Abstract
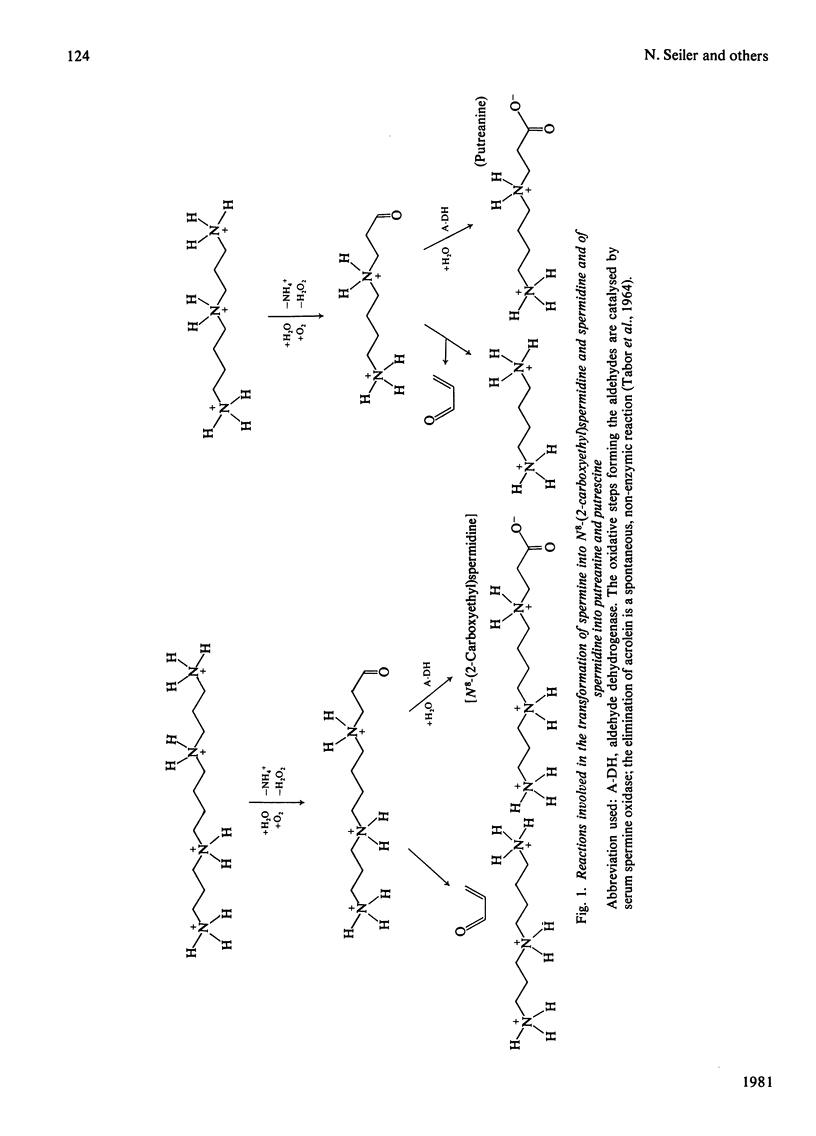
Evidence obtained from experiments with rats and mice is presented suggesting that the naturally occurring amino acids putreanine and N8-(2-carboxyethyl)spermidine, and most probably also related compounds deriving from the polyamines spermidine and spermine by oxidative metabolism, are formed within two anatomical compartments. In the first step polyamines are converted into aldehydes by serum spermine oxidase in the circulation. A certain portion of these aldehydes can be taken up by liver and other organs and transformed by aldehyde dehydrogenase into the corresponding amino acids. Putreanine is not only derived from spermidine, but can also be formed from N8-(2-carboxyethyl)spermidine by oxidative deamination, catalysed by serum spermine oxidase, and subsequent spontaneous elimination of acrolein.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLASCHKO H. The amine oxidases of mammalian blood plasma. Adv Comp Physiol Biochem. 1962;1:67–116. [PubMed] [Google Scholar]
- Bolkenius F. N., Seiler N. Acetylderivatives as intermediates in polyamine catabolism. Int J Biochem. 1981;13(3):287–292. doi: 10.1016/0020-711x(81)90080-x. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Park M. H., Chung S. I., Schrode J., Lester E. P., Cooper H. L. Polyamines as physiological substrates for transglutaminases. J Biol Chem. 1980 Apr 25;255(8):3695–3700. [PubMed] [Google Scholar]
- Imaoka N., Matsuoka Y. Isolation and identification of spermic acid, N,N'-bis (2-carboxyethyl)-1,4-diaminobutane, from bovine brain. J Neurochem. 1974 May;22(5):859–860. doi: 10.1111/j.1471-4159.1974.tb04305.x. [DOI] [PubMed] [Google Scholar]
- Kakimoto Y., Nakajima T., Kumon A., Matsuoka Y., Imaoka N., Sano I. Putreanine, N-(4-aminobutyl)-3-aminopropionic acid. An amino acid occurring uniquely in the mammalian central nervous system. J Biol Chem. 1969 Nov 10;244(21):6003–6007. [PubMed] [Google Scholar]
- Kremzner L. T., Hiller J. M., Simon E. J. Metabolism of polyamines in mouse neuroblastoma cells in culture: formation of GABA and putreanine. J Neurochem. 1975 Dec;25(6):889–894. doi: 10.1111/j.1471-4159.1975.tb04423.x. [DOI] [PubMed] [Google Scholar]
- Kremzner L. T., Sturman J. A. Putreanine in developing monkey brain: relation to polyamines. J Neurochem. 1979 Nov;33(5):1115–1117. doi: 10.1111/j.1471-4159.1979.tb05249.x. [DOI] [PubMed] [Google Scholar]
- Lebsack M. E., Anderson A. D. Further characterization of the inhibition of aldehyde dehydrogenase activity by pargyline. Res Commun Chem Pathol Pharmacol. 1979 Nov;26(2):263–275. [PubMed] [Google Scholar]
- Marton L. J., Heby O., Wilson C. B., Lee P. L. A method for the determination of polyamines in cerebrospinal fluid. FEBS Lett. 1974 Sep 15;46(1):305–307. doi: 10.1016/0014-5793(74)80393-5. [DOI] [PubMed] [Google Scholar]
- Nakajima T. Formation of putreanine, N-(4-aminobutyl)-3-aminopropionic acid from spermidine in rat brain and liver. J Neurochem. 1973 Mar;20(3):735–742. doi: 10.1111/j.1471-4159.1973.tb00034.x. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Matsuoka Y. Distribution of putreanine in organs of rats and rabbits. J Neurochem. 1971 Dec;18(12):2547–2548. doi: 10.1111/j.1471-4159.1971.tb00211.x. [DOI] [PubMed] [Google Scholar]
