Abstract
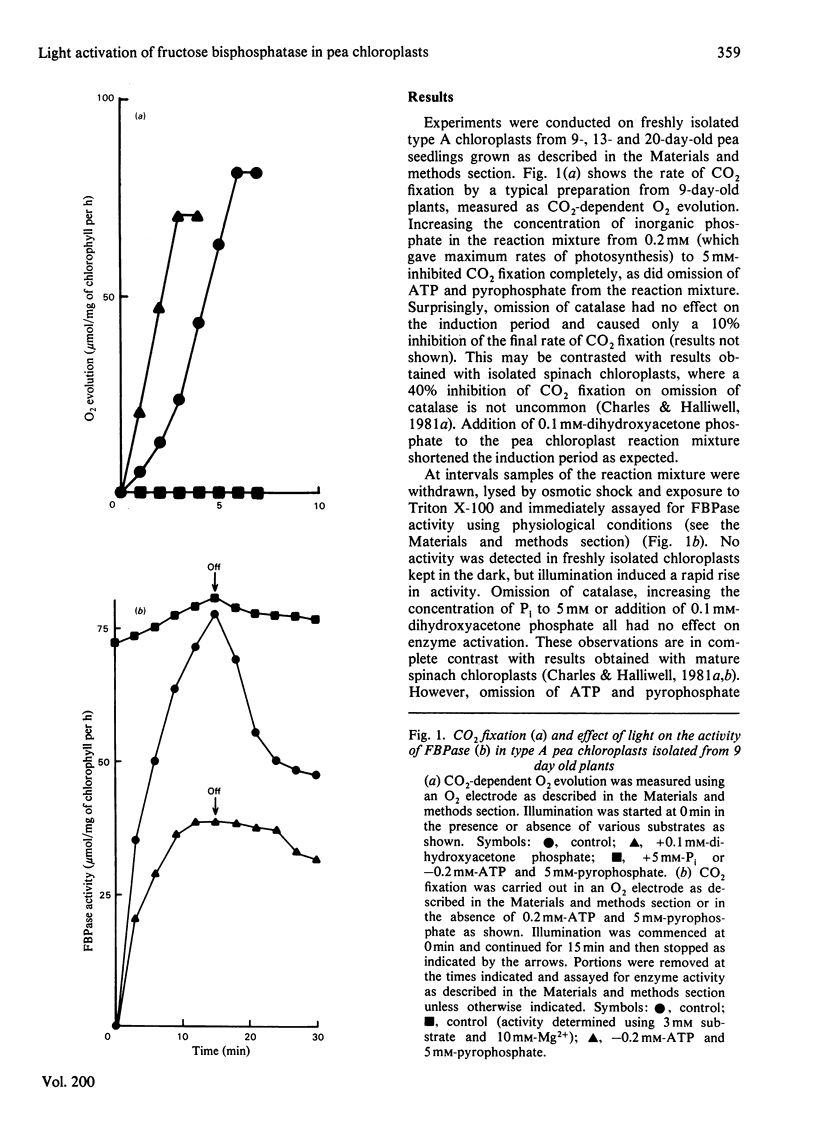
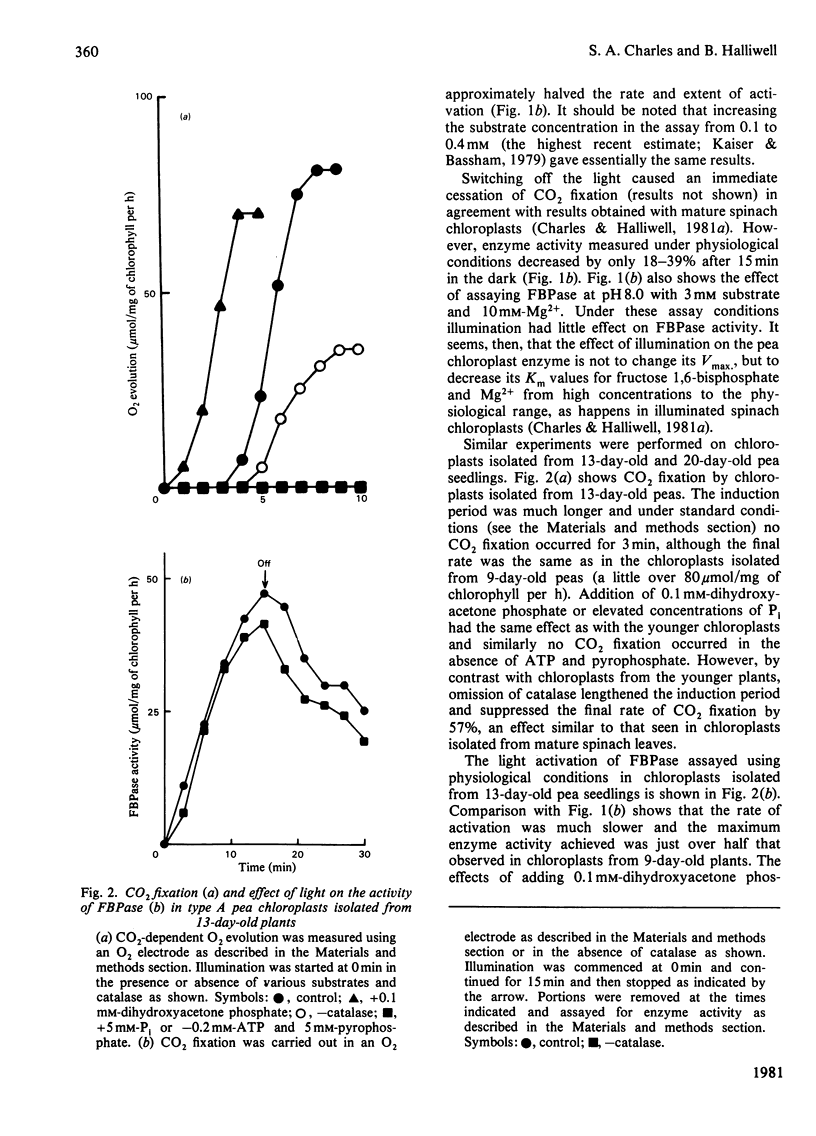
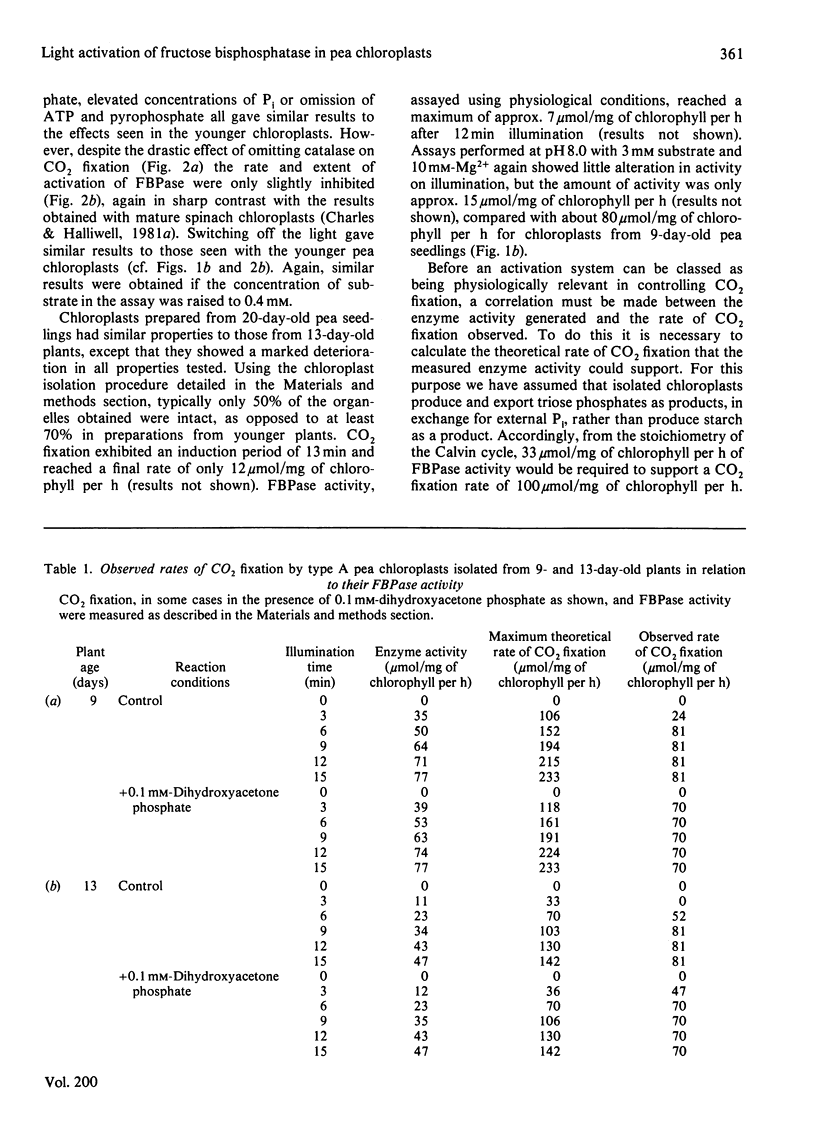
The fructose bisphosphatase (EC 3.1.3.11) activity of type A chloroplasts isolated from young (9-day-old) pea (Pisum sativum var. Progress no. 9) plants, assayed at physiological pH, substrate and Mg2+ concentrations, increased rapidly on illumination. The enzyme activity detected was more than sufficient to account for observed rates of Co2 fixation both during the induction period and during steady-state CO2 fixation, whether or not dihydroxyacetone phosphate had been added to the preparation. Omission of catalase from the suspension medium had no effect. On switching off the light, CO2 fixation by the chloroplasts ceased at once, yet fructose bisphosphatase activity decreased much more slowly. Changes in enzyme activity were much less marked if assays were conducted at 3 mM substrate and 10 mM-Mg2+. Chloroplasts from older (13--20-day-old) peas only fixed CO2 rapidly if catalase was present in the assay medium. The fructose bisphosphatase activity detected under physiological assay conditions was again more than sufficient to account for observed rates of Co2 fixation. In the presence of added dihydroxyacetone phosphate, however, the rate of Co2 fixation appeared to be determined by the rate of light activation of fructose bisphosphatase. In general, the rates of Co2 fixation and enzyme activation, and the final enzyme activity achieved, decreased markedly with increasing age of the plants. The role of light activation of fructose bisphosphatase as a means of controlling the rate of CO2 fixation in pea chloroplasts is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Chin H. M., Gupta V. K. Modulation of Chloroplast Fructose-1,6-bisphosphatase Activity by Light. Plant Physiol. 