Abstract
The early initiation of binge‐drinking and biological sex are critical risk factors for the development of affective disturbances and cognitive decline, as well as neurodegenerative diseases including Alzheimer's disease. Further, a history of excessive alcohol consumption alters normal age‐related changes in the pattern of protein expression in the brain, which may relate to an acceleration of cognitive decline. Here, we aimed to disentangle the interrelation between a history of binge‐drinking during adolescence, biological sex and normal aging on the manifestation of negative affect, cognitive decline and associated biochemical pathology. To this end, adolescent male and female C57BL/6J mice (PND 28–29) underwent 30 days of alcohol binge‐drinking using a modified drinking‐in‐the‐dark (DID) paradigm. Then, mice were assayed for negative affect, sensorimotor gating and cognition at three developmental stages during adulthood—mature adulthood (6 months), pre‐middle age (9 months) and middle age (12 months). Behavioural testing was then followed by immunoblotting to index the protein expression of glutamate receptors, neuropathological markers [Tau, p (Thr217)‐Tau, p (Ser396)‐Tau, BACE, APP, Aβ], as well as ERK activation within the entorhinal cortex, prefrontal cortex and amygdala. Across this age span, we detected only a few age‐related changes in our measures of negative affect or spatial learning/memory in the Morris water maze and all of these changes were sex‐specific. Prior adolescent binge‐drinking impaired behaviour only during reversal learning in 9‐month‐old females and during radial arm maze testing in 12‐month‐old females. In contrast to behaviour, we detected a large number of protein changes related to prior binge‐drinking history, several of which manifested as early as 6 months of age, with the prefrontal cortex particularly affected at this earlier age. While 6‐month‐old mice exhibited relatively few alcohol‐related protein changes within the entorhinal cortex and amygdala, the number of alcohol‐related protein changes within the entorhinal cortex increased with age, while the 12‐month‐old mice exhibited the largest number of protein changes within the amygdala. Approximately a third of the alcohol‐related protein changes were sex‐selective. Taken together, the results of our longitudinal study using a murine model of binge‐drinking indicate that a prior history of heavy alcohol consumption, beginning in adolescence, is sufficient to induce what we presume to be latent changes in protein indices of cellular activity, glutamate transmission and neuropathology within key brain regions governing cognition, executive function and emotion that appear to precede the onset of robust behavioural signs of dysregulated affect and cognitive impairment.
Keywords: Alzheimer's disease, glutamate receptors, Morris water maze, negative affect, radial arm maze
Immunoblotting for glutamate receptor‐related signaling and markers of neuropathology conducted on the prefrontal cortex, entorhinal cortex and amygdala of 6, 9 and 12‐month‐old C57BL/6J mice with a prior history of binge‐drinking during adolescence revealed a number of protein changes, many of which were sex‐, age‐ and/or region‐selective. Curiously, despite these changes in protein expression, we detected little evidence for overt effects of prior binge‐drinking history on working, reference or spatial memory, particularly in 6‐ and 9‐month‐old mice. These data indicate that adolescent binge‐drinking can induce very long‐lasting changes in indices of glutamate signaling and neuropathology within key brain regions governing executive function, learning, memory and affect and that these protein adaptations may precede behavioral anomalies.
1. INTRODUCTION
Adolescence is a pivotal stage of behavioural and neurological development, characterized by significant maturation of brain regions governing emotion and cognition. 1 , 2 , 3 This developmental period is also characterized by an increased propensity for risky behaviours, including alcohol binge‐drinking. 4 , 5 , 6 Although adults consume alcohol more frequently, adolescents between the ages of 12 to 20 years old account for approximately 11% of the total alcohol intake in the United States, predominately through binge‐drinking. 7 This pattern of drinking is typically defined as four or more drinks per occasion for women and five or more for men. 8 Over 90% of adolescent alcohol intake occurs through binge‐drinking episodes. 7 , 9 , 10 The neurotoxic impact of this binge pattern of consumption, marked by repeated heavy drinking episodes followed by periods of cessation, has been shown to exacerbate the potential for neurological harm 11 and is highly associated with the development of an alcohol use disorder (AUD) in adolescence. 12
Extant literature from human studies consistently indicates that a history of binge‐drinking heightens susceptibility to mood disorders and cognitive deficits during alcohol withdrawal, with females experiencing these effects more severely than males. 13 , 14 , 15 , 16 These observed sex differences are particularly troubling considering that women are more frequently diagnosed with depression and anxiety, disorders that are commonly exacerbated by their history of heavy alcohol consumption. 17 , 18 The cognitive repercussions of excessive alcohol in females are similarly disproportionate, with greater deficits in memory retention and spatial navigation capabilities. 19 Moreover, women with a history of excessive alcohol consumption also show an increased incidence of cancer, accelerated liver problems and exacerbated cardiovascular complications. 20 , 21 , 22 The severity of these health concerns is even more alarming given the recent epidemiological trends revealing an 84% surge in excessive drinking among women, more than double the increase observed in men over the same period. 15 , 23 Additionally, there is a high co‐occurrence of AUD and early‐onset dementia, with AUD being a factor in nearly 60% of these dual diagnoses. 24 This association is particularly concerning for women who have a twofold increased risk of developing alcohol‐related dementias, including Alzheimer's disease (AD), compared to men, 25 and is compounded by evidence that AUD among older female veterans is correlated with a more than threefold increase in dementia risk. 26
Evidently, an early onset of binge‐drinking and biological sex serve as strong and important predictors for alcohol‐related affective and cognitive disturbances, including Alzheimer's disease and related dementias (ADRDs). Although the biological underpinnings of these behavioural impairments are still not entirely understood, it is theorized that adolescent binge‐drinking leads to neuronal alterations and the eventual degradation of key brain regions governing affective and cognitive processes. 27 , 28 , 29 Notably, adolescents with AUD exhibit disrupted neurodevelopmental trajectories, impacting the executive functions of the prefrontal cortex (PFC), including decision‐making and impulse control, with females showing more pronounced volumetric reductions within the PFC versus males. 30 Adolescents with AUD also exhibit volumetric reductions of the hippocampus (HPC), a key region central to memory and learning. 31 , 32 The entorhinal cortex (EC) connects to the HPC via the perforant pathway 33 and is important for memory consolidation and spatial navigation. 34 Notable cellular changes, including the reduction of neuronal nuclei size in layers II and III, have been observed in the EC of young and middle‐aged humans 33 and replicated in adolescent rats following a history of chronic alcohol use. 35 Further, the EC is especially vulnerable in AD, sustaining the most extensive cortical damage, 36 with animal studies suggesting that EC disruptions may serve as an early marker of ADRD pathology. 37 The amygdala (AMY), a core region for emotional processing and memory, 38 has also been identified as an early site of AD‐related pathological changes, including synaptic disruptions and volumetric reductions in humans. 39 In parallel, transgenic mouse models exhibit similar AD‐related molecular markers such as amyloid pathology within the AMY, along with cognitive impairments apparent from as early as 4 months of age. 40 Notably, these AD‐related changes are most evident in the AMY of adult female 3xTg‐AD transgenic mice following binge alcohol exposure during adolescence, in contrast to male mice, which, despite having a similar history of adolescent alcohol use, fail to show persistent increases in AD biomarkers in adulthood. 41 Such sex‐dependent differences in ADRD‐related biomarker prevalence emphasizes the AMY's vulnerability and its role in the development ADRD‐related pathology.
