = 1), dietary patterns (n = 1) and variety (n = 1), DASH diet (n = 1), Japanese Food Guide Spinning Top (JFG‐ST) diet (n = 1), Nordic diet (n = 1), healthy diet index (n = 1) and sleep patterns (n = 1).
Despite the many studies in this domain (38 in total), only smoking habits had consistent results with follow‐up measurements of HGS, pointing to a lack of association between those variables. Nonetheless, smoking has been extensively associated with adverse health outcomes such as an increased risk of CVDs, negative changes in the circulatory system and oxidative stress, which may end up negatively affecting the musculoskeletal system. 21 , 22 The effects of physical activity (PA) were the most frequently analysed lifestyle factors in this review. However, the results were mixed, as were the measures used in studies that looked at levels of PA, inactivity/sedentary lifestyle, work‐related PA and/or changes in PA.
Reproductive factors included the following variables: sex hormone levels including testosterone (n = 2), menopause (n = 2), age at hysterectomy (n = 1), gynaecological/breast conditions (n = 1), hormone replacement therapy (n = 1) and lactation duration across pregnancies (n = 1). Due to the small number of studies in this domain, no consistent association was found.
Environmental factors included exposure to natural environments (n = 1) and occupational exposure to chemicals (n = 1). The lack of studies in this area prevented drawing any further conclusions.
Discussion
Findings from this review suggest that factors associated with stronger HGS measurements over time include higher birth weight whereas depressive symptoms in adulthood were consistently associated with weaker grip. Surprisingly, there was no association between current or past smoking and HGS. Increased age, male sex, cardiovascular diseases and higher levels of inflammatory markers were repeatedly associated with a steeper decline in HGS. Finally, education level, medication use and self‐rated health were not associated with the change in grip.
Low HGS has been linked to premature mortality and a wide range of health conditions during ageing, yet little is known about the mechanisms determining weakness to date. 3 To the best of our knowledge, this is the first study to systematically and comprehensively review the evidence for predictors of HGS from longitudinal studies. Determinants research can be used to identify high‐risk groups, thus supporting better decision‐making and targeted interventions. 18 , 70 Determinants such as age and sex are not modifiable; however, there were several modifiable determinants of HGS identified in this review, which can be used to guide health interventions from early life to adulthood. 70 Consistent associations with inflammatory markers, cardiovascular diseases and depressive symptoms suggest those factors should be prioritized in interventions. Additionally, the bidirectional associations with mental health and cardiovascular diseases indicate that joint efforts to tackle mental and physical health declines can produce mutual benefits. 21 , 43 , 57 , 58 , 71 This review also sheds light on gaps in the literature, particularly genetics, reproductive and environmental factors, given the small number of studies in those domains.
The results from this review seem to agree with the conceptual model proposed by McGrath et al., 3 where metabolic and neurological systems drive the associations between HGS and health outcomes. Some of the included studies showed parallel associations between HGS and depression 57 , 58 and cardiovascular diseases, 21 , 43 indicating that there likely are common mediating factors affecting nervous and muscular systems function and, thereby, muscle strength and health. 3 Shared aetiological factors such as ageing and inflammation may help to better understand the aetiology of weakness. 3 , 72
All the 11 major determinants identified in this study seem to have some kind of association with HGS over time. It is noteworthy, however, that only about 10% of the 103 exposure variables had consistent associations with HGS over time reported in studies. Between 21% and 35% of the variables did not show any association with HGS follow‐up measurements or the rate of decline, but results, as well as methodological approaches, were mostly not uniform, and thus, those variables should not be dismissed. The same applies to the 8–21% that showed weak evidence of positive associations and the 17–29% that showed negative associations, that is, more research is needed to confirm those results. Lastly, 24–32% of variables had associations deemed as inconsistent or indeterminate, mainly due to sex‐graded or non‐linear associations.
