TO THE EDITOR:
Defects in cell death pathways, particularly in apoptosis signaling, can lead to poor treatment response in patients with B-cell precursor (BCP)–acute lymphoblastic leukemia (ALL). Induction of apoptosis by B-cell lymphoma 2 homology 3 (BH3) mimetics targeting antiapoptotic B-cell lymphoma 2 (BCL-2) family proteins is a promising treatment approach for hematological malignancies. The BCL-2 selective inhibitor venetoclax shows heterogeneous efficacy in BCP-ALL, and mitochondrial BCL-2 dependency is a marker of response.1,2 However, regulation of BCL-2 dependence and the role of other antiapoptotic regulators mediating resistance to BCL-2 inhibition is not entirely understood. Certain cancers are characterized by B-cell lymphoma-extra large (BCL-XL) dependency,3,4 suggesting that concomitant BCL-XL inhibition is more effective than targeting BCL-2 alone. Despite this, BCL-XL plays a role in healthy tissues, particularly in platelet survival, and the BCL-2/BCL-XL inhibitor navitoclax is limited by thrombocytopenia.5,6 Given the side effects are manageable, combined targeting of different antiapoptotic BCL-2 family proteins could improve therapeutic efficacy compared with selective agents. In addition to BCL-XL, myeloid cell leukemia 1 (MCL-1) plays a role in mediating resistance to BCL-2 inhibition, making it a valuable target.2,6, 7, 8, 9, 10, 11, 12, 13
AZD4320, a dual inhibitor of BCL-2 and BCL-XL, has shown anticancer activity in preclinical hematological cancer models, achieving tumor growth inhibition with once-weekly dosing that allowed for platelet recovery despite transient thrombocytopenia.14 Despite its activity and manageable thrombocytopenia, AZD4320 was not further progressed into clinical development because of dose-limiting cardiovascular toxicity. However, its dendrimer-conjugate AZD0466 has optimized drug release and reduced cardiovascular side effects.15 In this study, we investigated susceptibilities of BCP-ALL to inhibitors of BCL-2 family proteins, searched for markers of response, elucidated mechanisms of action, and evaluated combinatorial activities.
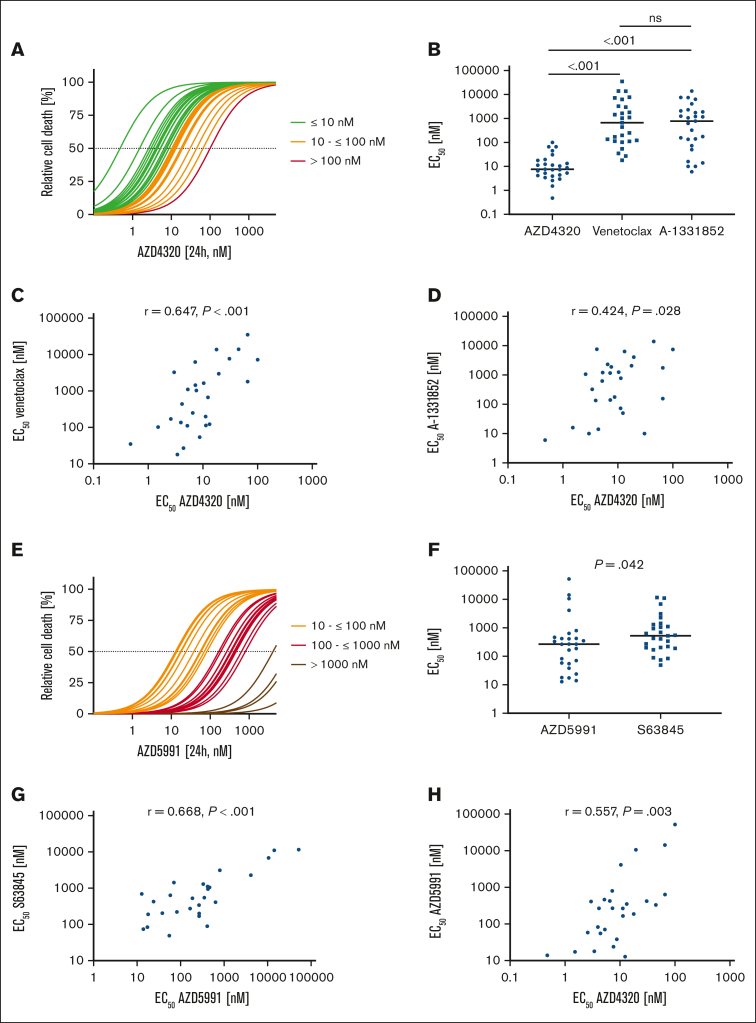