Cellular and biochemical studies of rodent models of AUD support a cause‐effect relationship between alcohol experience and perturbed emotionality and cognitive deficits. 41 , 42 , 43 , 44 Our laboratory's previous work with C57BL6/J (B6) mice demonstrated that a 2‐week history of binge‐drinking initiated in adolescence can elicit biochemical changes within several mesocorticolimbic regions, 45 , 46 , 47 which manifest during protracted withdrawal as a spectrum of behavioural anomalies from hyperactivity to depression‐like symptoms. 45 , 47 , 48 , 49 Moreover, both adolescent and adult female mice not only consume greater amounts of alcohol but also tend to exhibit more pronounced signs of alcohol‐induced behavioural anomalies than males. 42 , 48 , 49 A history of repeated alcohol exposure is well‐characterized to augment both pre‐ and postsynaptic indices of glutamate transmission throughout the brain 50 , 51 , 52 and alcohol‐induced glutamate excitotoxicity is theorized to contribute significantly to alcohol‐related neurodegeneration underpinning the loss of executive function, volitional control and cognitive decline. 53 , 54 Consistent with this, young adult (~2.5 months old) B6 mice with a prior 2‐week history of adolescent binge‐drinking exhibit increased protein expression of key glutamatergic signalling proteins, including group 1 metabotropic glutamate receptors (mGlu1, mGlu5), ionotropic glutamate receptor subunits (AMPA and NMDA), the glutamate receptor‐associated scaffolding proteins Homer 1b/c and Homer 2a/b, that correlate with heightened negative affect. 44 , 45 , 46 , 47 , 55 , 56 , 57 Such findings argue that a history of adolescent binge‐drinking can produce latent effects in the brain and behaviour that manifest later in adulthood. 41 , 58 , 59 , 60 However, our more recent study indicates that the brain and behavioural disturbances instigated by a 2‐week history of adolescent binge‐drinking may not persist throughout adulthood, as they were less apparent in adult mice tested ~ 4 months of age. 42
Building upon our previous research, the present study explored the biobehavioural effects of a more prolonged, 1‐month, history of binge‐drinking that commences in early adolescence by tracking the course of affective and cognitive anomalies at three later developmental stages, 6, 9 and 12 months of age (i.e. from mature adulthood to middle age 61 ). Guided by our prior immunoblotting work 44 , 45 , 46 , 47 , 56 and evidence that a history of binge‐drinking during mature adulthood is sufficient to elevate certain protein indices of ADRD‐related neuropathology within the brain during early withdrawal, 48 we examined for changes in glutamate receptor‐related protein expression within the PFC, HPC, EC and AMY, as well as indices of ADRD‐related neuropathology, including BACE isozyme 62 , 63 and phospho‐tau expression. 64 , 65 , 66 We hypothesized that a 1‐month long history of binge‐drinking during adolescence and into young adulthood would accelerate the onset and progression of normal age‐related cognitive and affective anomalies, particularly in female subjects. Secondly, we hypothesized that behavioural anomalies would be associated with heightened indices of glutamatergic signalling and markers of neuropathology. To the best of our knowledge, this study is the first to examine the biobehavioural consequences of a history of binge‐drinking during adolescence across multiple developmental time‐points in later life of relevance to the aetiology and ontogeny of AUDs.
2. METHODS
2.1. Subjects
Male and female C57BL/6J (B6) mice, PND21–25, were purchased from the Jackson Laboratory (Sacramento, CA) and allowed to acclimate to the colony room for 1 week prior to commencing drinking procedures (see below). Mice were housed in same‐sex groups of four in standard polycarbonate cages on a ventilated rack in a climate‐ and humidity‐controlled holding room. Cages were lined with sawdust bedding and contained nesting material and a plastic enrichment device in accordance with vivarium protocols. All mice were housed under a reverse light cycle (lights off: 1100 hours; lights on: 2300 hours), with food and water available ad libitum throughout the study. Mice arrived in cohorts of 48 (24 females and 24 males), with cohorts spaced approximately 1 month apart to accommodate drinking procedures. All experimental procedures were in compliance with The Guide for the Care and Use of Laboratory Animals (2014) and approved by the Institutional Animal Care and Use Committee of the University of California, Santa Barbara. A summary of the procedural time‐line and the sample sizes employed for this study are provided in Figure 1.
FIGURE 1.
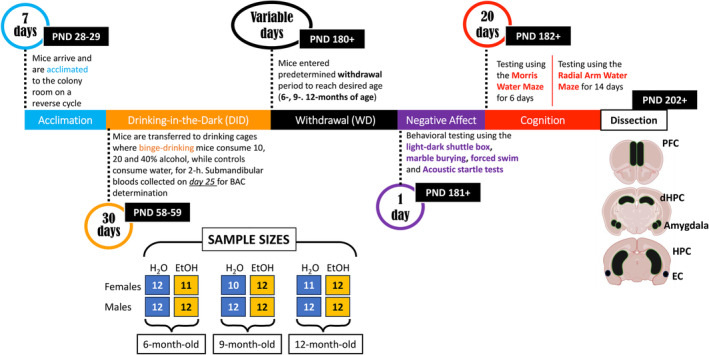
Illustration of the procedural timeline of the experiments conducted in the current study and representation of the sample size distributions used across the different experimental groups.
2.2. Drinking‐in‐the‐dark (DID) procedures
Half of the mice in each cohort (i.e. 12 males and 12 females) were randomly assigned to either an alcohol or water‐drinking group. The first 2 cohorts of mice to arrive at our facility were designated to be tested at 12 months of age, while the next 2 cohorts of mice were designated to be tested at 9 months of age, while the last 2 cohorts were designated to be tested at 6 months of age. This assignment was done to simply the organization of this large study and reduce the time interval between the testing of the different age groups to minimize environmental confounds during cognitive testing.
For all age‐designations, mice assigned to the alcohol‐drinking group were subjected to 30 consecutive days of alcohol‐drinking using a multi‐bottle‐choice DID procedure, beginning at approximately PND28–32. At 2 h after lights out (i.e. 1300 hours), alcohol‐drinking (EtOH) animals were transferred to individual drinking cages that were lined with sawdust bedding and topped with a wire lid, situated on a free‐standing rack within the colony room. Mice were allowed to habituate to the drinking cage for 1 h, at which time, alcohol‐drinking mice (EtOH) were allowed concurrent access to unadulterated ethanol 10, 20 and 40% (v/v) solutions in tap water as the results of prior studies in our laboratory indicate greater alcohol intake, on average, when mice are offered a choice between concentrations than under single‐bottle procedures (e.g. 46 , 49 , 67 ). The location of sipper tubes was randomized daily. Animals were allowed to drink for 2 h (1400–1600 hours). During the 1‐h habituation and 2‐h alcohol drinking periods, water control mice (H2O) underwent our simplified water drinking procedures in which daily handling and removal from the home cage were controlled for by placing H2O mice, with their cage mates, into a novel drinking cage on the same free‐standing rack as the EtOH mice for 1 h and then presenting them with a single sipper tube containing water for the 2‐h drinking period (e.g. 42 , 49 , 56 , 57 , 68 ). At 1600 hours, the sipper tubes were removed from the drinking cages and both the EtOH and H2O mice were then transferred back into their home cages.
For all cohorts, the alcohol‐containing sipper tubes were weighed prior to, and immediately following, each 2‐h drinking session to determine the volume consumed. The alcohol/water in the bottles was refreshed and all the mice were weighed every 3–4 days during the month‐long drinking procedures. The recorded body weights of the mice were used to calculate alcohol intake on a g/kg body weight basis.
2.3. Blood ethanol concentration
On the 25th drinking day, submandibular blood samples were collected from the alcohol‐drinking mice only, immediately after the 2‐h alcohol‐drinking period and samples were stored at −20°C until processing (7–10 days following collection). Headspace gas chromatography using a Shimadzu GC‐2014 gas chromatography system (Shimadzu, Columbia, MD) was employed to analyse blood ethanol concentrations (BECs) as in recent reports (e.g. 42 , 43 , 48 ). BECs were determined via the GC Solutions 2.10.00 software in samples diluted at 1:9 with non‐bacteriostatic saline (50 μL of sample). Toluene was used as the pre‐solvent and the determination of ethanol from each sample was derived using the standard curve equation determined prior to analyses of the blood samples. A new standard curve was formulated for each cohort of blood samples to ensure maximal accuracy. After the ethanol peak area was determined, the peak area was used to determine the ethanol concentration and subsequently the percent of ethanol in the blood.
2.4. Behavioural test battery for negative affect
To test the hypothesis that a 1‐month history of binge‐drinking during the period of adolescence into young adulthood might induce long‐lasting changes in negative affect, a 1‐day behavioural test battery for negative affect was conducted when the mice were 6, 9 or 12 months of age (respectively, 6M, 9M and 12M). As in our prior studies (e.g. 42 , 48 , 49 , 69 ), this behavioural test battery consisted of the light–dark shuttle‐box, marble‐burying, acoustic startle and forced swim test, which were run in series and mice remained in their home cages in the procedural room between paradigms. The light–dark box, marble‐burying and forced swim tests were selected based on earlier work indicating that they are more reliably sensitive to the anxiogenic effects of early alcohol withdrawal in our binge‐drinking models than other assays of anxiety‐like behaviour and negative affect (e.g. elevated plus‐maze; see 46 , 49 , 69 ). While we have not detected effects of alcohol withdrawal on acoustic startle exhibited by mice with adolescent‐ or early adult‐onset binge‐drinking history (e.g. 46 , 49 , 69 ), a robust alcohol‐related deficit is observed in 18‐month‐old mice. 43 Thus, we included a test of acoustic startle and prepulse inhibition of acoustic startle in this study. The behavioural testing equipment was cleaned in‐between each use with Rescue Disinfectant Veterinary Wipes (Virox Animal Health, Oakville, ON, Canada). The details of each specific assay are provided below. Consistent with recent studies, 42 , 48 males and females were tested for negative affect on separate days to minimize any pheromonal influences on affective behaviour 70 and the experimenters were blind to the prior drinking history of the mice throughout all aspects of behavioural testing in this study.