1979 Sep;64(3):491–494. doi: 10.1104/pp.64.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles S. A., Halliwell B. Action of calcium ions on spinach (Spinacia oleracea) chloroplast fructose bisphosphatase and other enzymes of the Calvin cycle. Biochem J. 1980 Jun 15;188(3):775–779. doi: 10.1042/bj1880775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles S. A., Halliwell B. Effect of hydrogen peroxide on spinach (Spinacia oleracea) chloroplast fructose bisphosphatase. Biochem J. 1980 Aug 1;189(2):373–376. doi: 10.1042/bj1890373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles S. A., Halliwell B. Properties of freshly purified and thiol-treated spinach chloroplast fructose bisphosphatase. Biochem J. 1980 Mar 1;185(3):689–693. doi: 10.1042/bj1850689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groden D., Beck E. H2O2 destruction by ascorbate-dependent systems from chloroplasts. Biochim Biophys Acta. 1979 Jun 5;546(3):426–435. doi: 10.1016/0005-2728(79)90078-1. [DOI] [PubMed] [Google Scholar]
- Hall D. O. Nomenclature for isolated chloroplasts. Nat New Biol. 1972 Jan 26;235(56):125–126. doi: 10.1038/newbio235125a0. [DOI] [PubMed] [Google Scholar]
- Halliwell B. The chloroplast at work. A review of modern developments in our understanding of chloroplast metabolism. Prog Biophys Mol Biol. 1978;33(1):1–54. doi: 10.1016/0079-6107(79)90024-5. [DOI] [PubMed] [Google Scholar]
