Abstract
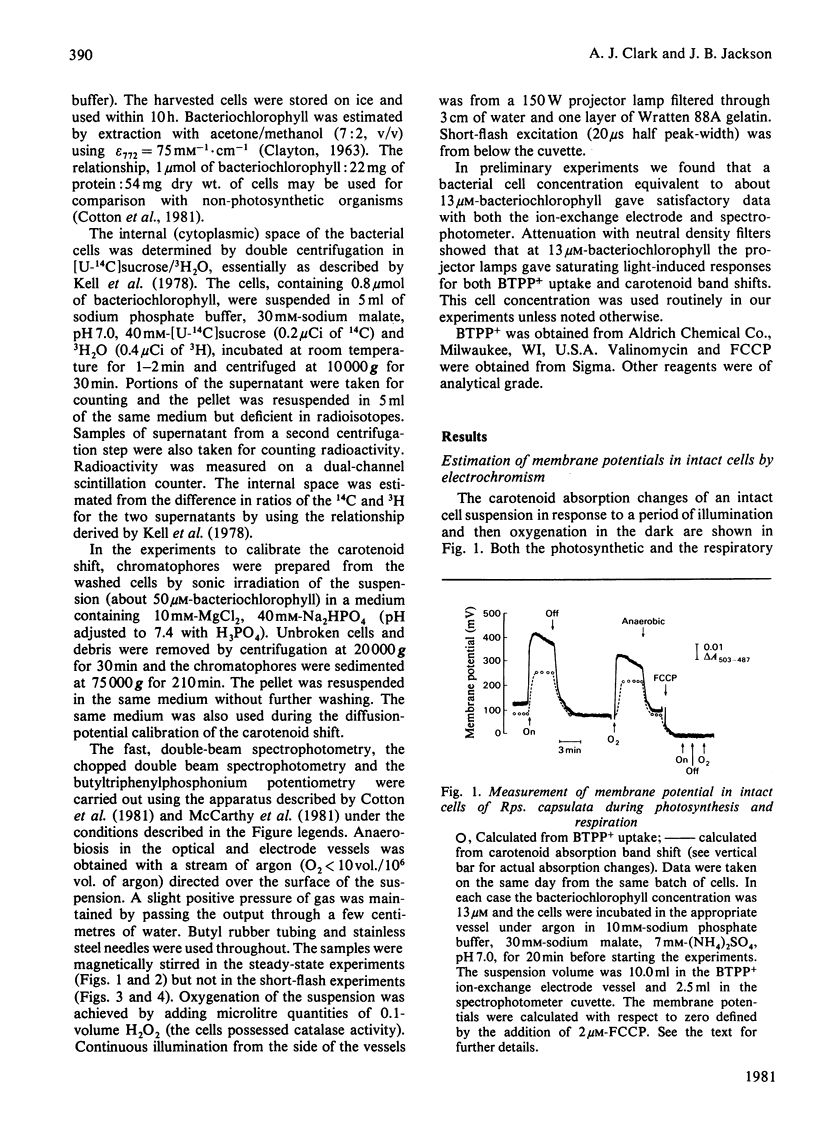
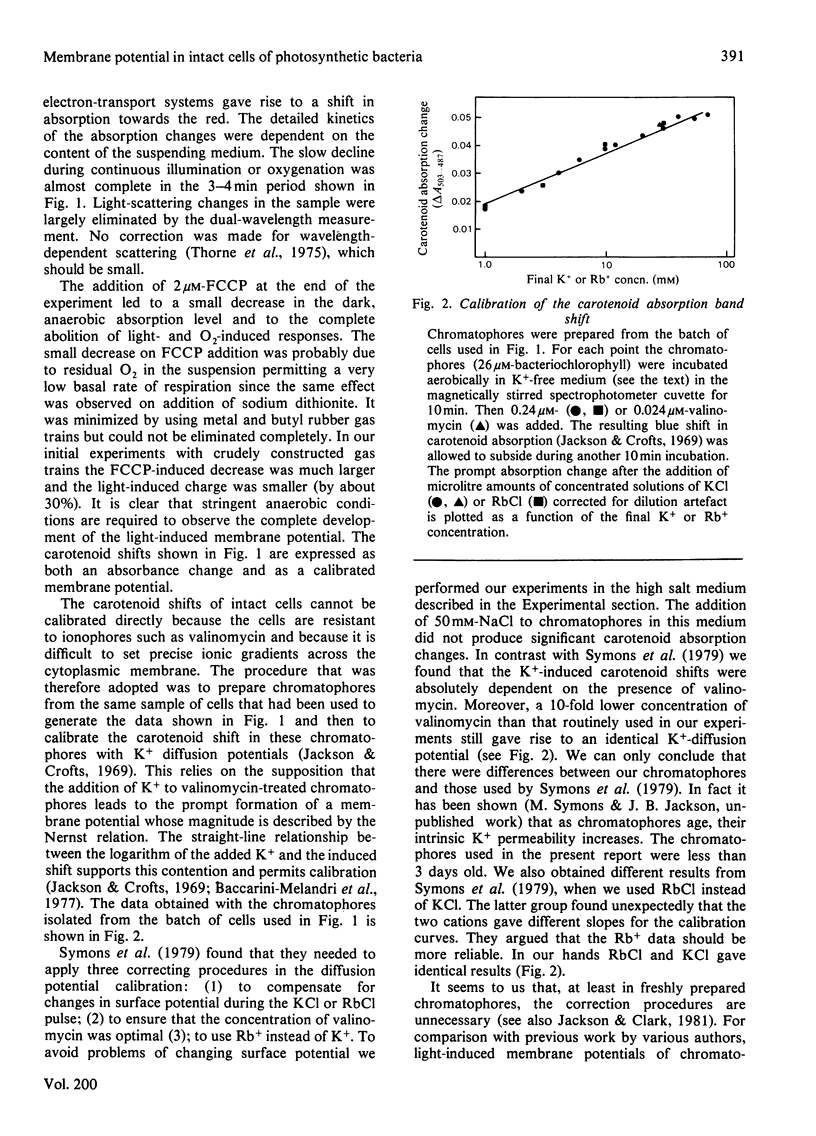
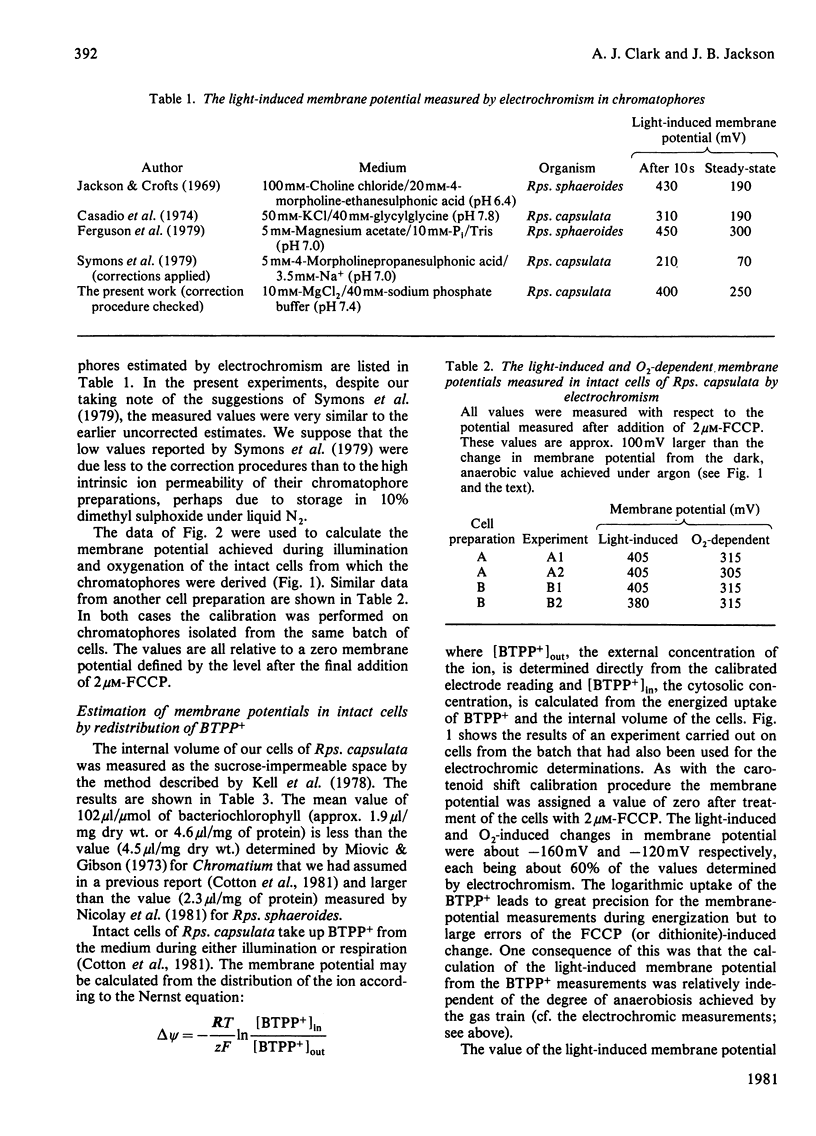
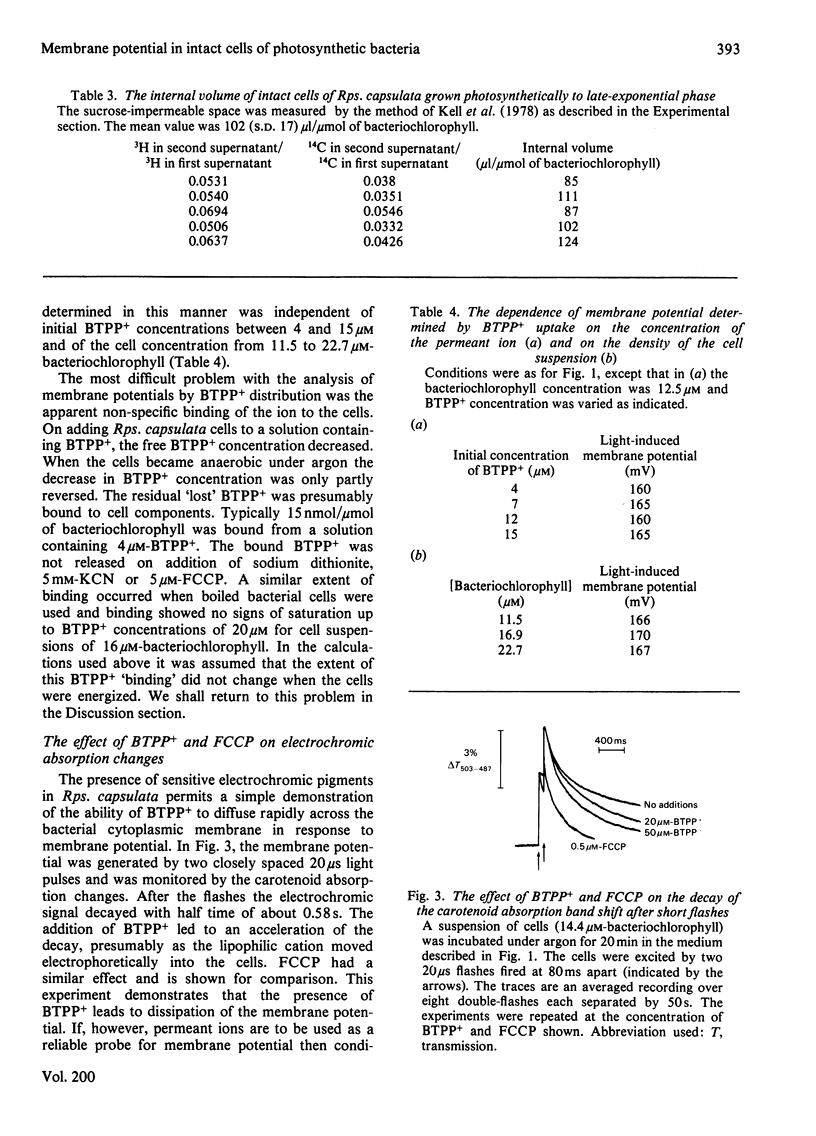
1. The membrane potential in intact cells of Rhodopseudomonas capsulata during photosynthesis and during dark respiration has been measured by two independent methods. 2. The light-induced and O2-induced shifts in the carotenoid absorption spectrum were measured in the intact cells. The shift was calibrated with K+-diffusion potentials in chromatophores derived from those cells. The light-induced and O2-induced membrane potentials were -290 mV and -230 mV respectively. 3. The energized uptake of butyltriphenylphosphonium ions was measured in the same batch of cells. The light-induced and O2-induced membrane potentials calculated from the Nernst equation were -160 mV and -120 mV respectively. 4. It is concluded that the two kinds of probe measure the electric potentials across different domains of the cytoplasmic membrane, but it is difficult to reconcile the existence of such domains with simple electrical analogues of the membrane and aqueous phases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzone G. F., Pozzan T., Massari S. Proton electrochemical gradient and phosphate potential in mitochondria. Biochim Biophys Acta. 1978 Feb 9;501(2):307–316. doi: 10.1016/0005-2728(78)90036-1. [DOI] [PubMed] [Google Scholar]
- Baccarini Melandri A., Casadio R., Melandri B. A. Thermodynamics and kinetics of photophosphorylation in bacterial chromatophores and their relation with the transmembrane electrochemical potential difference of protons. Eur J Biochem. 