2.4.1. Light–dark shuttle‐box
The light–dark shuttle‐box was used to measure photophobia, with decreased activity in the light‐side interpreted as reflecting an anxiety‐like phenotype. 71 , 72 Animals were placed into a polycarbonate box (46 cm long × 22 cm wide × 24 cm high) that was divided into two environments, one side is white with a clear lid and the other side was black with a black lid (respectively, light versus dark side) that were accessible through a central divider with an opening. Testing commenced by placing the mice into the dark environment. The latency to enter the light side, total time spent in the light side and total number of light entries were recorded over a 5‐min period using digital video cameras mounted above the test apparatus and ANYMaze software (Stoelting, Wood Dale, IL).
2.4.2. Marble‐burying test
The marble‐burying test is particularly sensitive to the anxiogenic effects of alcohol withdrawal, based on our prior work with adolescent and young adult (i.e. 2–3‐month‐old) mice (e.g. 42 , 45 , 46 , 47 , 49 , 56 , 69 ). For this assay, mice were placed in a polycarbonate cage (12 cm × 8 cm × 6 cm), with 5‐cm deep sawdust bedding on top of which 20 black marbles were arranged equidistantly. Mice were left undisturbed for a period of 20 min at which time, the number of marbles buried (i.e. 75% covered by bedding) was counted by an experimenter who was blind to the drinking history of the mice.
2.4.3. Acoustic startle and pre‐pulse inhibition of acoustic startle
The apparatus and procedures employed to assay the magnitude of acoustic startle and prepulse inhibition of acoustic startle were similar to those described previously by our group (e.g. 73 , 74 , 75 ). Six different trial types were presented: startle pulse (st110, 110 dB/40 ms, low prepulse stimulus given alone (st74, 74 dB/20 ms), high prepulse stimulus given alone (st90, 90 dB/20 ms), st74 or st90 given 100 ms before the onset of the startle pulse (pp74 and pp90, respectively) and no acoustic stimulus (i.e. only background noise was presented; st0). St110, st0, pp74 and pp90 trials were applied 10 times, st74 and st90 trials were applied five times, and all trials were given in random order. The average intertrial interval was 15 s (10–20 s), and the background noise of each chamber was 70 dB. The data for startle amplitude were averaged across each of the stimulus trial types for statistical analyses of startle magnitude. The percent inhibition of the 110 dB startle by the 74‐ and 90‐dB prepulse intensities was also calculated for each animal.
2.4.4. Forced swim test
The forced swim test is a commonly employed assay for the reversal of passive coping behaviour by anti‐depressant treatments. 76 Excessive swimming behaviour in this assay can be reversed by pretreatment with anxiolytic medications 47 and thus, has been used by our group as an additional measure of anxiety‐like behaviour during alcohol withdrawal (e.g. 42 , 48 , 49 , 56 , 57 ). The swim ‘tank’ consists of an 11‐cm‐diameter cylindrical glass container, filled to 15 cm from the rim, with room temperature water. Mice are lowered into the tank and tested over a 6‐min period during which AnyMaze™ tracking software records the latency to first immobile episode, total time spent immobile and the number of immobile episodes. Immobility is defined as the lack of vertical or horizontal displacement of the animal's centre of gravity for at least 5 s. Upon the conclusion of this assay, animals were allowed to dry prior to being returned to their home cage and the holding room.
2.5. Morris water maze
The day following testing for negative affect, mice were assayed for spatial learning and memory using Morris water maze procedures akin to those published previously by our laboratory (e.g. 48 , 73 , 77 ). The maze consisted of a stainless‐steel circular tank (200 cm in diameter, 60 cm in height; filled with room temperature water to a depth of 40 cm), with salient intra‐maze cues located on all four sides of the tank (star, square, sun and stripes). To ensure equivalent visual processing in all mice at the outset of each experiment, a ‘flag test’ was first performed, in which the clear platform was placed in the tank in the NW quadrant with a patterned flag attached that extended 6 in. above the water. Over the course of the next 4 days, the clear platform (unflagged) remained in a fixed location in the NE quadrant (i.e. a quadrant distinct from that employed in the flag test). Each day, mice were trained four times a day (once at each compass point) to locate the hidden platform. During each trial, mice were randomly placed in the pool at one of the four compass points and swimming was recorded digitally by a video camera mounted on the ceiling directly above the pool (ANY‐Maze, Stoelting). Training sessions were 2 min in duration and mice were tested in series at each compass release point. Mice unable to locate the platform during the allotted time were guided to the platform using forceps, where they remained for 30 s. At 24 h after the last training trial, a 2‐min memory probe test was performed in which the platform was removed from the pool and the amount of time taken by the mouse to swim toward the former platform location and the number of entries into the former platform location was recorded. The next day, a reversal training session was conducted in which the platform (unflagged) was situated in the SW quadrant (i.e. the quadrant opposite to that employed during the training phase of the experiment). Again, mice were trained to locate the platform over four, 2‐min, sessions (one training trial for each compass point) to locate the repositioned platform. 48 , 77
2.6. Water version of the radial arm maze
Following the Morris maze testing, working and reference memory were determined using a water version of the radial arm maze with procedures similar to those employed in our prior studies. 48 , 77 The maze consisted of 8 arms with clear, hidden, escape platforms at the ends of 4 of the arms. The start arm was the same for all the mice and remained constant throughout. Each mouse was assigned different platform locations that remained fixed throughout the experiment and the baited arms were semi‐randomly assigned across subjects. A subject had 180 s to locate a platform. If the mouse was unsuccessful at locating a platform in the allotted time, it was guided to the nearest available platform using forceps. Once a platform was found, the animal remained on it for 15 s and was then returned to an empty, heated, holding cage for 30 s. During that time, the located platform was removed from the maze. The animal was then placed back into the start arm and allowed to locate another platform. Each day, this sequence of events repeated until the mouse located all four platforms. Thus, each mouse underwent four trials per day, with the working memory system taxed increasingly with each trial. As in the land version of this maze, animals have to avoid arms that never contained a reinforcer (reference memory) and enter only once into arms that contained a reinforcer (working memory). Day 1 was considered a training session because the animal had no previous experience in the maze. Days 2–7 were testing sessions and errors were quantified for each day using the orthogonal measures of working and reference memory errors, 78 as conducted previously by our group 48 , 77 and others. 79 Working memory correct errors were the number of first and repeat entries into any arm from which a platform had been removed during that session. Reference memory errors were the number of first entries into any arm that never contained a platform. Working memory incorrect errors were the number of repeat entries into an arm that never contained a platform in the past (thus, repeat entries into a reference memory arm).
2.7. Tissue dissection and immunoblotting
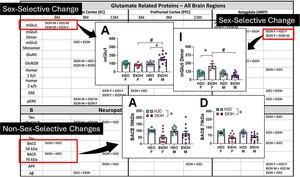
As recent immunoblotting studies indicated interactions between age, sex and a history of binge‐drinking on the expression of glutamate receptor‐related proteins, as well as protein indices of ADRD‐related neuropathology, within the PFC and hippocampus of 6M and 18M B6 mice, 44 we determined whether a prior history of binge‐drinking during early life could accelerate age‐related changes in these proteins in a sex‐dependent manner. For this, mice employed in the behavioural study were decapitated approximately 24 h following the last radial arm maze session. Brains were extracted and cooled on ice, then the brain was sectioned in 1‐mm‐thick coronal slices. The PFC was dissected out using blunt forceps, the EC and amygdala were dissected using an 18‐gauge needle and then both the ventral and dorsal hippocampus removed with blunt forceps and tissue from both hippocampal subregions combined into a single sample (see Figure 1). Unfortunately, the hippocampal samples were accidentally subjected to over‐heating, resulting in the degradation of protein and could not be processed. Thus, only the data for the PFC, EC and amygdala are presented herein.