First, we studied the antileukemia activities of inhibitors targeting different BCL-2 family proteins in BCP-ALL cell lines (n = 7) and patient-derived xenograft (PDX) samples (n = 27). Because we analyzed exclusively in vitro and ex vivo effects of the inhibitors in this study, we used the nonconjugated dual BCL-2/BCL-XL inhibitor AZD4320. The efficacy of AZD4320 was compared with the sensitivities of navitoclax (BCL-2/BCL-XLi) and AZD5991 (MCL-1i), as well as with the sensitivities of venetoclax (BCL-2i), A-1331852 (BCL-XLi), and S63845 (MCL-1i), which we have previously published.7 Analyzing half maximal effective concentration (EC50) values based on the cell death rates upon drug exposure, we identified sensitivity toward dual BCL-2/BCL-XL inhibition (AZD4320) in all PDX samples (median EC50, 7.60 nM; Figure 1A). Comparing the activities of inhibitors revealed that dual BCL-2/BCL-XL inhibition with AZD4320 is more potent in PDX samples than using navitoclax (supplemental Figure 2A-B) and more effective than antagonizing BCL-2 (venetoclax) or BCL-XL (A-1331852) alone (Figure 1B). In this study, we focused primarily on AZD4320 due to its higher efficacy than navitoclax. However, it is important to evaluate a broader range of dual BCL-2/BCL-XL inhibitors in future studies to fully understand their potential and optimize therapeutic strategies in BCP-ALL. In cell lines, heterogeneous responses were observed for the inhibitors used, with no significant differences when comparing their median sensitivities (supplemental Figure 1A-B). Importantly, we found a clear association of the activities of AZD4320 and venetoclax in PDX samples (Figure 1C) and a trend in cell lines (supplemental Figure 1C). Moreover, we found an association between the activities of AZD4320 and A-1331852 in PDX samples (Figure 1D) but not in cell lines (supplemental Figure 1D). In addition, we found a trend between the sensitivity of navitoclax and those of AZD4320, venetoclax, and A-1331852 (supplemental Figure 2C-E). For MCL-1 inhibition using AZD5991, we found heterogeneous sensitivities (Figure 1E; supplemental Figure 1E). When comparing the effects of 2 MCL-1 inhibitors, AZD5991 showed slightly lower EC50 values than S63845 in PDX samples, but the opposite was seen in cell lines (Figure 1F; supplemental Figure 1F). Importantly, we observed a robust correlation of the sensitivities of both MCL-1 inhibitors (Figure 1G; supplemental Figure 1G), substantiating their on-target activities. Moreover, we found a correlation between the sensitivities of AZD4320 and AZD5991 in PDX samples (Figure 1H). Although a positive correlation coefficient was observed also in cell lines, the correlation was not statistically significant (supplemental Figure 1H).
Figure 1.