1977 Sep;78(2):389–402. doi: 10.1111/j.1432-1033.1977.tb11751.x. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. TOWARD THE ISOLATION OF A PHOTOCHEMICAL REACTION CENTER IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1963 Nov 29;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- Casadio R., Baccarini-Melandri A., Melandri B. A. On the determination of the transmembrane pH difference in bacterial chromatophores using 9-aminoacridine. Eur J Biochem. 1974 Aug 15;47(1):121–128. doi: 10.1111/j.1432-1033.1974.tb03675.x. [DOI] [PubMed] [Google Scholar]
- Felle H., Porter J. S., Slayman C. L., Kaback H. R. Quantitative measurements of membrane potential in Escherichia coli. Biochemistry. 1980 Jul 22;19(15):3585–3590. doi: 10.1021/bi00556a026. [DOI] [PubMed] [Google Scholar]
- Ferguson S. J., Jones O. T., Kell D. B., Sorgato M. C. Comparison of permeant ion uptake and carotenoid band shift as methods for determining the membrane potential in chromatophores from Rhodopseudomonas sphaeroides Ga. Biochem J. 1979 Apr 15;180(1):75–85. doi: 10.1042/bj1800075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLT S. C., MARR A. G. LOCATION OF CHLOROPHYLL IN RHODOSPIRILLUM RUBRUM. J Bacteriol. 1965 May;89:1402–1412. doi: 10.1128/jb.89.5.1402-1412.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. B., Crofts A. R. The high energy state in chromatophores from Rhodopseudomonas spheroides. FEBS Lett. 1969 Aug;4(3):185–189. doi: 10.1016/0014-5793(69)80230-9. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Crofts A. R. The kinetics of light induced carotenoid changes in Rhodopseudomonas spheroides and their relation to electrical field generation across the chromatophore membrane. Eur J Biochem. 