Interestingly, there was no consensus regarding the association between PA and HGS over time, despite being the most extensively studied associated factor in this review. A systematic review of cross‐sectional studies showed that the practice of regular mild‐to‐moderate or moderate‐to‐vigorous PA was associated with greater HGS levels. 18 In this review, 57.1% of studies found a positive association between higher PA levels and follow‐up measurements. However, there seemed to be a lack of association with the rate of decline in HGS (in 44.4% of studies). Similar findings were reported in studies from Australia and the Netherlands, which analysed the course of physical functioning over time using both subjective and objective measures of muscle function and performance. 73 , 74 Nevertheless, in the Dutch study, those who remained inactive through midlife to older ages (40–55+ years) showed 1.5–1.6 higher odds of physical decline than those who were physically active in one or both of those periods. 74 As argued by Peeters et al., 73 cumulative activity, rather than activity at any given time, may have a greater impact on physical functioning, considering the fluctuations in PA levels across the lifespan. In this review, few studies looked at PA history (n = 3), using different measurement methods, which limits the interpretation of findings. Nonetheless, evidence suggests that PA type, intensity, frequency and duration are other important factors to consider in this relationship. 21 , 75 , 76
A common finding across themes was that researchers could generally observe cross‐sectional associations with HGS but not longitudinal associations with HGS change. As an example, there was weak evidence of a non‐association of height with the rate of change, even though height is a widely recognized influencing factor of HGS. 8 , 77 Such findings might suggest that the rate of decline is more linked to age and sex than any other factors. Associations with age and sex were more consistent; however, they also depended on the follow‐up period and the age range of the sample. 25 , 30 , 31 Because HGS remains fairly stable in shorter periods, especially between the second and the fifth decade of life, those who followed participants for shorter periods would have not been able to evaluate the associations effectively. 8 , 78
This review highlights that the rate of decline in HGS varies notably across age groups. Establishing norms for HGS or identifying consistent patterns across populations is challenging due to variations in other potential influencing factors such as sex, region, health conditions and levels of physical activity across different studies. 79 The European Working Group on Sarcopenia in Older People reported a variation in loss of strength in the order of 1.5–5% from the age of 50 years onwards. 78 Studies included in this review show a variation of 0.03–0.4 kg decrease in strength for each year above the fifth decade. 23 , 24 , 67 Although this effect may be viewed as insignificant (<5–6.5 kg difference), there is currently no consensus regarding the minimal clinically important difference in HGS. 80 Furthermore, the accumulation of continuous loss over the years can become significant.
While everyone will decline in strength with age, those who maintained a higher measurement over time are likely to remain stronger than those who had weaker grip strength. 28 , 73 As an example, findings from this review suggest that while males may exhibit a more pronounced decline in HGS, they still maintain a significant strength advantage over females. Females are at higher risk of developing musculoskeletal disorders later in life than males. 11 Therefore, ensuring optimal muscle strength as early as possible is crucial. Positive results found between better parameters in early life, such as birth weight and stronger grip strength point out that interventions should cover much younger ages. However, interventions for improving muscle strength (as well as muscle strength research, as demonstrated in this review) are currently focused on older populations. 73
This is the first systematic summary of the predictive factors of HGS. We reviewed 117 studies assessing longitudinal factors associated with HGS. Despite the careful search strategy construction, some studies may have been missed. Another limitation of this review is its semiquantitative design, which is based on previously established definitions of consistency of results that are, nonetheless, open to interpretation and debate. 18 , 19 , 20 The comparison between studies is challenging due to the varied age range and sex distribution of the samples, differences in protocols of HGS measurement (equipment used, type of HGS measure, hand(s) measured, units of measure), and covariates used in analyses (refer to Appendix SB, Table S B.2 ). For instance, HGS tends to increase with the number of trials, as participants become more familiar with the dynamometer. Even within multiple attempts, the maximal HGS will be higher than the average HGS. 35 , 79 In addition, maximal HGS is approximately 10% higher in the dominant hand than the non‐dominant hand. 35 Finally, comparing results between studies is limited by other factors such as the inter‐instrument reliability of dynamometers. 8 , 35 Those issues have prompted several authors to advocate for a standardized approach. 35 , 37 , 79 A review of measurement of grip strength has recommended instruments such as the Jamar dynamometer, the Baltimore Therapeutic Equipment (BTE) Work Simulator, the BTE Primus, and the Martin Vigorimeter. These instruments have acceptable validity and reliability but use different units of measurement, a consideration when assessing measures against normative standards. 35 Proposed protocols also emphasize recording hand dominance, conducting multiple measurements on each hand and recording maximal HGS as the greatest grip force generated across all trials. 79
As also observed in previous systematic reviews about HGS, the wide range of exposure variables and many differences in how those variables were measured and analysed against HGS precludes the use of statistical techniques to estimate meaningful and valid pooled estimates of the associations between exposure and outcome. 37 The mixed findings or weak evidence for most of the associations only highlight the methodological issues. As stated by McGrath, 79 ‘A consensus statement for better standardising HGS protocols is warranted to decrease internal threats to validity, create standardization in assessments, and allow for comparisons of HGS findings across investigations’.