Heterogeneous activities of inhibitors of antiapoptotic molecules in BCP-ALL PDX samples. (A) BCP-ALL PDX samples were cultured for 24 hours and exposed to 11 increasing concentrations (0.1, 1, 2.5, 5, 10, 25, 50, 100, 500, 1000, and 5000 nM) of the BCL-2/BCL-XL inhibitor AZD4320. Relative cell death rates were assessed by propidium iodide (PI) staining and normalized to dimethyl sulfoxide controls. EC50 values were calculated, showing a range from 0.47 to 100.30 nM and a median EC50 value of 7.60 nM. (B) Comparison of the sensitivities of inhibitors of BCL-2 and BCL-XL (AZD4320), BCL-2 (venetoclax), or BCL-XL (A-1331852). The scatterplot shows EC50 values of the inhibitors, with each data point representing the EC50 value of 1 PDX sample. The medians are shown as solid lines. Mann-Whitney U test; P values represent significance. (C) Association of venetoclax and AZD4320 sensitivities. (D) Association of A-1331852 and AZD4320 sensitivities. (E) BCP-ALL PDX samples were cultured for 24 hours and exposed to 11 increasing concentrations (0.1, 1, 2.5, 5, 10, 25, 50, 100, 500, 1000 and 5000 nM) of the MCL-1 inhibitor AZD5991. Relative cell death rates (PI) were assessed, and EC50 values were calculated, resulting in a range from 13.93 nM to 51.86 μM and a median of 267 nM. (F) Comparison of the EC50 values of both MCL-1 inhibitors. Mann-Whitney U test; P values, significance. (G) Association of S63845 and AZD5991 sensitivities. (H) Association of the sensitivities of the MCL-1 inhibitor AZD5991 and the BCL-2/BCL-XL inhibitor AZD4320. EC50 values of venetoclax, A-1331852, and S63845 have been published previously.7 Spearman correlation; r represents correlation coefficient; P value, significance.
With the aim to identify markers of response for the inhibitors, we evaluated whether their sensitivities were related to leukemia characteristics, but no clear associations were found (supplemental Figure 3A-B). Studies have shown that venetoclax sensitivity is associated with high BCL-2 and low MCL-1 levels.1,16 Additionally, the "mediators of apoptosis combinatorial score," reflecting the ratio of BCL-2, BCL-XL, and MCL-1 in leukemic stem cells, predicts the response to venetoclax combined with azacitidine in patients with acute myeloid leukemia.17 Here, we analyzed the basal expression levels and ratios of the target proteins of the inhibitors by western blot and complex formation or release of proapoptotic BCL-2-interacting mediator of cell death (BIM), an important downstream activator of BCL-2-associated X protein,18 by BIM coimmunoprecipitation analyses. Importantly, we found varying levels of the antiapoptotic target proteins, their ratios, and their binding to BIM among different leukemias in cell lines (supplemental Figure 4A) and PDX samples (supplemental Figure 4C). However, we did not find a clear association between these parameters and inhibitor sensitivities in cell lines (supplemental Figure 4A-B) or PDX samples (supplemental Figure 4C-D). Notably, our study found no significant differences in EC50 values between samples from patients with or without relapse, although this analysis does not fully capture the complexity of relapsed ALL. In acute myeloid leukemia, acquired multidrug resistance has recently been linked to low apoptotic priming.19 Additionally, our recent findings in ALL show that cells with acquired venetoclax resistance show decreased BCL-2 dependence, higher MCL-1 dependence, and increased mitochondrial metabolic activity, indicating that leukemia cells can adapt their antiapoptotic dependencies and metabolic pathways to survive therapeutic pressure.13 In this study, the intrinsic sensitivity of leukemia samples to BH3 mimetics could not be fully explained by leukemia characteristics, such as cytogenetic alterations, or by levels or ratios of the target proteins or their complexes with BIM, suggesting additional contributing factors. However, further studies with larger cohorts are needed to conclusively determine whether these or other parameters within the apoptotic regulatory network could serve as predictive biomarkers for the response of ALL toward BH3 mimetics. Integrating results from both cell lines and PDX models provides crucial insights into drug efficacy, resistance mechanisms, and toxicity, guiding effective therapeutic strategies for ALL.