1971 Jan 1;18(1):120–130. doi: 10.1111/j.1432-1033.1971.tb01222.x. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Crofts A. R., von Stedingk L. V. Ion transport induced by light and antibiotics IN CHROMATOPHORES FROM Rhodospirillum rubrum. Eur J Biochem. 1968 Oct 17;6(1):41–54. doi: 10.1111/j.1432-1033.1968.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Junge W., Witt H. T. On the ion transport system of photosynthesis--investigations on a molecular level. Z Naturforsch B. 1968 Feb;23(2):244–254. doi: 10.1515/znb-1968-0222. [DOI] [PubMed] [Google Scholar]
- Kell D. B., Ferguson S. J., John P. Measurement by a flow dialysis technique of the steady-state proton-motive force in chromatophores from Rhodospirillum rubrum. Comparison with phosphorylation potential. Biochim Biophys Acta. 1978 Apr 11;502(1):111–126. doi: 10.1016/0005-2728(78)90136-6. [DOI] [PubMed] [Google Scholar]
- Kell D. B. On the functional proton current pathway of electron transport phosphorylation. An electrodic view. Biochim Biophys Acta. 1979 Jul 3;549(1):55–99. doi: 10.1016/0304-4173(79)90018-1. [DOI] [PubMed] [Google Scholar]
- Michels P. A., Konings W. N. The electrochemical proton gradient generated by light in membrane vesicles and chromatophores from Rhodopseudomonas sphaeroides. Eur J Biochem. 1978 Apr;85(1):147–155. doi: 10.1111/j.1432-1033.1978.tb12222.x. [DOI] [PubMed] [Google Scholar]
