Abstract
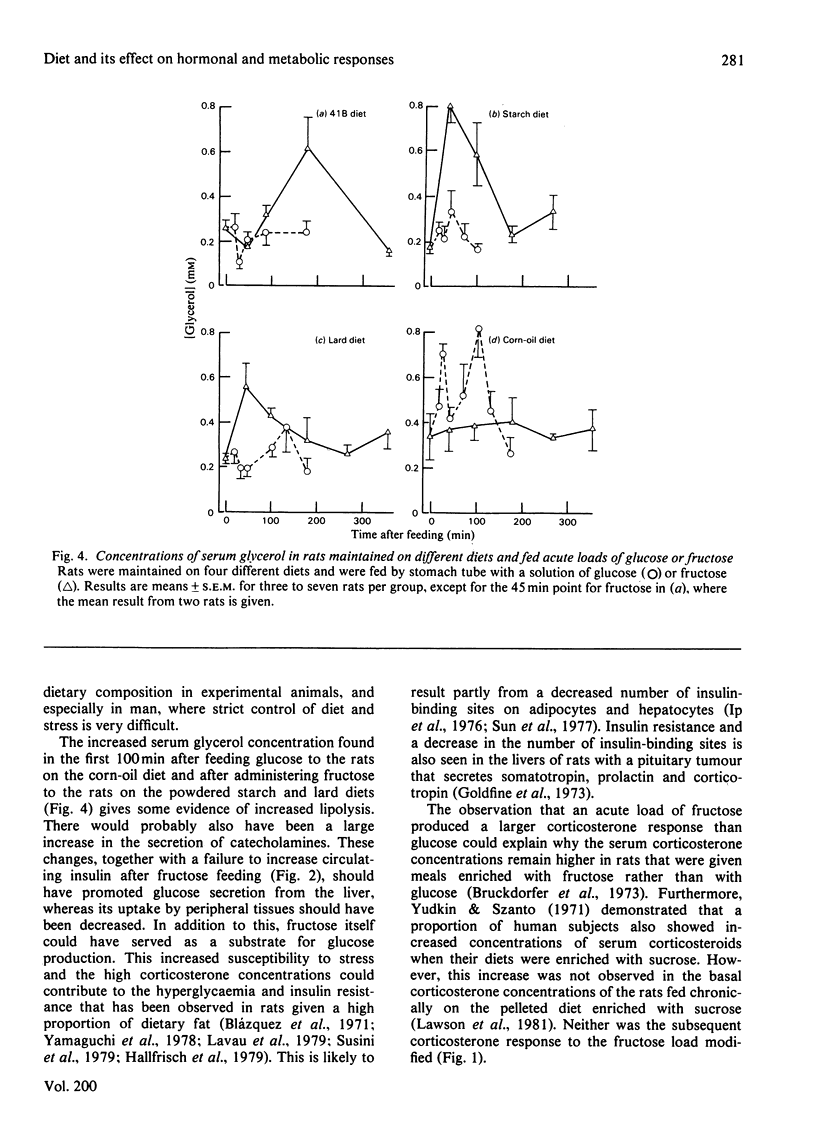
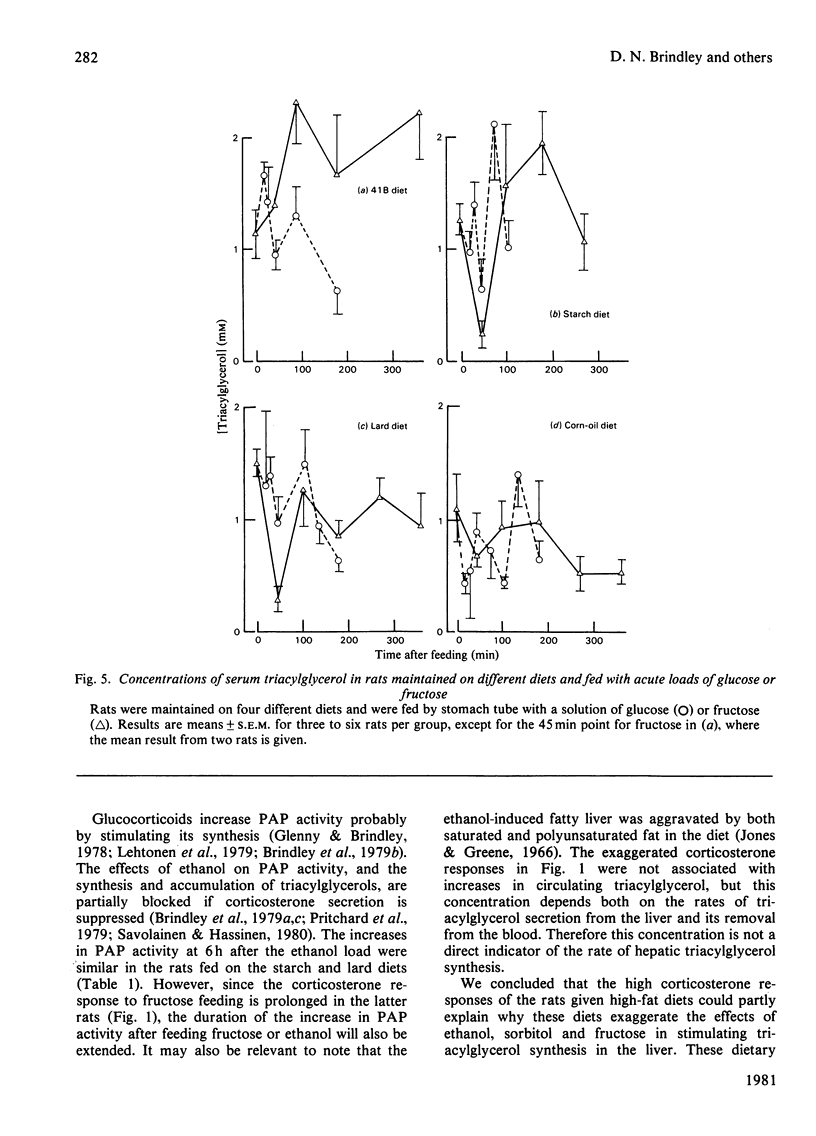
1. Male rats were fed for 14 days on powdered diets containing (by weight) 53% of starch, or on diets in which 20g of starch per 100g of diet was replaced by lard or corn oil. They were then fed acutely by stomach tube with a single dose of glucose, fructose or ethanol of equivalent energy contents, or with 0.15m-NaCl. The serum concentrations of corticosterone, insulin, glucose, glycerol, triacylglycerol and cholesterol were measured up to 6h after this treatment. 2. Feeding saline (0.9% NaCl) acutely to the rats maintained on the three powdered diets produced a small transient increase in circulating corticosterone that was similar to that in rats maintained on the normal 41B pelleted diet. 3. Feeding glucose acutely to the rats on the powdered diets produced peak concentrations of corticosterone that were 2–3-fold higher than those seen in rats maintained on the 41B diet. The duration of this response increased in the order starch diet<lard diet<corn-oil diet. This abnormal corticosterone response to glucose feeding appeared to be responsible for an increased activity in phosphatidate phosphohydrolase in the livers of rats fed the starch and lard diets of 2.9- and 4.9-fold respectively. The latter increase was similar to that produced by ethanol, whereas glucose did not increase the phosphohydrolase activity in the liver of rats maintained on the 41B diet. 4. Feeding fructose acutely produced even more marked increases than glucose in the concentrations of circulating corticosterone in rats given the powdered diets, but unlike glucose did not increase circulating insulin. The duration of the corticosterone response again increased in the order starch diet<lard diet<corn-oil diet. The concentrations of circulating glucose were increased by fructose feeding in rats maintained on these diets, but they were not altered in the rats maintained on the 41B pellets. A prolonged increase in serum corticosterone concentrations was also observed when fructose was fed to rats maintained on pelleted diets enriched with corn oil or beef tallow rather than with starch or sucrose. However, these effects were less marked than those seen with rats fed on the powdered diets. 5. These results are discussed in relation to the mechanism whereby high dietary fat exaggerates the effects of ethanol, fructose and sorbitol in stimulating triacylglycerol synthesis in the liver.