Conclusions
This semiquantitative review highlighted evidence for associations between various demographic, socioeconomic, genetic, early life, body composition, health markers/biomarkers, health condition, psychosocial, lifestyle, reproductive and environmental factors with HGS. Our results confirm the complexity of processes contributing to muscle strength capacity. It may be that age and sex are the main drivers of the decline in strength overall, but there are other factors contributing to this phenomenon, including birth weight, depression, inflammation and cardiovascular factors.
Several insights for future research can be derived from the present findings. Even among variables that have been repeatedly analysed in studies, we were not able to analyse effect sizes due to methodological heterogeneity, which limits the practical application of findings. Furthermore, many variables have been understudied and, therefore, should be emphasized in future studies, especially modifiable factors that are related to the development of chronic diseases, such as body fat, diet, sleep and stress. Other modifiable factors that showed indeterminate associations with HGS over time, such as physical activity and BMI, also warrant further investigation and standardization of analysis. As these factors tend to change over time, it would be interesting to look at historical exposure and its effect on HGS rather than looking at time‐specific exposure. Ultimately, this area of research should give greater focus to younger populations, before the fifth decade of age. Identifying what determines better muscle strength before the decline leads off is crucial for improving intervention and prevention strategies. To overcome those challenges and improve the quality of evidence, there is a need for collaboration and consensus among researchers, health practitioners and stakeholders on standardized protocols for HGS testing and reporting.
Funding
G. D. M. is an NHMRC Leadership Fellow (grant number APP2009577). G. I. M. is supported by a National Health and Medical Research Council Investigator Grant (grant number APP2008702). L. W. R. and S. B. are PhD student recipients of UQ Research Training Stipend Earmarked scholarships.
Supporting information
Appendix S1. Supporting Information
Acknowledgements
Not applicable.
Ribeiro LW, Berndt S, Mielke GI, Doust J, Mishra GD. Factors associated with handgrip strength across the life course: A systematic review. Journal of Cachexia, Sarcopenia and Muscle 2024; 10.1002/jcsm.13586.
References
- 1. Officer A, Manandhar M. In Life‐Course Aa , ed. Decade of Healthy Ageing 2020–2030. Geneva, Switzerland: WHO; 2019. [Google Scholar]
- 2. Rijk JM, Roos PR, Deckx L, van den Akker M, Buntinx F. Prognostic value of handgrip strength in people aged 60 years and older: a systematic review and meta‐analysis. Geriatr Gerontol Int 2016;16:5–20. [DOI] [PubMed] [Google Scholar]
- 3. McGrath R, Johnson N, Klawitter L, Mahoney S, Trautman K, Carlson C, et al. What are the association patterns between handgrip strength and adverse health conditions? A topical review. SAGE Open Med 2020;8:2050312120910358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, di Iorio A, et al. Age‐associated changes in skeletal muscles and their effecton mobility: an operational diagnosis of sarcopenia. J Appl Physiol 2003;95:1851–1860. [DOI] [PubMed] [Google Scholar]
- 5. Zanker J, Sim M, Anderson K, Balogun S, Brennan‐Olsen SL, Dent E, et al. Consensus guidelines for sarcopenia prevention, diagnosis and management in Australia and New Zealand. J Cachexia Sarcopenia Muscle 2023;14:142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soysal P, Hurst C, Demurtas J, Firth J, Howden R, Yang L, et al. Handgrip strength and health outcomes: umbrella review of systematic reviews with meta‐analyses of observational studies. J Sport Health Sci 2021;10:290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sui SX, Holloway‐Kew KL, Hyde NK, Williams LJ, Tembo MC, Mohebbi M, et al. Handgrip strength and muscle quality in Australian women: cross‐sectional data from the Geelong Osteoporosis Study. J Cachexia Sarcopenia Muscle 