To index total NMDA receptor expression, we immunoblotted for the obligatory GluN1 subunit. We also immunoblotting for GluN2b expression as GluN2b is well‐characterized to be highly alcohol‐sensitive (e.g. 80 ), is up‐regulated in a number of brain regions in adult mice with a history of binge‐drinking 44 , 45 , 46 , 47 , 56 , 67 , 81 , 82 and most relevant to this study, is upregulated in adult rodents following a history of adolescent alcohol exposure. 83 Likewise, both the mGlu1 and mGlu5 subtypes of mGluRs are also typically up‐regulated in mice with a history of binge‐drinking, 44 , 67 , 81 , 82 to include adult mice with a history of binge‐drinking during adolescence. 45 , 46 , 47 , 56 The signalling and localization of both NMDA and group 1 mGluRs are regulated in brain by the Homer1b/c and Homer2a/b members of the Homer family of scaffolding proteins, 84 , 85 of which Homer2a/b is highly alcohol‐sensitive and gates the rewarding/reinforcing and sedative properties of alcohol, 67 , 81 , 82 , 86 , 87 as well as the manifestation of negative affect during protracted withdrawal from adolescent binge‐drinking. 68 Thus, we immunoblotted also for Group 1 mGluRs and their Homer scaffolding proteins. As it was predicted that an upregulation of glutamate receptor expression would increase the activational state of our regions of interest, we examined for p (Tyr204) ERK1/2 expression as an index of cellular activity. A number of proteins currently serve as strong and reliable biomarkers of AD in human brain 88 , 89 , 90 , 91 , 92 , 93 that can accumulate in brain during normal aging in both humans 94 , 95 , 96 , 97 and induced by prior alcohol experience in laboratory rodents. 44 , 98 , 99 , 100 Thus, we assayed also for the following proteins: amyloid precursor proteins (APP), amyloid‐β peptides (Aβ), hyper‐phosphorylated tau proteins and beta secretase (BACE).
The tissue homogenization and immunoblotting procedures employed in the present study were very similar to those detailed in our earlier reports. 44 , 77 , 101 , 102 The following rabbit primary antibodies were used: mGlu5 (metabotropic glutamate receptor 5; 1:1000 dilution; Millipore; AB5675), GluN1 (NMDA receptor subunit 1; 1:500 dilution; Cell Signaling Technology; 5704S), Homer2a/b (1:500 dilution; Synaptic Systems; 160 203), p (Tyr204)ERK1/2 (1:750 dilution; R&D systems; AF1018), APP (1:1000 dilution; Millipore‐Sigma; 07‐667), amyloid beta (1:500 dilution; Abcam, ab180956), p (Ser396)‐tau (1:750 dilution; Abcam; ab109390) and p (Thr217)‐tau (1:500 dilution; Invitrogen, 44‐744). The following mouse primary antibodies were also employed: mGlu1 (metabotropic glutamate receptor 1; 1:500 dilution; BD Biosciences; 610965), GluN2b (NMDA subunit 2b; 1:500 dilution; Invitrogen; MA1‐2014), Homer1b/c (1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA; sc‐25271), ERK1/2 (1:1000 dilution; Invitrogen, MA5‐15605), tau (1:750 dilution; Invitrogen, AHB0042) and BACE (1:500 dilution; Millipore Sigma; MAB5308). Note that as reported in our earlier study, 102 our selected mGlu1 antibody failed to reliably detect the dimer form of the receptor on every immunoblot. As such, only the monomer form of mGlu1 is reported herein. Calnexin expression was employed to control for protein loading and transfer using either a rabbit or mouse primary anti‐calnexin antibody (for rabbit, 1:1000 dilution; Enzo Life Sciences; ADI‐SPA‐860; for mouse, 1:500 dilution; Invitrogen, MA5‐31501). Following primary antibody incubation, the membranes were washed with phosphate‐buffered saline with tween (PBST), incubated in either a goat anti‐rabbit IRDye 800CW secondary antibody (1:10,000 dilution; Li‐Cor; 925‐3221) or a goat anti‐mouse IRDye 680RD secondary antibody (1:10,000 dilution; Li‐Cor; 925‐68070), and imaged on an Odyssey Infrared Imaging System (Li‐Cor Biosciences, Lincoln, NE, USA). Raw values for each band were measured, and first normalized to their corresponding calnexin signal and then to the average value of the water control for that particular age and sex (see more details below).
2.8. Data analyses
We initially conducted a comprehensive three‐way factorial analysis of variance (ANOVA) with the variables of sex (males vs. females), age (6, 9, 12 months) and drinking history (EtOH vs. H2O) as independent variables to examine their impact on measures of negative affect. This analysis identified a single significant sex difference (see Table S1). Due to the complexity of our experimental design, we opted to conduct separate statistical analyses for males and females to enhance the clarity and interpretability of our behavioural findings. For variables associated with negative affect, we employed an age (6M, 9M and 12M) × drinking history (H2O vs. EtOH) univariate ANOVA, with age and drinking history as between‐subject factors. Data from the acoustic startle test were analysed using an age × drinking history × stimulus ANOVA, with repeated measures on the stimulus factor (4 levels). Data for prepulse inhibition of acoustic startle were analysed using an age × drinking history × prepulse ANOVA, with repeated measures on the prepulse factor (2 levels). Data from the maze tests were examined using an age × drinking history ANOVA, with day or trial as a repeated measure, when appropriate. For the immunoblotting data, we employed a sex × drinking history ANOVA. As this study employed 12 experimental conditions, immunoblotting procedures were performed independently for 6M, 9M and 12M mice, separately for both sexes. For each individual gel, results were normalized separately by sex in relation to the average of the control group (i.e. water‐drinking mice). This approach yielded interaction effects that mirrored observations related to the sex factor. Thus, we report on the interaction effect (sex × drinking history ANOVA), as well as the main effect of drinking history. A complete set of statistical outcomes, including both significant and non‐significant findings, is presented in Table S1 for the behavioural outcomes and in Tables 1, 2 and 3 for the immunoblotting data.
TABLE 1.
Summary of the quantitative analysis of proteins implicated in (A) glutamate function (B) and neuropathological processes within the entorhinal cortex. Significant results are bolded.
| A | Glutamate‐related proteins—entorhinal cortex | |||
|---|---|---|---|---|
| Protein of interest | Age (months) | Main effect: drinking history | Interaction effect: sex by drinking history | Significant group comparisons |
| mGlu1 | 6M | Not significant |
Significant F(1,34) = 7.71, p = 0.009, η 2 = 0.185 |
Female EtOH = Female H2O (p = 0.342) Male EtOH > Male H 2 O (p = 0.006) Female EtOH < Male EtOH (p < 0.001) Female H2O = Male H2O (p = 1.000) |
| 9M |
Not significant p = 0.255, η 2 = 0.039 |
Not significant F(1,33) = 3.74, p = 0.062, η 2 = 0.102 |