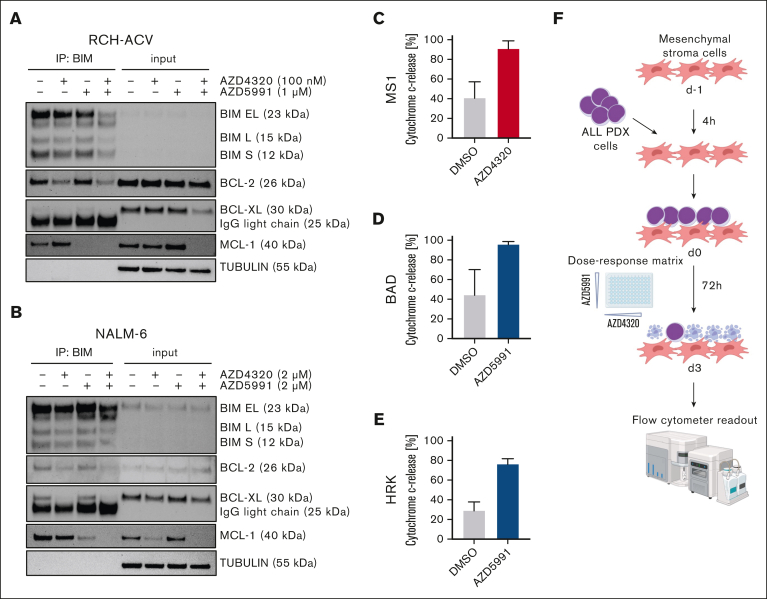
Next, we sought to analyze the molecular mechanisms by which AZD4320 and AZD5991 mediate cell death in ALL cells. Recent studies have shown that BH3 mimetics can induce apoptosis signaling by releasing proapoptotic proteins from their antiapoptotic binding partners.7,20 After BCL-2/BCL-XL (AZD4320) inhibition, we found substantially reduced binding of BIM to both BCL-2 and BCL-XL in RCH-ACV (Figure 2A; supplemental Figure 5A) and NALM-6 (Figure 2B; supplemental Figure 5B) cells. Additionally, on-target activity was observed by selective MCL-1 inhibition using AZD5991. Remarkably, coincubation with both compounds resulted in the release of BIM from all 3 antiapoptotic regulators (Figure 2A-B; supplemental Figure 5).
Figure 2.
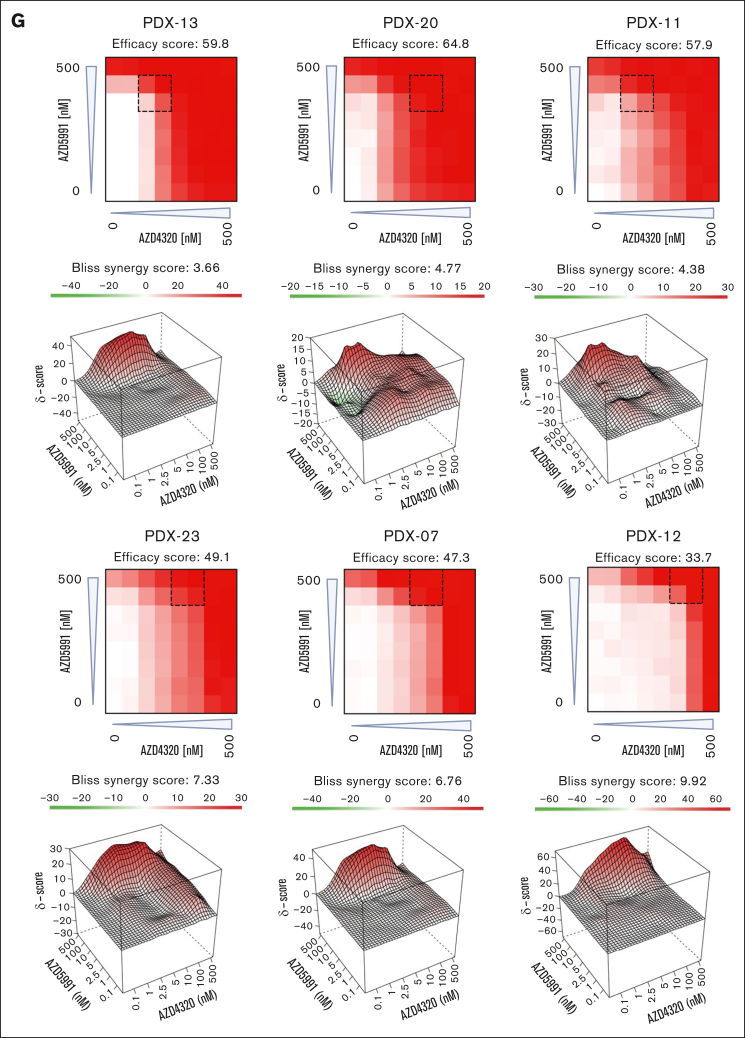
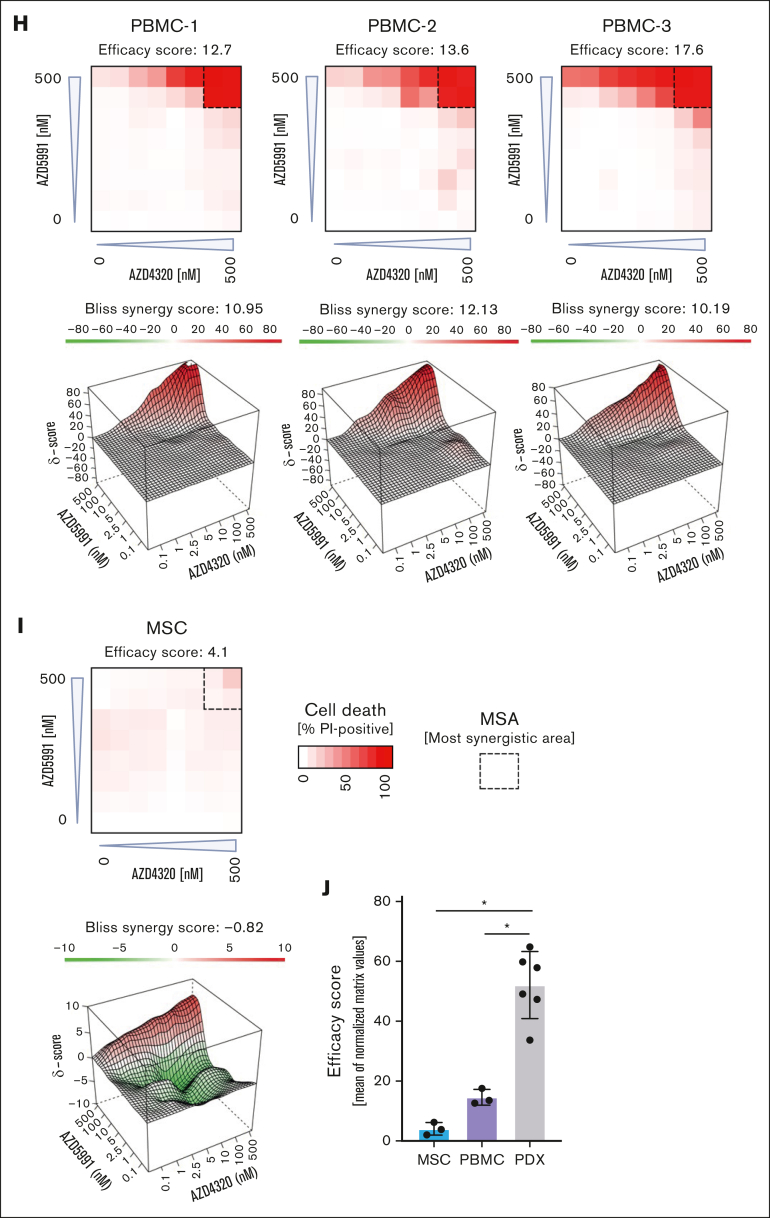
Cell death signaling induced by combined targeting of antiapoptotic proteins. (A-B) The interactions of proapoptotic and antiapoptotic proteins were analyzed upon exposure of leukemia cells to apoptosis-inducing drugs by coimmunoprecipitation (IP). RCH-ACV cells (A) were exposed to AZD4320 (100 nM) and/or AZD5991 (1 μM) and NALM-6 cells (B) to AZD4320 (2 μM) and/or AZD5991 (2 μM) for 4 hours. The IP lanes show the interactions of BCL-2, BCL-XL, and MCL-1 with BIM, and the input lanes show whole protein lysates. Representative immunoblots of 2 independent experiments are shown. (C-E) Dynamic BH3 profiling was performed in RCH-ACV cells. The cells were treated with 10-nM AZD4320 (C) or 100-nM AZD5991 (D-E) for 2 hours. After permeabilization, the cells were exposed to 0.3 μM of the proapoptotic BH3-peptide BAD (binding to BCL-2, BCL-W, and BCL-XL), 30-μM HRK (BCL-XL), or 10-μM MS1 (MCL-1), followed by fixation and staining with an anticytochrome c antibody that selectively binds to mitochondrial cytochrome c. Delta priming was calculated as the