Full text
PDF


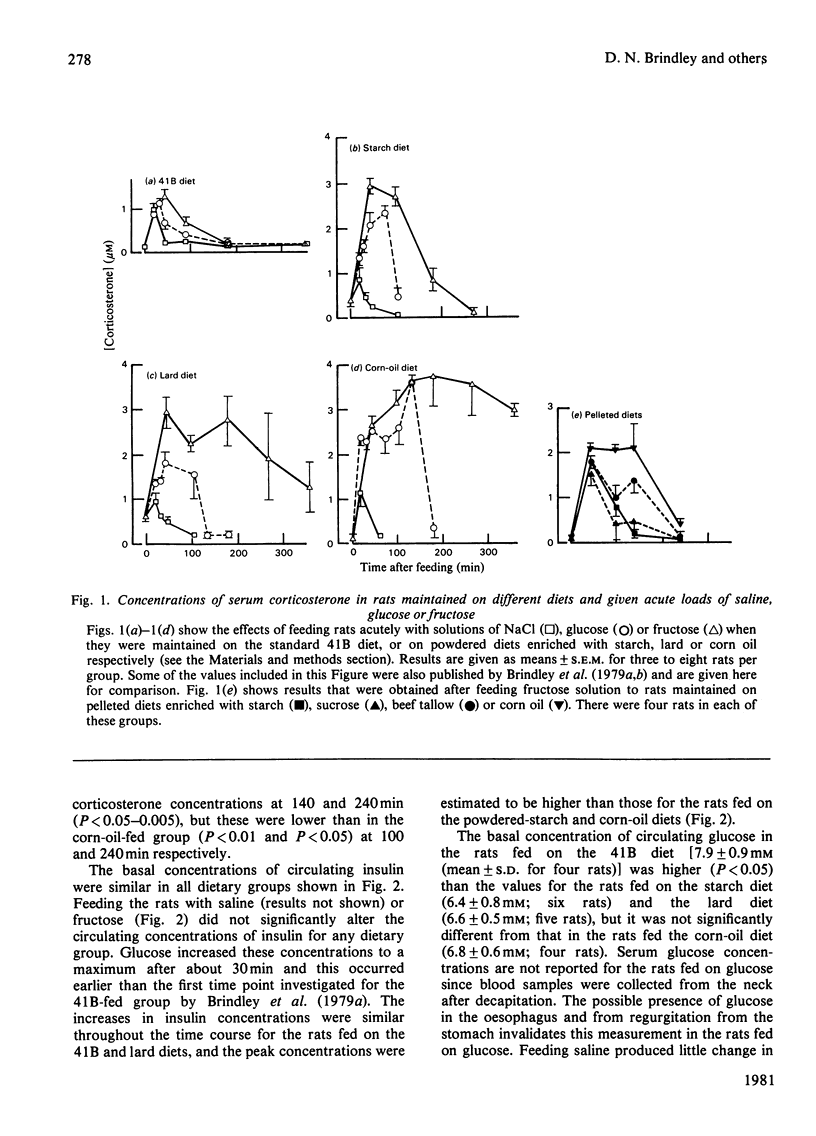
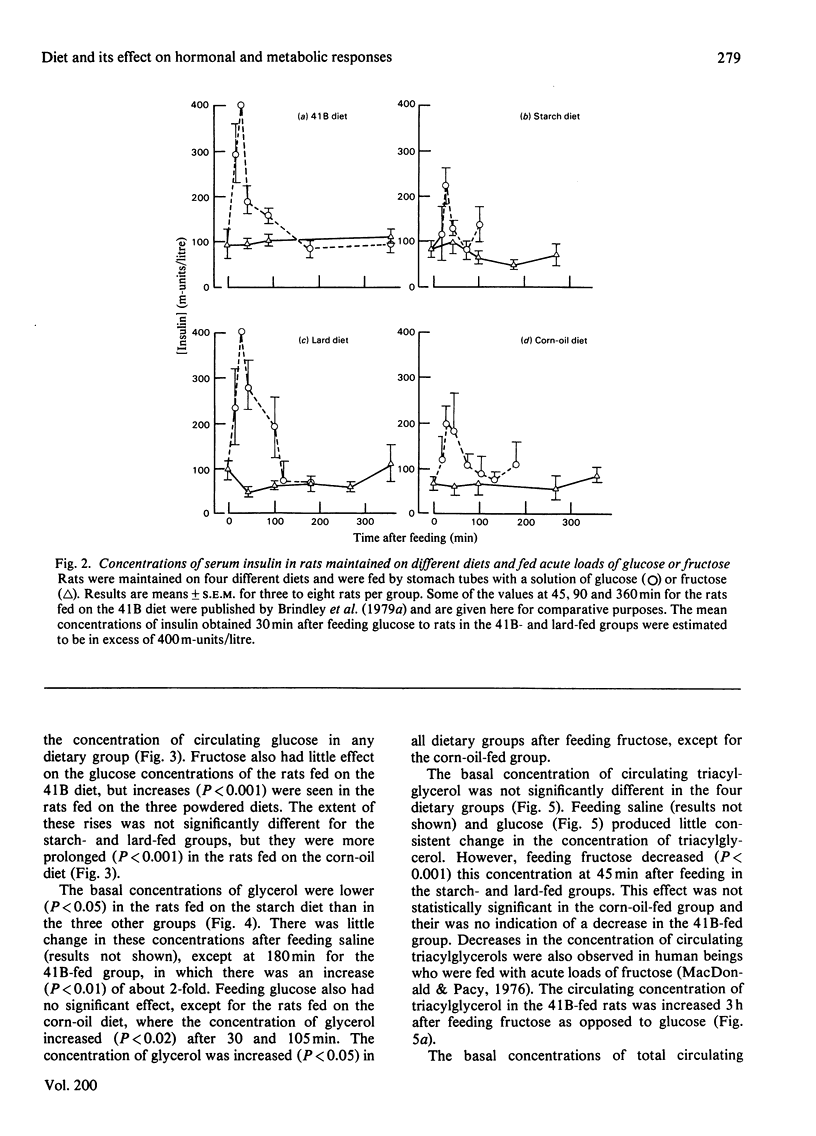
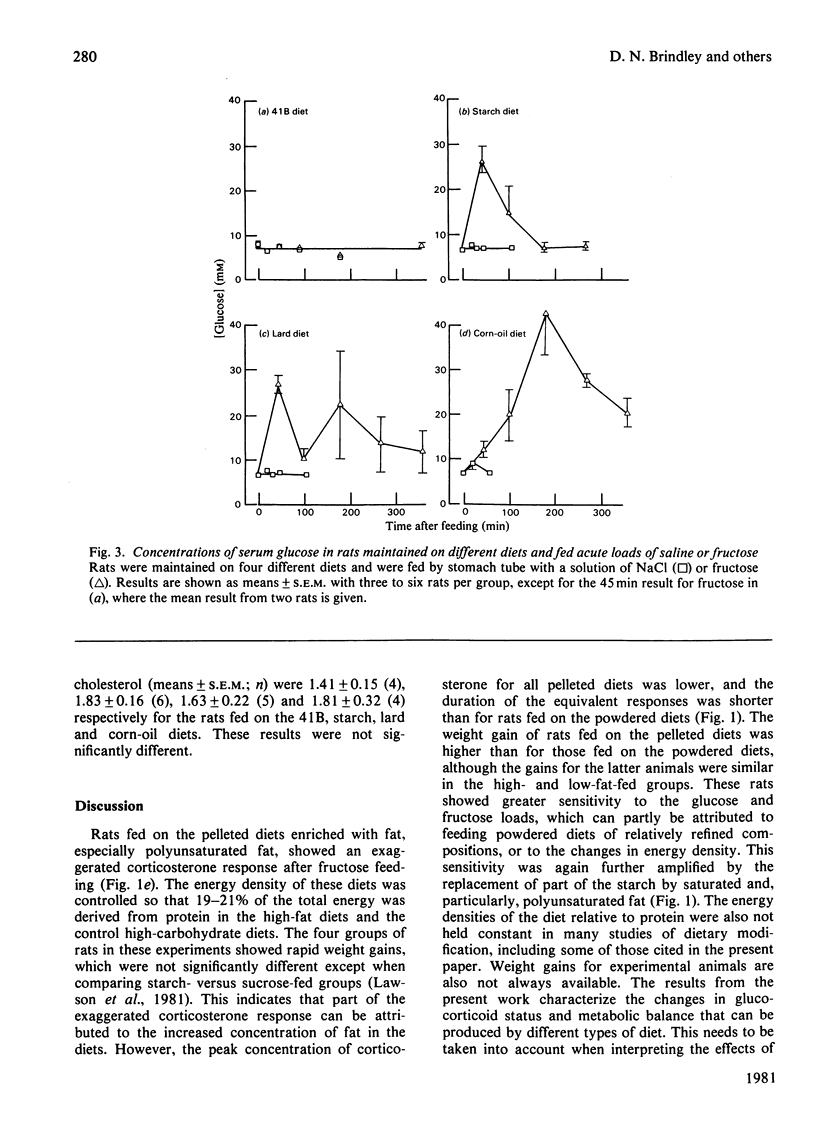

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blázquez E., Castro M., Herrera E. Effect of a high-fat diet on pancreatic insulin release, glucose tolerance and hepatic gluconeogenesis in male rats. Rev Esp Fisiol. 1971 Dec;27(4):297–304. [PubMed] [Google Scholar]
- Brindley D. N., Cooling J., Burditt S. L., Pritchard P. H., Pawson S., Sturton R. G. The involvement of glucocorticoids in regulating the activity of phosphatidate phosphohydrolase and the synthesis of triacylglycerols in the liver. Effects of feeding rats with glucose, sorbitol, fructose, glycerol and ethanol. Biochem J. 1979 Apr 15;180(1):195–199. doi: 10.1042/bj1800195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckdorfer K. R., Kari-Kari B. P., Khan I. H., Yudkin J. Activity of lipogenic enzymes and plasma triglyceride levels in the rat and the chicken as determined by the nature of the dietary fat and dietary carbohydrate. Nutr Metab. 1972;14(4):228–237. doi: 10.1159/000175385. [DOI] [PubMed] [Google Scholar]
- CARROLL K. K., NOBLE R. L. Effects of feeding rape oil on some endocrine functions of the rat. Endocrinology. 1952 Dec;51(6):476–486. doi: 10.1210/endo-51-6-476. [DOI] [PubMed] [Google Scholar]
- Carroll C., Williams L. Modification of ethanol-induced changes in rat liver composition by the carbohydrate-fat component of the diet. J Nutr. 1971 Aug;101(8):997–1012. doi: 10.1093/jn/101.8.997. [DOI] [PubMed] [Google Scholar]
- Carroll K. K. Experimental evidence of dietary factors and hormone-dependent cancers. Cancer Res. 1975 Nov;35(11 Pt 2):3374–3383. [PubMed] [Google Scholar]
- Chen N. S., Chen N. C., Johnson R. J., McGinnis J., Dyer I. A. Effect of dietary composition on hepatic lipid accumulation of rats with chronic ethanol intake. J Nutr. 1977 Jun;107(6):1114–1119. doi: 10.1093/jn/107.6.1114. [DOI] [PubMed] [Google Scholar]