None | |
| 12M |
Not significant p = 0.960, η 2 = 0.000 |
Not significant F(1,31) = 1.11, p = 0.301, η 2 = 0.034 |
None | |
| mGlu5 dimer | 6M |
Not significant p = 0.737, η 2 = 0.003 |
Not significant F(1,39) = 0.01, p = 0.927, η 2 = 0.000 |
None |
| 9M |
Not significant p = 0.376, η 2 = 0.024 |
Not significant F(1,33) = 1.04, p = 0.315, η 2 = 0.031 |
None | |
| 12M |
Not significant p = 0.262, η 2 = 0.043 |
Not significant F(1,29 = 0.14, p = 0.716, η 2 = 0.005 |
None | |
| mGlu5 monomer | 6M |
Not significant p = 0.771, η 2 = 0.002 |
Not significant F(1,35) = 1.99, p = 0.167, η 2 = 0.054 |
None |
| 9M |
Significant F(1,34) = 4.73, p = 0.037, η 2 = 0.122 |
Not significant F(1,34) = 1.02, p = 0.319, η 2 = 0.029 |
EtOH < H 2 O (p = 0.037) | |
| 12M |
Significant F(1,27) = 12.59, p = 0.001, η 2 = 0.318 |
Not significant F(1,27) = 2.04, p = 0.164, η 2 = 0.070 |
EtOH > H 2 O (p = 0.001) | |
| GluN1 | 6M |
Not significant p = 0.065, η 2 = 0.087 |
Not significant F(1,38) = 0.63, p = 0.434, η 2 = 0.016 |
None |
| 9M |
Not significant p = 0.967, η 2 = 0.000 |
Not significant F(1,38) = 0.07, p = 0.797, η 2 = 0.002 |
None | |
| 12M |
Not significant p = 0.245, η 2 = 0.050 |
Significant F(1,27) = 1.91, p = 0.010, η 2 = 0.220 |
Female EtOH = Female H2O (p = 0.317) Male EtOH > Male H 2 O (p = 0.005) Female EtOH < Male EtOH (p = 0.001) Female H2O = Male H2O (p = 1.000) |
|
| GluN2B | 6M |
Not significant p = 0.075, η 2 = 0.003 |
Not significant F(1,40) = 0.68, p = 0.416, η 2 = 0.017 |
None |
| 9M |
Significant F(1,34) = 7.71, p = 0.002, η 2 = 0.244 |
Significant F(1,34) = 7.71, p = 0.009, η 2 = 0.185 |
Female EtOH = Female H2O (p = 0.681) Male EtOH < Male H 2 O (p < 0.001) Female EtOH > Male EtOH (p < 0.001) Female H2O = Male H2O (p = 1.000) |
|
| 12M |
Not significant p = 0.123, η 2 = 0.092 |
Significant F(1,25) = 11.24, p = 0.003, η 2 = 0.310 |
Female EtOH = Female H2O (p = 0.270) Male EtOH > Male H 2 O (p < 0.001) Female EtOH < Male EtOH (p < 0.001) Female H2O = Male H2O (p = 1.000) |
|
| Homer 1b/c | 6M |
Not significant p = 0.758, η 2 = 0.003 |
Not significant F(1,37) = 0.16, p = 0.693, η 2 = 0.004 |
None |
| 9M |
Not significant p = 0.596, η 2 = 0.009 |
Not significant F(1,32) = 0.47, p = 0.499, η 2 = 0.014 |
None | |
| 12M |
Not significant p = 0.191, η 2 = 0.053 |
Not significant F(1,32) = 0.53, p = 0.474, η 2 = 0.016 |
None | |
| Homer 2a/b | 6M |
Not significant p = 0.560, η 2 = 0.010 |
Not significant F(1,36) = 0.08, p = 0.738, η 2 = 0.002 |
None |
| 9M |
Not significant p = 0.642, η 2 = 0.007 |
Not significant F(1,32) = 1.76, p = 0.195, η 2 = 0.052 |
None | |
| 12M |
Significant F(1,25) = 4.59, p = 0.042, η 2 = 0.155 |
Not significant F(1,25) = 1.58, p = 0.221, η 2 = 0.059 |
EtOH > H 2 O (p = 0.042) | |
| ERK | 6M |
Not significant p = 0.069, η 2 = 0.080 |
Not significant F(1,40) = 0.71, p = 0.406, η 2 = 0.017 |
None |
| 9M |
Not significant p = 0.600, η 2 = 0.008 |
Not significant F(1,33) = 0.93, p = 0.342, η 2 = 0.027 |
None | |
| 12M |
Significant F(1,28) = 8.59, p = 0.007, η 2 = 0.235 |
Significant F(1,28) = 15.52, p < 0.001, η 2 = 0.357 |
Female EtOH < Female H 2 O (p < 0.001) Male EtOH = Male H2O (p = 0.400) Female EtOH < Male EtOH (p < 0.001) Female H2O = Male H2O (p = 1.000) |
|
| pERK | 6M |
Not significant p = 0.281, η 2 = 0.040 |
Not significant F(1,29) = 0.40, p = 0.531, η 2 = 0.014 |
None |
| 9M |
Significant F(1,31) = 5.70, p = 0.023, η 2 = 0.155 |
Significant F(1,31) = 7.30, p = 0.011, η 2 = 0.191 |
Female EtOH = Female H2O (p = 0.827) Male EtOH > Male H 2 O (p = 0.001) Female EtOH < Male EtOH (p < 0.001) Female H2O = Male H2O (p = 1.000) |
|
| 12M |
Not significant p = 0.717, η 2 = 0.005 |
Not significant F(1,29) = 1.75, p = 0.196, η 2 = 0.057 |
None | |
| B | Neuropathological protein expression—entorhinal cortex | |||
|---|---|---|---|---|
| Protein of interest | Age (months) | Main effect: drinking history | Interaction effect: sex by drinking history | Significant group comparisons |
| Tau | 6M |
Not significant p = 0.288, η 2 = 0.032 |
Not significant F(1,35) = 1.15, p = 0.291, η 2 = 0.032 |
None |
| 9M |
Not significant p = 0.550, η 2 = 0.011 |
Not significant F(1,33) = 0.55, p = 0.463, η 2 = 0.016 |
None | |
| 12M |
Not significant p = 0.053, η 2 = 0.132 |
Not significant F(1,27) = 0.03, p = 0.877, η 2 = 0.001 |
None | |
| pThr(217) Tau | 6M |
Not significant p = 0.840, η 2 = 0.001 |
Not significant F(1,34) = 0.04, p = 0.838, η 2 = 0.001 |
None |
| 9M |
Significant F(1,33) = 6.75, p = 0.014, η 2 = 0.170 |
Significant F(1,33) = 5.96, p = 0.020, η 2 = 0.153 |
Female EtOH = Female H2O (p = 0.908) Male EtOH > Male H 2 O (p = 0.002) Female EtOH < Male EtOH (p < 0.001) Female H2O = Male H2O (p = 1.000) |
|
| 12M |
Significant F(1,28) = 16.51, p < 0.001, η 2 = 0.371 |
Not significant F(1,28) = 2.26, p = 0.144, η 2 = 0.075 |
EtOH > H 2 O (p < 0.001) | |
| pSer(396) Tau | 6M |
Not significant p = 0.934, η 2 = 0.000 |
Not significant F(1,37) = 1.03, p = 0.317, η 2 = 0.027 |
None |
| 9M |
Significant F(1,33) = 15.17, p < 0.001, η 2 = 0.315 |
Not significant F(1,33) = 1.99, p = 0.168, η 2 = 0.057 |
EtOH < H 2 O (p < 0.001) | |
| 12M |
Not significant p = 0.349, η 2 = 0.030 |
Not significant F(1,23) = 3.43, p = 0.077, η 2 = 0.111 |
None | |
| BACE 56 kDa | 6M |
Significant F(1,32) = 5.27, p = 0.028, η 2 = 0.141 |
Not significant F(1,32) = 0.82, p = 0.371, η 2 = 0.025 |
EtOH < H 2 O (p = 0.028) |
| 9M |
Not significant p = 0.609, η 2 = 0.008 |
Not significant F(1,32) = 0.174, p = 0.679, η 2 = 0.005 |
None | |
| 12M |
Not significant p = 0.172, η 2 = 0.061 |
Not significant F(1,26) = 2.32, p = 0.140, η 2 = 0.071 |
None | |
| BACE 70 kDa | 6M |
Significant F(1,36) = 17.63, p < 0.001, η 2 = 0.329 |
Not significant F(1,36) = 0.12, p = 0.731, η 2 = 0.003 |
EtOH < H 2 O (p < 0.001) |
| 9M |
Not significant p = 0.606, η 2 = 0.009 |
Not significant F(1,31) = 0.52, p = 0.476, η 2 = 0.016 |
None | |
| 12M |
Significant F(1,28) = 5.53, p = 0.026, η 2 = 0.157 |
Not significant F(1,28) = 0.79, p = 0.380, η 2 = 0.023 |
EtOH > H 2 O (p = 0.026) | |
| APP | 6M |
Not significant p = 0.191, η 2 = 0.044 |
Not significant F(1,39) = 0.23, p = 0.638, η 2 = 0.006 |
None |
| 9M |
Not significant p = 0.476, η 2 = 0.014 |
Not significant F(1,36) = 0.00, p = 0.958, η 2 = 0.000 |
None | |
| 12M |
Significant F(1,32) = 15.82, p < 0.001, η 2 = 0.331 |
Not significant F(1,32) = 0.47, p = 0.500, η 2 = 0.014 |
EtOH > H 2 O (p < 0.001) | |
| Aβ | 6M |
Not significant p = 0.081, η 2 = 0.089 |
Not significant F(1,33) = 2.94, p = 0.096, η 2 = 0.082 |
None |
| 9M |
Significant F(1,37) = 10.09, p = 0.003, η 2 = 0.214 |
Not significant F(1,37) = 0.04, p = 0.836, η 2 = 0.001 |
EtOH < H 2 O (p = 0.003) | |
| 12M |
Not significant p = 0.328, η 2 = 0.029 |
Not significant F(1,33) = 3.70, p = 0.063, η 2 = 0.101 |
None | |
TABLE 2.