percentage of drug-induced cytochrome c release minus the percentage of cytochrome c release induced by the control. The bar graphs show mean values ± standard deviations derived from 3 independent experiments in triplicates. (F) Schematic overview of ex vivo coculture of BCP-ALL PDX cells on MSCs. On day –1, MSCs were seeded in 96-well plates before the addition of ALL PDX cells. On day 0, samples were exposed to increasing concentrations (0.1, 1, 2.5, 5, 10, 100, and 500 nM) of AZD4320 and/or AZD5991 in a drug matrix for 72 hours. Cell death rates were determined by PI staining and flow cytometry measurement after drug exposure. Created with BioRender.com. (G-I) Heat maps showing cell death rates from dose-response matrix analyses of ALL PDX samples (G), PBMCs (H), and MSCs (I). Cell death rates were assessed by PI staining after 72 hours of drug exposure and coculture with MSCs. Efficacy scores were calculated as the mean of normalized cell death rates across the matrix. Synergy effects were visualized using SynergyFinder, and synergy scores were analyzed using the Bliss independence model. Dashed lines indicate the MSA. (J) Comparison of efficacy scores of the drug matrix analyses of AZD4320 and AZD5991 between ALL PDX samples, PBMCs, and MSCs. Bar graphs show mean ± standard deviation. Mann-Whitney U test; ∗P < .05. DMSO, dimethyl sulfoxide; MSA, most synergistic area.
To further validate these findings, we conducted dynamic BH3 profiling21,22 in RCH-ACV. Upon treatment with the dual BCL-2/BCL-XL inhibitor (AZD4320), we observed a marked increase in dependency on MCL-1 (Figure 2C). Conversely, when the cells were treated with the MCL-1 inhibitor AZD5991, an increased combined dependence on BCL-2 and BCL-XL was observed (Figure 2D-E). These results of alternating dependencies strongly suggest that cotargeting all 3 antiapoptotic proteins could overcome intrinsic resistance to apoptosis.