2020;11:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dodds RM, Syddall HE, Cooper R, Kuh D, Cooper C, Sayer AA. Global variation in grip strength: a systematic review and meta‐analysis of normative data. Age Ageing 2016;45:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dodds RM, Denison HJ, Ntani G, Cooper R, Cooper C, Sayer AA, et al. Birth weight and muscle strength: a systematic review and meta‐analysis. J Nutr Health Aging 2012;16:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Araujo AB, Chiu GR, Kupelian V, Hall SA, Williams RE, Clark RV, et al. Lean mass, muscle strength, and physical function in a diverse population of men: a population‐based cross‐sectional study. BMC Public Health 2010;10:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Azzolino D, Spolidoro GCI, Saporiti E, Luchetti C, Agostoni C, Cesari M. Musculoskeletal changes across the lifespan: nutrition and the life‐course approach to prevention. Front Med (Lausanne) 2021;8:697954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia‐Hermoso A, Izquierdo M, Ramirez‐Velez R. Tracking of physical fitness levels from childhood and adolescence to adulthood: a systematic review and meta‐analysis. Transl Pediatr 2022;11:474–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clark JM, Sanders S, Carter M, Honeyman D, Cleo G, Auld Y, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc 2020;108:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scells H, Zuccon G. searchrefiner: a query visualisation and understading tool for systematic reviews. CIKM '18: Proceedings of the 27th ACM International Conference on Information and Knowledge Management. 2018:1939–42.
- 15. Clark J, Glasziou P, Mar CD, Bannach‐Brown A, Stehlik P, Scott AM. A full systematic review was completed in 2 weeks using automation tools: a case study. J Clin Epidemiol 2020;121:81–90. [DOI] [PubMed] [Google Scholar]
- 16. Moons KGM, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med 2014;11:e1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayden JA, van der Windt D, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–286. [DOI] [PubMed] [Google Scholar]
- 18. de Lima TR, Silva DAS, de Castro JAC, Christofaro DGD. Handgrip strength and associated sociodemographic and lifestyle factors: a systematic review of the adult population. J Bodyw Mov Ther 2017;21:401–413. [DOI] [PubMed] [Google Scholar]
- 19. Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity of children and adolescents. Med Sci Sports Exerc 2000;32:963–975. [DOI] [PubMed] [Google Scholar]
- 20. Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults' participation in physical activity: review and update. Med Sci Sports Exerc 2002;34:1996–2001. [DOI] [PubMed] [Google Scholar]
- 21. Stenholm S, Tiainen K, Rantanen T, Sainio P, Heliövaara M, Impivaara O, et al. Long‐term determinants of muscle strength decline: prospective evidence from the 22‐year Mini‐Finland follow‐up survey. J Am Geriatr Soc 2012;60:77–85. [DOI] [PubMed] [Google Scholar]
- 22. Sternäng O, Reynolds CA, Finkel D, Ernsth‐bravell M, Pedersen NL, Dahl aslan AK. Factors associated with grip strength decline in older adults. Age Ageing 2015;44:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Syddall HE, Westbury LD, Shaw SC, Dennison EM, Cooper C, Gale CR. Correlates of level and loss of grip strength in later life: findings from the English Longitudinal Study of Ageing and the Hertfordshire Cohort Study. Calcif Tissue Int 2018;102:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O'Keefe P, Mann FD, Clouston S, Voll S, Muniz‐Terrera G, Lewis N, et al. Getting a grip on secular changes: age‐period‐cohort modeling of grip strength in the English Longitudinal Study of Ageing. J Gerontol A Biol Sci Med Sci 2022;77:1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller DK, Malmstrom TK, Miller JP, Andresen EM, Schootman M, Wolinsky FD. Predictors of change in grip strength over 3 years in the African American health project. J Aging Health 2010;22:183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Forrest KYZ, Zmuda JM, Cauley JA. Patterns and correlates of muscle strength loss in older women. Gerontol 2007;53:140–147. [DOI] [PubMed] [Google Scholar]