Summary of the quantitative analysis of proteins implicated in (A) glutamate function (B) and neuropathological processes within the prefrontal cortex. Significant results are bolded.
| A | Glutamate‐related proteins—prefrontal cortex | |||
|---|---|---|---|---|
| Protein of interest | Age (months) | Main effect: drinking history | Interaction effect: sex by drinking history | Significant group comparisons |
| mGlu1 | 6M |
Significant F(1,37) = 4.59, p = 0.039, η 2 = 0.110 |
Not significant F(1,37) = 1.06, p = 0.311, η 2 = 0.028 |
EtOH > H 2 O (p = 0.039) |
| 9M |
Not significant p = 0.318, η 2 = 0.024 |
Not significant F(1,42) = 0.01, p = 0.915, η 2 = 0.000 |
None | |
| 12M |
Not significant p = 0.922, η 2 = 0.000 |
Not significant F(1,32) = 3.84, p = 0.059, η 2 = 0.107 |
None | |
| mGlu5 dimer | 6M |
Not significant p = 0.155, η 2 = 0.057 |
Not significant F(1,35) = 0.38, p = 0.543, η 2 = 0.011 |
None |
| 9M |
Not significant p = 0.330, η 2 = 0.025 |
Not significant F(1,38) = 0.21, p = 0.650, η 2 = 0.005 |
None | |
| 12M |
Not significant p = 0.806, η 2 = 0.002 |
Not significant F(1,28) = 0.41, p = 0.527, η 2 = 0.014 |
None | |
| mGlu5 monomer | 6M |
Significant F(1,33) = 6.41, p = 0.016, η 2 = 0.163 |
Not significant F(1,33) = 0.53, p = 0.470, η 2 = 0.016 |
EtOH > H 2 O (p = 0.016) |
| 9M |
Not significant p = 0.159, η 2 = 0.050 |
Significant F(1,39) = 5.40, p = 0.025, η 2 = 0.122 |
Female EtOH = Female H2O (p = 0.540) Male EtOH < Male H 2 O (p = 0.010) Female EtOH > Male EtOH (p = 0.001) Female H2O = Male H2O (p = 1.000) |
|
| 12M |
Not significant p = 0.535, η 2 = 0.013 |
Not significant F(1,30) = 0.03, p = 0.957, η 2 = 0.000 |
None | |
| GluN1 | 6M |
Not significant p = 0.062, η 2 = 0.089 |
Not significant F(1,38) = 3.71, p = 0.062, η 2 = 0.089 |
None |
| 9M |
Significant F(1,40) = 5.02, p = 0.031, η 2 = 0.112 |
Not significant F(1,40) = 3.79, p = 0.059, η 2 = 0.086 |
EtOH < H 2 O (p = 0.031) | |
| 12M | Not significantp = 0.282, η 2 = 0.032 |
Not significant F(1,36) = 1.23, p = 0.276, η 2 = 0.033 |
None | |
| GluN2B | 6M |
Significant F(1,34) = 9.36, p = 0.004, η 2 = 0.216 |
Not significant F(1,34) = 1.76, p = 0.194, η 2 = 0.049 |
EtOH > H 2 O (p = 0.004) |
| 9M |
Not significant .199, η 2 = 0.049 |
Significant F(1,33) = 13.36, p < 0.001, η 2 = 0.288 |
Female EtOH < Female H 2 O (p = 0.002) Male EtOH = Male H2O (p = 0.086) Female EtOH < Male EtOH (p < 0.001) Female H2O = Male H2O (p = 1.000) |
|
| 12M |
Not significant p = 0.535, η 2 = 0.013 |
Not significant F(1,29) = 0.355, p = 0.556, η 2 = 0.012 |
None | |
| Homer 1b/c | 6M |
Significant F(1,35) = 19.09, p < 0.001, η 2 = 0.353 |
Not significant F(1,35) = 1.75, p = 0.195, η 2 = 0.048 |
EtOH > H 2 O (p < 0.001) |
| 9M |
Not significant p = 0.813, η 2 = 0.001 |
Not significant F(1,38) = 3.37, p = 0.074, η 2 = 0.081 |
None | |
| 12M |
Not significant p = 0.409, η 2 = 0.022 |
Not significant F(1,31) = 0.47, p = 0.500, η 2 = 0.015 |
None | |
| Homer 2a/b | 6M |
Significant F(1,38) = 16.93, p < 0.001, η 2 = 0.308 |
Not significant F(1,38) = 0.78, p = 0.383, η 2 = 0.020 |
EtOH > H 2 O (p < 0.001) |
| 9M |
Not significant p = 0.214, η 2 = 0.038 |
Not significant F(1,40) = 1.37, p = 0.249, η 2 = 0.033 |
None | |
| 12M |
Not significant p = 0.115, η 2 = 0.076 |
Not significant F(1,32) = 0.000, p = 0.986, η 2 = 0.000 |
None | |
| ERK | 6M |
Significant F(1,41) = 9.76, p = 0.003, η 2 = 0.192 |
Not significant F(1,41) = 0.63, p = 0.433, η 2 = 0.015 |
EtOH > H 2 O (p = 0.003) |
| 9M |
Not significant p = 0.169, η 2 = 0.047 |
Not significant F(1,40) = 0.73, p = 0.398, η 2 = 0.018 |
None | |
| 12M |
Not significant p = 0.822, η 2 = 0.002 |
Not significant F(1,33) = 0.106, p = 0.746, η 2 = 0.003 |
None | |
| pERK | 6M |
Significant F(1,35) = 44.94, p < 0.001, η 2 = 0.562 |
Not significant F(1,35) = 2.73, p = 0.107, η 2 = 0.072 |
EtOH > H 2 O (p < 0.001) |
| 9M |
Not significant p = 0.640, η 2 = 0.005 |
Not significant F(1,41) = 2.52, p = 0.120, η 2 = 0.058 |
None | |
| 12M |
Not significant p = 0.574, η 2 = 0.011 |
Significant F(1,28) = 4.57, p = 0.042, η 2 = 0.140 |
Female EtOH = Female H2O (p = 0.092) Male EtOH < Male H2O (p = 0.225) Female EtOH > Male EtOH (p = 0.007) Female H2O = Male H2O (p = 1.000) |
|
| B | Neuropathological protein expression—prefrontal cortex | |||
|---|---|---|---|---|
| Protein of interest | Age (months) | Main effect: drinking history | Interaction effect: sex by drinking history | Significant group comparisons |
| Tau | 6M |
Not significant p = 0.841, η 2 = 0.001 |
Not significant F(1,34) = 0.30, p = 0.590, η 2 = 0.009 |
None |
| 9M |
Not significant p = 0.101, η 2 = 0.073 |
Not significant F(1,36) = 0.23, p = 0.633, η 2 = 0.006 |
None | |
| 12M |
Not significant p = 0.161, η 2 = 0.062 |
Not significant F(1,31) = 0.07, p = 0.793, η 2 = 0.002 |
None | |
| pThr(217) Tau | 6M |
Significant F(1,34) = 4.86, p = 0.034, η 2 = 0.125 |
Not significant F(1,34) = 0.31, p = 0.580, η 2 = 0.009 |
EtOH > H 2 O (p = 0.034) |
| 9M |
Not significant p = 0.570, η 2 = 0.009 |
Not significant F(1,36) = 0.31, p = 0.580, η 2 = 0.009 |
None | |
| 12M |
Not significant p = 0.118, η 2 = 0.072 |
Not significant F(1,33) = 0.10, p = 0.758, η 2 = 0.003 |
None | |
| pSer(396) Tau | 6M |
Not significant p = 0.079, η 2 = 0.090 |
Not significant F(1,33) = 1.84, p = 0.184, η 2 = 0.053 |
None |
| 9M |
Not significant p = 0.066, η 2 = 0.086 |
Not significant F(1,38) = 0.05, p = 0.817, η 2 = 0.001 |
None | |
| 12M |
Significant F(1,28) = 10.01, p = 0.004, η 2 = 0.263 |
Not significant F(1,28) = 0.93, p = 0.342, η 2 = 0.032 |
EtOH < H 2 O (p = 0.004) | |
| BACE 56 kDa | 6M |
Not significant p = 0.243, η 2 = 0.041 |
Not significant F(1,33) = 0.11, p = 0.743, η 2 = 0.003 |
None |
| 9M |
Not significant p = 0.118, η 2 = 0.067 |
Not significant F(1,36) = 0.25, p = 0.621, η 2 = 0.007 |
None | |
| 12M |
Not significant p = 0.887, η 2 = 0.001 |
Not significant F(1,34) = 1.38, p = 0.249, η 2 = 0.039 |
None | |
| BACE 70 kDa | 6M |
Not significant p = 0.179, η 2 = 0.052 |
Not significant F(1,34) = 0.35, p = 0.558, η 2 = 0.010 |
None |
| 9M |
Not significant p = 0.910, η 2 = 0.000 |
Not significant F(1,42) = 0.10, p = 0.754, η 2 = 0.002 |
None | |
| 12M |
Not significant p = 0.708, η 2 = 0.004 |
Not significant F(1,37) = 0.79, p = 0.379, η 2 = 0.021 |
None | |
| APP | 6M |
Not significant p = 0.330, η 2 = 0.024 |
Not significant F(1,39) = 0.03, p = 0.854, η 2 = 0.001 |
None |
| 9M |
Not significant p = 0.382, η 2 = 0.021 |
Not significant F(1,37) = 0.01, p = 0.922, η 2 = 0.000 |
None | |
| 12M |
Significant F(1,33) = 5.36, p = 0.027, η 2 = 0.140 |
Not significant F(1,33) = 1.20, p = 0.282, η 2 = 0.035 |
EtOH > H 2 O (p = 0.027) | |
| Aβ | 6M |
Not significant p = 0.552, η 2 = 0.010 |
Not significant F(1,35) = 0.25, p = 0.619, η 2 = 0.007 |
None |
| 9M |
Not significant p = 0.903, η 2 = 0.000 |
Not significant F(1,34) = 1.05, p = 0.313, η 2 = 0.030 |
None | |
| 12M |
Not significant p = 0.945, η 2 = 0.000 |
Not significant F(1,36) = 0.19, p = 0.666, η 2 = 0.005 |
None | |
TABLE 3.