- Heldt H. W. Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett. 1969 Sep;5(1):11–14. doi: 10.1016/0014-5793(69)80280-2. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Portis A. R., Lilley R. M., Mosbach A., Chon C. J. Assay of nucleotides and other phosphate-containing compounds in isolated chloroplasts by ion exchange chromatography. Anal Biochem. 1980 Jan 15;101(2):278–287. doi: 10.1016/0003-2697(80)90187-6. [DOI] [PubMed] [Google Scholar]
- Kaiser W. M., Bassham J. A. Light-Dark Regulation of Starch Metabolism in Chloroplasts: I. Levels of Metabolites in Chloroplasts and Medium during Light-Dark Transition. Plant Physiol. 1979 Jan;63(1):105–108. doi: 10.1104/pp.63.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause G. H. Light-induced movement of magnesium ions in intact chloroplasts. Spectroscopic determination with Eriochrome Blue SE. Biochim Biophys Acta. 1977 Jun 9;460(3):500–510. doi: 10.1016/0005-2728(77)90088-3. [DOI] [PubMed] [Google Scholar]
- Leegood R. C., Walker D. A. Autocatalysis and light activation of enzymes in relation to photosynthetic induction in wheat chloroplasts. Arch Biochem Biophys. 1980 Apr 1;200(2):575–582. doi: 10.1016/0003-9861(80)90389-6. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Chon C. J., Mosbach A., Heldt H. W. The distribution of metabolites between spinach chloroplasts and medium during photosynthesis in vitro. Biochim Biophys Acta. 1977 May 11;460(2):259–272. doi: 10.1016/0005-2728(77)90212-2. [DOI] [PubMed] [Google Scholar]