- 27. Forrest KYZ, Bunker CH, Sheu Y, Wheeler VW, Patrick AL, Zmuda JM. Patterns and correlates of grip strength change with age in Afro‐Caribbean men. Age Ageing 2012;41:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age‐related decline of grip strength: cross‐sectional and longitudinal perspectives. J Gerontol 1990;45:M82–M88. [DOI] [PubMed] [Google Scholar]
- 29. Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM. Grip strength changes over 27 yr in Japanese‐American men. J Applied Physiol 1998;85:2047–2053. [DOI] [PubMed] [Google Scholar]
- 30. Kurina LM, Gulati M, Everson‐Rose SA, Chung PJ, Karavolos K, Cohen NJ, et al. The effect of menopause on grip and pinch strength: results from the Chicago, Illinois, site of the Study of Women's Health Across the Nation. Am J Epidemiol 2004;160:484–491. [DOI] [PubMed] [Google Scholar]
- 31. Proctor DN, Fauth EB, Hoffman L, Hofer SM, McClearn GE, Berg S, et al. Longitudinal changes in physical functional performance among the oldest old: insight from a study of Swedish twins. Aging Clin Exp Res 2006;18:517–530. [DOI] [PubMed] [Google Scholar]
- 32. Granic A, Davies K, Jagger C, Kirkwood TBL, Syddall HE, Sayer AA. Grip strength decline and its determinants in the very old: longitudinal findings from the Newcastle 85+ Study. PLoS ONE 2016;11:e0163183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oksuzyan A, Maier H, McGue M, Vaupel JW, Christensen K. Sex differences in the level and rate of change of physical function and grip strength in the Danish 1905‐cohort study. J Aging Health 2010;22:589–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Botoseneanu A, Bennett JM, Nyquist L, Shinkai S, Fujiwara Y, Yoshida H, et al. Cardiometabolic risk, socio‐psychological factors, and trajectory of grip strength among older Japanese adults. J Aging Health 2015;27:1123–1146. [DOI] [PubMed] [Google Scholar]
- 35. Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 2011;40:423–429. [DOI] [PubMed] [Google Scholar]
- 36. Stenholm S, Maggio M, Lauretani F, Bandinelli S, Ceda GP, di Iorio A, et al. Anabolic and catabolic biomarkers as predictors of muscle strength decline: the InCHIANTI study. Rejuvenation Res 2010;13:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cooper R, Kuh D, Cooper C, Gale CR, Lawlor DA, Matthews F, et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing 2011;40:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hirani V, Naganathan V, Blyth F, Le Couteur DG, Seibel MJ, Waite LM, et al. Low hemoglobin concentrations are associated with sarcopenia, physical performance, and disability in older Australian men in cross‐sectional and longitudinal analysis: the Concord Health and Ageing in Men Project. J Gerontol A Bio Sci Med Sci 2016;71:1667–1675. [DOI] [PubMed] [Google Scholar]
- 39. Sanders JL, Ding V, Arnold AM, Kaplan RC, Cappola AR, Kizer JR, et al. Do changes in circulating biomarkers track with each other and with functional changes in older adults? J Gerontol A Bio Sci Med Sci. 2014;69:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schalk BWM, Deeg DJH, Penninx BWJH, Bouter LM, Visser M. Serum albumin and muscle strength: a longitudinal study in older men and women. J Am Geriatr Soc 2005;53:1331–1338. [DOI] [PubMed] [Google Scholar]
- 41. Vidoni ML, Pettee Gabriel K, Luo ST, Simonsick EM, Day RS. Relationship between homocysteine and muscle strength decline: the Baltimore Longitudinal Study of Aging. J Gerontol A Bio Sci Med Sci. 2018;73:546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schaap LA, Pluijm SMF, Deeg DJH, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 2006;119:526.e9–526.e17. [DOI] [PubMed] [Google Scholar]