Summary of the quantitative analysis of proteins implicated in (A) glutamate function (B) and neuropathological processes within the amygdala. Significant results are bolded.
| A | Glutamate‐related proteins—amygdala | |||
|---|---|---|---|---|
| Protein of interest | Age (months) | Main effect: drinking history | Interaction effect: sex by drinking history | Significant group comparisons |
| mGlu1 | 6M |
Significant F(1,30) = 8.49, p = 0.007, η 2 = 0.221 |
Significant F(1,30) = 4.50, p = 0.042, η 2 = 0.130 |
Female EtOH > Female H 2 O (p = 0.001) Male EtOH = Male H2O (p = 0.580) Female EtOH > Male EtOH (p = 0.002) Female H2O = Male H2O (p = 1.000) |
| 9M |
Not significant p = 0.618, η 2 = 0.007 |
Not significant F(1,34) = 0.81, p = 0.375, η 2 = 0.023 |
None | |
| 12M |
Significant F(1,33) = 7.93, p = 0.008, η 2 = 0.194 |
Not significant F(1,33) = 0.04, p = 0.848, η 2 = 0.001 |
EtOH > H 2 O (p = 0.008) | |
| mGlu5 dimer | 6M |
Not significant p = 0.360, η 2 = 0.024 |
Significant F(1,35) = 5.94, p = 0.020, η 2 = 0.145 |
Female EtOH > Female H 2 O (p = 0.029) Male EtOH = Male H2O (p = 0.271) Female EtOH > Male EtOH (p < 0.001) Female H2O = Male H2O (p = 1.000) |
| 9M |
Significant F(1,34) = 4.38, p = 0.044, η 2 = 0.114 |
Not significant F(1,34) = 0.75, p = 0.394, η 2 = 0.021 |
EtOH < H 2 O (p = 0.044) | |
| 12M |
Significant F(1,33) = 9.59, p = 0.004, η 2 = 0.225 |
Significant F(1,33) = 25.93, p < 0.001, η 2 = 0.440 |
Female EtOH > Female H 2 O (p < 0.001) Male EtOH = Male H2O (p = 0.108) Female EtOH > Male EtOH (p < 0.001) Female H2O = Male H2O (p = 1.000) |
|
| mGlu5 monomer | 6M |
Not significant p = 0.378, η 2 = 0.022 |
Not significant F(1,35) = 0.23, p = 0.637, η 2 = 0.006 |
None |
| 9M |
Not significant p = 0.932, η 2 = 0.000 |
Not significant F(1,33) = 1.85, p = 0.183, η 2 = 0.053 |
None | |
| 12M |
Not significant p = 0.932, η 2 = 0.000 |
Not significant F(1,33) = 1.85, p = 0.183, η 2 = 0.053 |
None | |
| GluN1 | 6M |
Not significant p = 0.052, η 2 = 0.104 |
Not significant F(1,35) = 0.35, p = 0.561, η 2 = 0.010 |
None |
| 9M |
Not significant p = 0.139, η 2 = 0.063 |
Not significant F(1,34) = 0.01, p = 0.759, η 2 = 0.003 |
None | |
| 12M |
Not significant p = 0.585, η 2 = 0.008 |
Not significant F(1,37) = 3.09, p = 0.087, η 2 = 0.077 |
None | |
| GluN2B | 6M |
Not significant p = 0.362, η 2 = 0.025 |
Not significant F(1,33) = 0.15, p = 0.902, η 2 = 0.000 |
None |
| 9M |
Significant F(1,30) = 14.53, p < 0.001, η 2 = 0.326 |
Not significant F(1,30) = 0.57, p = 0.456, η 2 = 0.019 |
EtOH < H 2 O (p < 0.001) | |
| 12M |
Not significant p = 0.079, η 2 = 0.114 |
Not significant F(1,26) = 2.10, p = 0.159, η 2 = 0.075 |
None | |
| Homer 1b/c | 6M |
Not significant p = 0.362, η 2 = 0.023 |
Not significant F(1,36) = 0.26, p = 0.616, η 2 = 0.007 |
None |
| 9M |
Not significant p = 0.128, η 2 = 0.069 |
Not significant F(1,33) = 4.01, p = 0.053, η 2 = 0.108 |
None | |
| 12M |
Significant F(1,34) = 15.55, p < 0.001, η 2 = 0.314 |
Not significant F(1,34) = 0.53, p = 0.473, η 2 = 0.015 |
EtOH > H 2 O (p < 0.001) | |
| Homer 2a/b | 6M |
Not significant p = 0.305, η 2 = 0.029 |
Not significant F(1,36) = 0.25, p = 0.619, η 2 = 0.007 |
None |
| 9M |
Not significant p = 0.652, η 2 = 0.006 |
Not significant F(1,32) = 3.32, p = 0.078, η 2 = 0.094 |
None | |
| 12M |
Not significant p = 0.658, η 2 = 0.007 |
Not significant F(1,29) = 0.34, p = 0.564, η 2 = 0.012 |
None | |
| ERK | 6M |
Not significant p = 0.830, η 2 = 0.001 |
Not significant F(1,39) = 0.15, p = 0.699, η 2 = 0.004 |
None |
| 9M |
Not significant p = 0.348, η 2 = 0.028 |
Not significant F(1,32) = 0.00, p = 0.978, η 2 = 0.000 |
None | |
| 12M |
Significant F(1,28) = 12.66, p = 0.001, η 2 = 0.311 |
Not significant F(1,28) = 1.02, p = 0.322, η 2 = 0.035 |
EtOH < H 2 O (p = 0.001) | |
| pERK | 6M |
Significant F(1,38) = 14.39, p = 0.001, η 2 = 0.275 |
Not significant F(1,38) = 0.51, p = 0.479, η 2 = 0.013 |
EtOH > H 2 O (p = 0.001) |
| 9M |
Not significant p = 0.160, η 2 = 0.061 |
Not significant F(1,32) = 1.76, p = 0.194, η 2 = 0.052 |
None | |
| 12M |
Significant F(1,28) = 9.84, p = 0.004, η 2 = 0.260 |
Not significant F(1,28) = 1.20, p = 0.282, η 2 = 0.041 |
EtOH < H 2 O (p = 0.004) | |
| B | Neuropathological protein expression—amygdala | |||
|---|---|---|---|---|
| Protein of interest | Age (months) | Main effect: drinking history | Interaction effect: sex by drinking history | Significant group comparisons |
| Tau | 6M |
Significant F(1,36) = 8.35, p = 0.007, η 2 = 0.188 |
Not significant F(1,36) = 0.39, p = 0.537, η 2 = 0.011 |
EtOH < H 2 O (p = 0.007) |
| 9M |
Not significant p = 0.646, η 2 = 0.007 |
Not significant F(1,29) = 0.76, p = 0.391, η 2 = 0.025 |
None | |
| 12M |
Significant F(1,27) = 9.30, p = 0.005, η 2 = 0.256 |
Not significant F(1,27) = 3.09, p = 0.090, η 2 = 0.103 |
EtOH > H 2 O (p = 0.005) | |
| pThr(217) Tau | 6M |
Not significant p = 0.890, η 2 = 0.000 |
Not significant F(1,39) = 1.87, p = 0.180, η 2 = 0.046 |
None |
| 9M |
Not significant p = 0.353, η 2 = 0.028 |
Not significant F(1,31) = 2.33, p = 0.137, η 2 = 0.070 |
None | |
| 12M |
Not significant p = 0.124, η 2 = 0.070 |
Not significant F(1,33) = 4.09, p = 0.051, η 2 = 0.110 |
None | |
| pSer(396) Tau | 6M |
Not significant p = 0.301, η 2 = 0.032 |
Not significant F(1,33) = 0.07, p = 0.788, η 2 = 0.002 |
None |
| 9M |
Significant F(1,32) = 11.53, p = 0.002, η 2 = 0.265 |
Not significant F(1,32) = 0.30, p = 0.590, η 2 = 0.009 |
EtOH < H 2 O (p = 0.002) | |
| 12M | Significant F(1,27) = 16.63, p < 0.001, η 2 = 0.381 |
Not significant F(1,28) = 0.29, p = 0.594, η 2 = 0.011 |
EtOH < H 2 O (p < 0.001) | |
| BACE 56 kDa | 6M |
Not significant p = 0.135, η 2 = 0.064 |
Not significant F(1,34) = 0.53, p = 0.470, η 2 = 0.015 |
None |
| 9M |
Not significant p = 0.875, η 2 = 0.001 |
Not significant F(1,28) = 1.60, p = 0.217, η 2 = 0.054 |
None | |
| 12M |
Significant F(1,34) = 5.36, p = 0.027, η 2 = 0.136 |
Not significant F(1,34) = 0.000, p = 0.992, η 2 = 0.000 |
EtOH > H 2 O (p = 0.027) | |
| BACE 70 kDa | 6M |
Not significant p = 0.860, η 2 = 0.001 |
Not significant F(1,30) = 2.17, p = 0.151, η 2 = 0.068 |
None |
| 9M |
Not significant p = 0.790, η 2 = 0.002 |
Not significant F(1,32) = 0.72, p = 0.404, η 2 = 0.022 |
None | |
| 12M |
Not significant p = 0.169, η 2 = 0.057 |
Not significant F(1,33) = 3.39, p = 0.074, η 2 = 0.093 |