- Glenny H. P., Bowley M., Burditt S. L., Cooling J., Pritchard P. H., Sturton R. G., Brindley D. N. The effect of dietary carbohydrate and fat on the activities of some enzymes responsible for glycerolipid synthesis in rat liver. Biochem J. 1978 Aug 15;174(2):535–541. doi: 10.1042/bj1740535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenny H. P., Brindley D. N. The effects of cortisol, corticotropin and thyroxine on the synthesis of glycerolipids and on the phosphatidate phosphohydrolase activity in rat liver. Biochem J. 1978 Dec 15;176(3):777–784. doi: 10.1042/bj1760777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine I. D., Kahn C. R., Neville D. M., Jr, Roth J., Garrison M. M., Bates R. W. Decreased binding of insulin to its receptors in rats with hormone induced insulin resistance. Biochem Biophys Res Commun. 1973 Aug 6;53(3):852–857. doi: 10.1016/0006-291x(73)90171-x. [DOI] [PubMed] [Google Scholar]
- Ip C., Tepperman H. M., Holohan P., Tepperman J. Insulin binding and insulin response of adipocytes from rats adapted to fat feeding. J Lipid Res. 1976 Nov;17(6):588–599. [PubMed] [Google Scholar]
- Ip C., Yip P., Bernardis L. L. Role of prolactin in the promotion of dimethylbenz[a]anthracene-induced mammary tumors by dietary fat. Cancer Res. 1980 Feb;40(2):374–378. [PubMed] [Google Scholar]
- Jones D. P., Greene E. A. Influences of dietary fat on alcoholic fatty liver. Am J Clin Nutr. 1966 May;18(5):350–357. doi: 10.1093/ajcn/18.5.350. [DOI] [PubMed] [Google Scholar]
- Knox A. M., Sturton R. G., Cooling J., Brindley D. N. Control of hepatic triacylglycerol synthesis. Diurnal variations in hepatic phosphatidate phosphohydrolase activity and in the concentrations of circulating insulin and corticosterone in rats. Biochem J. 1979 May 15;180(2):441–443. doi: 10.1042/bj1800441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. G., Wood C. K., Fallon H. J. The effect of acute and chronic ethanol intake on hepatic glycerolipid biosynthesis in the hamster. J Clin Invest. 1979 Jan;63(1):14–20. doi: 10.1172/JCI109268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavau M., Fried S. K., Susini C., Freychet P. Mechanism of insulin resistance in adipocytes of rats fed a high-fat diet. J Lipid Res. 1979 Jan;20(1):8–16. [PubMed] [Google Scholar]