- 43. Hurst C, Murray JC, Granic A, Hillman SJ, Cooper R, Sayer AA, et al. Long‐term conditions, multimorbidity, lifestyle factors and change in grip strength over 9 years of follow‐up: findings from 44,315 UK biobank participants. Age Ageing 2021;50:2222–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Granic A, Davies K, Martin‐Ruiz C, Jagger C, Kirkwood TBL, von Zglinicki T, et al. Grip strength and inflammatory biomarker profiles in very old adults. Age Ageing 2017;46:976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Snyder CK, Lapidus JA, Cawthon PM, Dam T‐TL, Sakai LY, Marshall LM. Serum albumin in relation to change in muscle mass, muscle strength, and muscle power in older men. J Am Geriatr Soc 2012;60:1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hilmer SN, Mager DE, Simonsick EM, Ling SM, Windham BG, Harris TB, et al. Drug burden index score and functional decline in older people. Am J Med 2009;122:1142–9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ferrari U, Then C, Rottenkolber M, Selte C, Seissler J, Conzade R, et al. Longitudinal association of type 2 diabetes and insulin therapy with muscle parameters in the KORA‐Age study. Acta Diabetol 2020;57:1057–1063. [DOI] [PubMed] [Google Scholar]
- 48. Ridgway CL, Ong KK, Tammelin T, Sharp SJ, Ekelund U, Jarvelin M‐R. Birth size, infant weight gain, and motor development influence adult physical performance. Med Sci Sports Exerc 2009;41:1212–1221. [DOI] [PubMed] [Google Scholar]
- 49. Kuh D, Hardy R, Blodgett JM, Cooper R. Developmental factors associated with decline in grip strength from midlife to old age: a British birth cohort study. BMJ Open 2019;9:e025755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Witham MD, Syddall HE, Dennison E, Cooper C, McMurdo MET, Sayer AA. ACE inhibitors, statins and thiazides: no association with change in grip strength among community dwelling older men and women from the Hertfordshire Cohort Study. Age Ageing 2014;43:661–666. [DOI] [PubMed] [Google Scholar]
- 51. Gray SL, Aragaki AK, LaMonte M, Cochrane BB, Kooperberg C, Robinson JG, et al. Statins, angiotensin‐converting enzyme inhibitors, and physical performance in older women. J Am Geriatr Soc 2012;60:2206–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bielemann RM, Gigante DP, Horta BL. Birth weight, intrauterine growth restriction and nutritional status in childhood in relation to grip strength in adults: from the 1982 Pelotas (Brazil) birth cohort. Nutrition 2016;32:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kuh D, Bassey J, Hardy R, Aihie Sayer A, Wadsworth M, Cooper C. Birth weight, childhood size, and muscle strength in adult life: evidence from a birth cohort study. Am J Epidemiol 2002;156:627–633. [DOI] [PubMed] [Google Scholar]
- 54. Ahlqvist VH, Persson M, Ortega FB, Tynelius P, Magnusson C, Berglind D. Birth weight and grip strength in young Swedish males: a longitudinal matched sibling analysis and across all body mass index ranges. Sci Rep 2019;9:9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nahhas RW, Choh AC, Lee M, Chumlea WM, Duren DL, Siervogel RM, et al. Bayesian longitudinal plateau model of adult grip strength. Am J Hum Biol 2010;22:648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Robinson SM, Simmonds SJ, Jameson KA, Syddall HE, Dennison EM, Cooper C, et al. Muscle strength in older community‐dwelling men is related to type of milk feeding in infancy. J Gerontol A Bio Sci Med Sci. 2012;67:990–996. [DOI] [PubMed] [Google Scholar]
- 57. Luo J, Yao W, Zhang T, Ge H, Zhang D. Exploring the bidirectional associations between handgrip strength and depression in middle and older Americans. J Psychosom Res 2022;152:110678. [DOI] [PubMed] [Google Scholar]
- 58. Lian Y, Wang G‐P, Chen G‐Q, Jia C‐X. Bidirectional associations between handgrip strength and depressive symptoms: a longitudinal cohort study. J Am Med Dir Assoc 2021;22:1744–1750.e1. [DOI] [PubMed] [Google Scholar]
- 59. Ylihärsilä H, Kajantie E, Osmond C, Forsén T, Barker DJP, Eriksson JG. Birth size, adult body composition and muscle strength in later life. Int J Obes (Lond) (2005) 2007;31:1392–1399. [DOI] [PubMed] [Google Scholar]
- 60. Ridgway CL, Sharp SJ, Derom C, Beunen G, Fagard R, Vlietinck R, et al. The contribution of prenatal environment and genetic factors to the association between birth weight and adult grip strength. PLoS ONE 2011;6:e17955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Raji MA, Kuo Y‐F, Snih SA, Markides KS, Peek MK, Ottenbacher KJ. Cognitive status, muscle strength, and subsequent disability in older Mexican Americans. J Am Geriatr Soc 2005;53:1462–1468. [DOI] [PubMed] [Google Scholar]