- Noto T., Tanaka T., Nakajima T. Urinary metabolites of polyamines in rats. J Biochem. 1978 Feb;83(2):543–552. doi: 10.1093/oxfordjournals.jbchem.a131942. [DOI] [PubMed] [Google Scholar]
- Perry T. L., Hansen S., Kloster M. Occurrence of putreanine in human brain. J Neurochem. 1972 May;19(5):1395–1396. doi: 10.1111/j.1471-4159.1972.tb01464.x. [DOI] [PubMed] [Google Scholar]
- Roth M., Hampaï A. Column chromatography of amino acids with fluorescence detection. J Chromatogr. 1973 Aug 29;83:353–356. doi: 10.1016/s0021-9673(00)97051-1. [DOI] [PubMed] [Google Scholar]
- SCHULER W. Zur Hemmung der Diaminooxydase (Histaminase). Experientia. 1952 Jun 15;8(6):230–232. doi: 10.1007/BF02170726. [DOI] [PubMed] [Google Scholar]
- Seiler N., Knödgen B. Determination of the naturally occurring monoacetyl derivatives of di- and polyamines. J Chromatogr. 1979 Oct 11;164(2):155–168. doi: 10.1016/s0378-4347(00)81184-6. [DOI] [PubMed] [Google Scholar]
- Seiler N., Knödgen B. High-performance liquid chromatographic procedure for the simultaneous determination of the natural polyamines and their monoacetyl derivatives. J Chromatogr. 1980 Dec 12;221(2):227–235. doi: 10.1016/s0378-4347(00)84307-8. [DOI] [PubMed] [Google Scholar]
- Seiler N. Use of the dansyl reaction in biochemical analysis. Methods Biochem Anal. 1970;18:259–337. doi: 10.1002/9780470110362.ch5. [DOI] [PubMed] [Google Scholar]
- Shirota F. N., DeMaster E. G., Nagasawa H. T. Propiolaldehyde, a pargyline metabolite that irreversibly inhibits aldehyde dehydrogenase. Isolation from a hepatic microsomal system. J Med Chem. 1979 May;22(5):463–464. doi: 10.1021/jm00191a001. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Hendley E. D. A simple and sensitive fluorescence assay for monoamine oxidase and diamine oxidase. J Pharmacol Exp Ther. 1968 Oct;163(2):386–392. [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., BACHRACH U. IDENTIFICATION OF THE AMINOALDEHYDES PRODUCED BY THE OXIDATION OF SPERMINE AND SPERMIDINE WITH PURIFIED PLASMA AMINE OXIDASE. J Biol Chem. 1964 Jul;239:2194–2203. [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- TABOR H., ROSENTHAL S. M., TABOR C. W. The biosynthesis of spermidine and spermine from putrescine and methionine. J Biol Chem. 1958 Oct;233(4):907–914. [PubMed] [Google Scholar]
- TABOR H., TABOR C. W. SPERMIDINE, SPERMINE, AND RELATED AMINES. Pharmacol Rev. 1964 Sep;16:245–300. [PubMed] [Google Scholar]
- TAYLOR J. D., WYKES A. A., GLADISH Y. C., MARTIN W. B. New inhibitor of monoamine oxidase. Nature. 1960 Sep 10;187:941–942. doi: 10.1038/187941a0. [DOI] [PubMed] [Google Scholar]
- Tsuji M., Nakajima T., Sano I. Putrescine, spermidine, N-acetylspermidine and spermine in the urine of patients with leukaemias and tumors. Clin Chim Acta. 1975 Mar 10;59(2):161–167. doi: 10.1016/0009-8981(75)90024-8. [DOI] [PubMed] [Google Scholar]
- WERNER G., SEILER N. UNTERSUCHUNGEN ZUM NACHWEIS EINER METHYLAMIN-OXYDASE. Biochem Z. 1963 Jul 10;337:383–396. [PubMed] [Google Scholar]
- ZELLER E. A., BARSKY J. In vivo inhibition of liver and brain monoamine oxidase by 1-Isonicotinyl-2-isopropyl hydrazine. Proc Soc Exp Biol Med. 1952 Nov;81(2):459–461. doi: 10.3181/00379727-81-19910. [DOI] [PubMed] [Google Scholar]