- Lederer J., Masri H., Niethals E. Action lipiogène du sorbitol associé aux graisses saturées dans le régime du rat mâle. Effet des oestrogènes. Ann Endocrinol (Paris) 1978;39(2):157–158. [PubMed] [Google Scholar]
- Lehtonen M. A., Savolainen M. J., Hassinen I. E. Hormonal regulation of hepatic soluble phosphatidate phosphohydrolase. Induction by cortisol in vivo and in isolated perfused rat liver. FEBS Lett. 1979 Mar 1;99(1):162–166. doi: 10.1016/0014-5793(79)80270-7. [DOI] [PubMed] [Google Scholar]
- Pritchard P. H., Bowley M., Burditt S. L., Cooling J., Glenny H. P., Lawson N., Sturton R. G., Brindley D. N. The effects of acute ethanol feeding and of chronic benfluorex administration on the activities of some enzymes of glycerolipid synthesis in rat liver and adipose tissue. Biochem J. 1977 Sep 15;166(3):639–642. doi: 10.1042/bj1660639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard P. H., Cooling J., Burditt S. L., Brindley D. N. Can the alterations in serum glucocorticoid concentrations explain the effects of ethanol and benfluorex on the synthesis of hepatic triacylglycerols? J Pharm Pharmacol. 1979 Jun;31(6):406–407. doi: 10.1111/j.2042-7158.1979.tb13534.x. [DOI] [PubMed] [Google Scholar]
- Savolainen M. J., Hassinen I. E. Effect of ethanol on hepatic phosphatidate phosphohydrolase: dose-dependent enzyme induction and its abolition by adrenalectomy and pyrazole treatment. Arch Biochem Biophys. 1980 May;201(2):640–645. doi: 10.1016/0003-9861(80)90554-8. [DOI] [PubMed] [Google Scholar]
- Savolainen M. J. Stimulation of hepatic phosphatidate phosphohydrolase activity by a single dose of ethanol;. Biochem Biophys Res Commun. 1977 Mar 21;75(2):511–518. doi: 10.1016/0006-291x(77)91071-3. [DOI] [PubMed] [Google Scholar]