To validate these findings in a more physiologically relevant environment, we performed dose-response matrix analyses23 in ALL PDX cells cocultured with primary bone marrow human telomerase reverse transcriptase-immortalized mesenchymal stroma cells (MSCs)24,25 (Figure 2F). Samples were exposed to 7 increasing concentrations of AZD4320 and AZD5991, and the combination effects were assessed. We discovered higher cell death with high efficacy and positive Bliss synergy scores in all BCP-ALL samples tested, including samples that are less sensitive to the inhibitors alone (Figure 2G; supplemental Figure 6). We also conducted dose-response matrix analyses with peripheral blood mononuclear cells (PBMCs), which showed lower sensitivities than ALL cells (Figure 2H). Importantly, the most synergistic area was typically achieved at lower concentrations in ALL samples than PBMCs, suggesting efficacy at lower doses. Finally, we performed dose-response matrix analyses on MSCs (Figure 2I), demonstrating that MSCs do not undergo cell death. This indicates that the observed cell death in ALL cells is due to the effects of the compounds on the ALL cells themselves and not due to the absence of MSC support. Comparing the effects of combined targeting of antiapoptotic molecules revealed higher efficacy in inducing cell death in ALL cells than in PBMCs and MSCs (Figure 2J). Notably, our results indicate significantly higher efficacy in ALL cells, particularly at lower concentrations, suggesting a potential for reduced dosing in therapeutic applications. Previous studies have reported that AZD4320 shows transient thrombocytopenia as the most notable adverse effect,14,15 whereas AZD5991 has demonstrated favorable tolerability in preclinical models.10 However, further research is needed, including larger studies and in vivo validation, to elucidate the therapeutic potential and safety profiles of these treatments.