- 62. Bertoni M, Maggi S, Manzato E, Veronese N, Weber G. Depressive symptoms and muscle weakness: a two‐way relation? Exp Gerontol 2018;108:87–91. [DOI] [PubMed] [Google Scholar]
- 63. Ikeda T, Tsuboya T. Effects of changes in depressive symptoms on handgrip strength in later life: a four‐year longitudinal study in England. J Affect Disord 2022;299:67–72. [DOI] [PubMed] [Google Scholar]
- 64. Jiang R, Westwater ML, Noble S, Rosenblatt M, Dai W, Qi S, et al. Associations between grip strength, brain structure, and mental health in >40,000 participants from the UK Biobank. BMC Med 2022;20:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. de Lima TR, González‐Chica DA, D'Orsi E, Sui X, Silva DAS. Individual and combined association between healthy lifestyle habits with muscle strength according to cardiovascular health status in adults and older adults. J Phys Act Health 2021;18:973–980. [DOI] [PubMed] [Google Scholar]
- 66. Sabia S, Elbaz A, Rouveau N, Brunner EJ, Kivimaki M, Singh‐Manoux A. Cumulative associations between midlife health behaviors and physical functioning in early old age: a 17‐year prospective cohort study. J Am Geriatr Soc 2014;62:1860–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Beller J, Miething A, Regidor E, Lostao L, Epping J, Geyer S. Trends in grip strength: age, period, and cohort effects on grip strength in older adults from Germany, Sweden, and Spain. SSM Popul Health 2019;9:100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. de Carvalho DHT, Scholes S, Santos JLF, de Oliveira C, Alexandre TS. Does abdominal obesity accelerate muscle strength decline in older adults? Evidence from the English Longitudinal Study of Ageing. J Gerontol A Bio Sci Med Sci 2019;74:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rantanen T, Penninx BW, Masaki K, Lintunen T, Foley D, Guralnik JM. Depressed mood and body mass index as predictors of muscle strength decline in old men. J Am Ger Soc 2000;48:613–617. [DOI] [PubMed] [Google Scholar]
- 70. Sallis JF, Owen N. Determinants of Physical Activity. Thousand Oaks, CA: SAGE Publications; 1999. [Google Scholar]
- 71. Kim GR, Sun J, Han M, Nam CM, Park S. Evaluation of the directional relationship between handgrip strength and cognitive function: the Korean Longitudinal Study of Ageing. Age Ageing 2019;48:426–432. [DOI] [PubMed] [Google Scholar]
- 72. Prado CM, Landi F, Chew STH, Atherton PJ, Molinger J, Ruck T, et al. Advances in muscle health and nutrition: a toolkit for healthcare professionals. Clin Nutr 2022;41:2244–2263. [DOI] [PubMed] [Google Scholar]
- 73. Peeters G, Dobson AJ, Deeg DJ, Brown WJ. A life‐course perspective on physical functioning in women. Bull World Health Organ 2013;91:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pluijm SMF, Visser M, Puts MTE, Dik MG, Schalk BWM, van Schoor N, et al. Unhealthy lifestyles during the life course: association with physical decline in late life. Aging Clin Exp Res 2007;19:75–83. [DOI] [PubMed] [Google Scholar]
- 75. Dodds R, Kuh D, Aihie Sayer A, Cooper R. Physical activity levels across adult life and grip strength in early old age: updating findings from a British birth cohort. Age Ageing 2013;42:794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dos Santos VR, Muniz CF, Gobbo LA. Influence of physical activity with moderate and vigorous intensities on the muscle strength of dynapenic older adults: prospective study. Sci Sports 2021;36:218–224. [Google Scholar]
- 77. Nevill AM, Holder RL. Modelling handgrip strength in the presence of confounding variables: results from the Allied Dunbar National Fitness Survey. Ergonomics 2000;43:1547–1558. [DOI] [PubMed] [Google Scholar]
- 78. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McGrath R. Are we maximizing the utility of handgrip strength assessments for evaluating muscle function? Aging Clin Exp Res 2021;33:1721–1723. [DOI] [PubMed] [Google Scholar]
- 80. Bohannon RW. Minimal clinically important difference for grip strength: a systematic review. J Phys Ther Sci 2019;31:75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information