- Sturton R. G., Brindley D. N. Problems encountered in measuring the activity of phosphatidate phosphohydrolase. Biochem J. 1978 Apr 1;171(1):263–266. doi: 10.1042/bj1710263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturton R. G., Pritchard P. H., Han L. Y., Brindley D. N. The involvement of phosphatidate phosphohydrolase and phospholipase A activities in the control of hepatic glycerolipid synthesis. Effects of acute feeding with glucose, fructose, sorbitol, glycerol and ethanol. Biochem J. 1978 Aug 15;174(2):667–670. doi: 10.1042/bj1740667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. V., Tepperman H. M., Tepperman J. A comparison of insulin binding by liver plasma membranes of rats fed a high glucose diet or a high fat diet. J Lipid Res. 1977 Jul;18(4):533–539. [PubMed] [Google Scholar]
- Susini C., Lavau M., Herzog J. Adrenaline responsiveness of glucose metabolism in insulin-resistant adipose tissue of rats fed a high-fat diet. Biochem J. 1979 May 15;180(2):431–433. doi: 10.1042/bj1800431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler R. G., Sprague E. A., Albanese R. A., Fuchs R., Thompson A. J. The association of elevated plasma cortisol and early atherosclerosis as demonstrated by coronary angiography. Atherosclerosis. 1977 Feb;26(2):151–162. doi: 10.1016/0021-9150(77)90098-3. [DOI] [PubMed] [Google Scholar]
- Whiting P. H., Bowley M., Sturton R. G., Pritchard P. H., Brindley D. N., Hawthorne J. N. The effect of chronic diabetes, induced by streptozotocin, on the activities of some enzymes of glycerolipid synthesis in rat liver. Biochem J. 1977 Nov 15;168(2):147–153. doi: 10.1042/bj1680147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Takashima S., Masuyama T., Matsuoka A. Effects of the electric stress on insulin secretion and glucose metabolism in rats fed with a high fat diet. Endocrinol Jpn. 1978 Oct;25(5):415–422. doi: 10.1507/endocrj1954.25.415. [DOI] [PubMed] [Google Scholar]
- Yudkin J., Szanto S. Hyperinsulinism and atherogenesis. Br Med J. 1971 Feb 6;1(5744):349–349. doi: 10.1136/bmj.1.5744.349. [DOI] [PMC free article] [PubMed] [Google Scholar]