In summary, the data of this study indicate that combined BCL-2 and BCL-XL inhibition is an efficient treatment for BCP-ALL and that concurrent targeting of MCL-1 increases the induction of leukemia cell death. Thus, the use of synergistic combination therapies could be further evaluated to avoid side effects and toxicity associated with high doses of individual substances.
Leukemia samples were collected from patients after obtaining informed written consent in accordance with the institutional ethics review board guidelines.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
Acknowledgments: The authors acknowledge S. Essig, S. Volk, and U. Formentini for their technical help.
This research was supported by the German Research Foundation (SFB 1074; B6) (K.-M.D. and L.H.M.), the International Graduate School in Molecular Medicine Ulm (C.F., A.N., and J.B.), and the Advanced Clinician Scientist Programme from the Medical Faculty, Ulm University (F.S.).
Contribution: M.C.W., C.F., A.N., J.B., S.E., and F.S. conducted experiments and analyzed data; K.-M.D., L.H.M., and F.S. conceived the study, interpreted data, and wrote the manuscript; and all authors approved the manuscript.
Footnotes
The data presented in this study are available on request from the corresponding author, Felix Seyfried (felix.seyfried@uniklinik-ulm.de).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Seyfried F, Demir S, Horl RL, et al. Prediction of venetoclax activity in precursor B-ALL by functional assessment of apoptosis signaling. Cell Death Dis. 2019;10(8):571. doi: 10.1038/s41419-019-1801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alford SE, Kothari A, Loeff FC, et al. BH3 inhibitor sensitivity and Bcl-2 dependence in primary acute lymphoblastic leukemia cells. Cancer Res. 2015;75(7):1366–1375. doi: 10.1158/0008-5472.CAN-14-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leverson JD, Phillips DC, Mitten MJ, et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med. 2015;7(279) doi: 10.1126/scitranslmed.aaa4642. [DOI] [PubMed] [Google Scholar]
- 4.Chonghaile TN, Roderick JE, Glenfield C, et al. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov. 2014;4(9):1074–1087. doi: 10.1158/2159-8290.CD-14-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson WH, O'Connor OA, Czuczman MS, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11(12):1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramsey HE, Fischer MA, Lee T, et al. A novel MCL1 inhibitor combined with venetoclax rescues venetoclax-resistant acute myelogenous leukemia. Cancer Discov. 2018;8(12):1566–1581. doi: 10.1158/2159-8290.CD-18-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seyfried F, Stirnweiss FU, Niedermayer A, et al. Synergistic activity of combined inhibition of anti-apoptotic molecules in B-cell precursor ALL. Leukemia. 2022;36(4):901–912. doi: 10.1038/s41375-021-01502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazaro-Navarro J, Pimentel-Gutierrez HJ, Gauert A, et al. Inhibiting casein kinase 2 sensitizes acute lymphoblastic leukemia cells to venetoclax via MCL1 degradation. Blood Adv. 2021;5(24):5501–5506. doi: 10.1182/bloodadvances.2021004513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhary GS, Al-Harbi S, Mazumder S, et al. MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis. 2015;6(1):e1593. doi: 10.1038/cddis.2014.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tron AE, Belmonte MA, Adam A, et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat Commun. 2018;9(1):5341. doi: 10.1038/s41467-018-07551-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caenepeel S, Brown SP, Belmontes B, et al. AMG 176, a selective MCL1 inhibitor, is effective in hematologic cancer models alone and in combination with established therapies. Cancer Discov. 2018;8(12):1582–1597. doi: 10.1158/2159-8290.CD-18-0387. [DOI] [PubMed] [Google Scholar]