- Miović M. L., Gibson J. Nucleotide pools and adenylate energy charge in balanced and unbalanced growth of Chromatium. J Bacteriol. 1973 Apr;114(1):86–95. doi: 10.1128/jb.114.1.86-95.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. A commentary on alternative hypotheses of protonic coupling in the membrane systems catalysing oxidative and photosynthetic phosphorylation. FEBS Lett. 1977;78(1):1–20. doi: 10.1016/0014-5793(77)80263-9. [DOI] [PubMed] [Google Scholar]
- Nicolay K., Lolkema J., Hellingwerf K. J., Kaptein R., Konings W. N. Quantitative agreement between the values for the light-induced delta pH in Rhodopseudomonas sphaeroides measured with automated follow-dialysis and 31P NMR. FEBS Lett. 1981 Jan 26;123(2):319–323. doi: 10.1016/0014-5793(81)80318-3. [DOI] [PubMed] [Google Scholar]
- Packham N. K., Greenrod J. A., Jackson J. B. Generation of membrane potential during photosynthetic electron flow in chromatophores from Rhodopseudomonas capsulata. Biochim Biophys Acta. 1980 Aug 5;592(1):130–142. doi: 10.1016/0005-2728(80)90120-6. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. Phase transitions and coupling in energy transducing membranes. FEBS Lett. 1978 Oct 15;94(2):295–297. doi: 10.1016/0014-5793(78)80960-0. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Saphon S., Jackson J. B., Lerbs V., Witt H. T. The functional unit of electrical events and phosphorylation in chromatophores from Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1975 Oct 10;408(1):58–66. doi: 10.1016/0005-2728(75)90158-9. [DOI] [PubMed] [Google Scholar]
- Schapendonk A. H., Hemrika-Wagner A. M., Theuvenet A. P., Sang H. W., Vredenberg W. J., Kraayenhof R. Energy-dependent changes of the electrokinetic properties of chloroplasts. Biochemistry. 1980 Apr 29;19(9):1922–1927. doi: 10.1021/bi00550a030. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Padan E., Rottenberg H., Gromet-Elhanan Z., Avron M. Delta pH and membrane potential in bacterial chromatophores. FEBS Lett. 1974 Dec 15;49(2):174–177. doi: 10.1016/0014-5793(74)80505-3. [DOI] [PubMed] [Google Scholar]