- Portis A. R. Evidence of a Low Stromal Mg Concentration in Intact Chloroplasts in the Dark: I. STUDIES WITH THE IONOPHORE A23187. Plant Physiol. 1981 May;67(5):985–989. doi: 10.1104/pp.67.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R., Jr, Heldt H. W. Light-dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochim Biophys Acta. 1976 Dec 6;449(3):434–436. doi: 10.1016/0005-2728(76)90154-7. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Walker D. A. The significance of light activation of enzymes during the induction phase of photosynthesis in isolated chloroplasts. Arch Biochem Biophys. 1980 Jul;202(2):617–623. doi: 10.1016/0003-9861(80)90469-5. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Wiskich J. T. Pyrophosphate inhibition of carbon dioxide fixation in isolated pea chloroplasts by uptake in exchange for endogenous adenine nucleotides. Plant Physiol. 1977 Mar;59(3):422–427. doi: 10.1104/pp.59.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Wiskich J. T. Stimulation of carbon dioxide fixation in isolated pea chloroplasts by catalytic amounts of adenine nucleotides. Plant Physiol. 1976 Aug;58(2):156–162. doi: 10.1104/pp.58.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R., Anderson L. E. Dark modulation of NADP-dependent malate dehydrogenase and glucose-6-phosphate dehydrogenase in the chloroplast. Biochim Biophys Acta. 1981 Jun 12;636(1):58–64. doi: 10.1016/0005-2728(81)90075-x. [DOI] [PubMed] [Google Scholar]
- Stankovic Z. S., Walker D. A. Photosynthesis by isolated pea chloroplasts: some effects of adenylates and inorganic pyrophosphate. Plant Physiol. 1977 Mar;59(3):428–432. doi: 10.1104/pp.59.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]