- 12.Kotschy A, Szlavik Z, Murray J, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538(7626):477–482. doi: 10.1038/nature19830. [DOI] [PubMed] [Google Scholar]
- 13.Enzenmuller S, Niedermayer A, Seyfried F, et al. Venetoclax resistance in acute lymphoblastic leukemia is characterized by increased mitochondrial activity and can be overcome by co-targeting oxidative phosphorylation. Cell Death Dis. 2024;15(7):475. doi: 10.1038/s41419-024-06864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balachander SB, Criscione SW, Byth KF, et al. AZD4320, a dual inhibitor of Bcl-2 and Bcl-xL, induces tumor regression in hematologic cancer models without dose-limiting thrombocytopenia. Clin Cancer Res. 2020;26(24):6535–6549. doi: 10.1158/1078-0432.CCR-20-0863. [DOI] [PubMed] [Google Scholar]
- 15.Patterson CM, Balachander SB, Grant I, et al. Design and optimisation of dendrimer-conjugated Bcl-2/xL inhibitor, AZD0466, with improved therapeutic index for cancer therapy. Commun Biol. 2021;4(1):112. doi: 10.1038/s42003-020-01631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Punnoose EA, Leverson JD, Peale F, et al. Expression profile of BCL-2, BCL-XL, and MCL-1 predicts pharmacological response to the BCL-2 selective antagonist venetoclax in multiple myeloma models. Mol Cancer Ther. 2016;15(5):1132–1144. doi: 10.1158/1535-7163.MCT-15-0730. [DOI] [PubMed] [Google Scholar]
- 17.Waclawiczek A, Leppa AM, Renders S, et al. Combinatorial BCL2 family expression in acute myeloid leukemia stem cells predicts clinical response to azacitidine/venetoclax. Cancer Discov. 2023;13(6):1408–1427. doi: 10.1158/2159-8290.CD-22-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavathiotis E, Suzuki M, Davis ML, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455(7216):1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olesinski EA, Bhatia KS, Wang C, et al. Acquired multidrug resistance in AML is caused by low apoptotic priming in relapsed myeloblasts. Blood Cancer Discov. 2024;5(3):180–201. doi: 10.1158/2643-3230.BCD-24-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greaves G, Milani M, Butterworth M, et al. BH3-only proteins are dispensable for apoptosis induced by pharmacological inhibition of both MCL-1 and BCL-XL. Cell Death Differ. 2019;26(6):1037–1047. doi: 10.1038/s41418-018-0183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montero J, Sarosiek KA, DeAngelo JD, et al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell. 2015;160(5):977–989. doi: 10.1016/j.cell.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatt S, Pioso MS, Olesinski EA, et al. Reduced mitochondrial apoptotic priming drives resistance to BH3 mimetics in acute myeloid Leukemia. Cancer Cell. 2020;38(6):872–890890.e6e876. doi: 10.1016/j.ccell.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ianevski A, Giri AK, Aittokallio T. SynergyFinder 3.0: an interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res. 2022;50(W1):W739–W743. doi: 10.1093/nar/gkac382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mihara K, Imai C, Coustan-Smith E, et al. Development and functional characterization of human bone marrow mesenchymal cells immortalized by enforced expression of telomerase. Br J Haematol. 2003;120(5):846–849. doi: 10.1046/j.1365-2141.2003.04217.x. [DOI] [PubMed] [Google Scholar]
- 25.Manabe A, Coustan-Smith E, Behm FG, Raimondi SC, Campana D. Bone marrow-derived stromal cells prevent apoptotic cell death in B-lineage acute lymphoblastic leukemia. Blood. 1992;79(9):2370–2377. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.