- Sorgato M. C., Ferguson S. J., Kell D. B., John P. The protonmotive force in bovine heart submitochondrial particles. Magnitude, sites of generation and comparison with the phosphorylation potential. Biochem J. 1978 Jul 15;174(1):237–256. doi: 10.1042/bj1740237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons M., Nuyten A., Sybesma C. On the calibration of the carotenoid band shift with diffusion potentials. FEBS Lett. 1979 Nov 1;107(1):10–14. doi: 10.1016/0014-5793(79)80451-2. [DOI] [PubMed] [Google Scholar]
- Thorne S. W., Horvath G., Kahn A., Boardman N. K. Light-dependent absorption and selective scattering changes at 518 nm in chloroplast thylakoid membranes. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3858–3862. doi: 10.1073/pnas.72.10.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver P. F., Wall J. D., Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- Witt H. T. Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. The central role of the electric field. Biochim Biophys Acta. 1979 Mar 14;505(3-4):355–427. doi: 10.1016/0304-4173(79)90008-9. [DOI] [PubMed] [Google Scholar]
- del Valle-Tascon S., van Grondelle R., Duysens L. N. Flash-induced photophosphorylation in Rhodospirillum rubrum chromatophores. I. The relationship between cytochrome c-420 content and photophosphorylation. Biochim Biophys Acta. 1978 Oct 11;504(1):26–39. doi: 10.1016/0005-2728(78)90004-x. [DOI] [PubMed] [Google Scholar]