None | |
| APP | 6M |
Not significant p = 0.451, η 2 = 0.015 |
Not significant F(1,38) = 0.97, p = 0.331, η 2 = 0.025 |
None |
| 9M |
Not significant p = 0.367, η 2 = 0.023 |
Not significant F(1,36) = 0.07, p = 0.792, η 2 = 0.002 |
None | |
| 12M | Significant F(1,32) = 45.09, p < 0.001, η 2 = 0.585 |
Significant F(1,32) = 11.42, p = 0.002, η 2 = 0.263 |
Female EtOH > Female H 2 O (p < 0.001) Male EtOH > Male H 2 O (p = 0.016) Female EtOH > Male EtOH (p < 0.001) Female H2O = Male H2O (p = 1.000) |
|
| Aβ | 6M |
Not significant p = 0.425, η 2 = 0.021 |
Not significant F(1,30) = 0.01, p = 0.924, η 2 = 0.000 |
None |
| 9M |
Not significant p = 0.144, η 2 = 0.060 |
Not significant F(1,35) = 2.14, p = 0.152, η 2 = 0.058 |
None | |
| 12M |
Significant F(1,34) = 7.99, p = 0.008, η 2 = 0.190 |
Not significant F(1,34) = 0.66, p = 0.422, η 2 = 0.019 |
EtOH > H 2 O (p = 0.008) | |
In cases where significant interactions were detected, we conducted simple main effect tests with least significant difference (LSD) adjustments. Conversely, when significant main effects (> 2 levels) were observed without an interaction, LSD post hoc tests were employed to clarify group differences. The F statistic, p values and partial eta‐squared values were reported for all statistical evaluations, with a pre‐set alpha level of 0.05 for significance. Greenhouse–Geisser corrections were applied where sphericity was violated, and outliers were initially addressed using the ±1.5 × IQR rule. However, in cases where the initial method significantly reduced the sample size, the more lenient ±3 × IQR rule was implemented for the most extreme outliers. As ANOVAs maintain robustness against violations of normality assumptions, 103 a formal assessment of data normality was not conducted. All statistical analyses were conducted using IBM SPSS Statistics (version 27.0 for Macintosh), Jamovi (version 2.3.21.0 for Macintosh), and the resulting graphs were produced with GraphPad Prism (version 10.2.2 for Macintosh).
3. RESULTS
3.1. Alcohol intake and BACs
A univariate sex × age ANOVA was conducted to determine group differences in the amount of alcohol consumed during the 30‐day drinking period. As shown in Figure 2A, no significant sex × age interaction was detected [ANOVA: p = 0.688, ɳ 2 = 0.011]; however, significant main effect of sex was observed [F(1,65) = 19.65, p < 0.001, ɳ 2 = 0.232], with females exhibiting higher alcohol consumption than males. We also detected a significant age effect [F(2,65) = 7.26, p = 0.001, ɳ 2 = 0.183] that reflected less alcohol consumed by 9M mice versus 6M (p = 0.001) and 12M mice (p = 0.003), with no significant differences in intake between 6M and 12M mice (p = 0.718).
FIGURE 2.
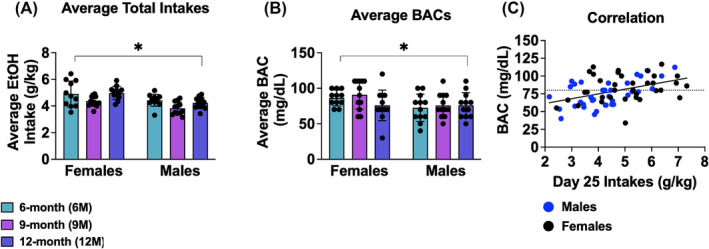
Depiction of the sex and age differences in alcohol intake and corresponding BAC levels. (A) Female mice consumed more alcohol on average over the 30‐day drinking period compared to males, with no significant interaction between sex and age. (B) BAC levels measured on day 25, with females displaying higher BACs than males, reflecting the sex‐specific intake patterns seen with total intake. (C) Scatter plot demonstrating a positive correlation between alcohol intake and BAC on day 25 in this study. The dotted line in (C) represents the legal intoxication threshold. Figures show means ± SEMs. *p < 0.05, female versus male.
Despite these differences in intake, blood alcohol concentrations (BAC) measured on day 25 of drinking did not show an age effect or interaction (Figure 2B; all p's > 0.338). Consistent with our intake data, a significant sex effect for BACs was noted [F(1,65) = 4.41, p = 0.040, η 2 = 0.064], with females exhibiting higher BACs than males. Furthermore, a positive correlation was confirmed between BAC levels and alcohol intake on day 25 (Figure 2C; r = 0.45, p < 0.001), indicating that intake levels reliably predicted BAC irrespective of age, suggesting that age‐related variations in consumption did not translate into differences in BAC.
To further explore potential sex differences in BAC distribution relative to binge‐level intoxication, we dichotomized the BAC variable into binge (≥80 mg/dL) and non‐binge (<80 mg/dL) categories and conducted a chi‐square test of association. The analysis revealed no significant difference in the proportion of males and females above or below the binge threshold [X 2 (1, N = 71) = 1.70, p = 0.192], indicating that the distribution of binge‐level intoxication was comparable between sexes.
3.2. Behavioural measures
For the sake of clarity and to facilitate visualization of significant group differences, only statistically significant main effects or interactions are described and depicted in the main text and we report both the results from the general linear model and estimates of effect sizes (ɳ 2). Non‐significant findings are summarized in the Supplemental Results and the reader is directed there for the full results of the statistical analyses of our behavioural measures (Table S1) and graphical depictions of the data.
3.2.1. Light–dark shuttle box test
For female mice, a significant main effect of age was found in the total time spent in the light side of the light–dark box [F(2,62) = 4.26, p = 0.019, ɳ 2 = 0.121; Figure 3A]. Tests for multiple comparisons indicated more time spent by 6M females when compared to both the 9M (p = 0.019) and 12M (p = 0.008) females. Additionally, significant main effects of age [F(2,62) = 10.37, p < 0.001, ɳ 2 = 0.251] and drinking history [F(1,62 = 5.57, p = 0.021, ɳ 2 = 0.082] were observed for the number of entries into the light side. For the main effect of age (Figure 3B), subsequent tests for multiple comparisons revealed that 6M female mice exhibited a greater number of entries into the light side, compared to the 9M females (p = 0.014) and both the 6M and 9M females made more entries to the light side than the 12M females (6M vs. 12M: p < 0.001; 9M vs. 12M: p = 0.047). For the main effect of drinking history in females (Figure 3C), binge‐drinking females made less entries into the light side relative to their water‐drinking counterparts. Figures depicting the means ± SEMS for all measures from the light dark shuttle box test are presented separately for males and females in Figure S1A–F.
FIGURE 3.
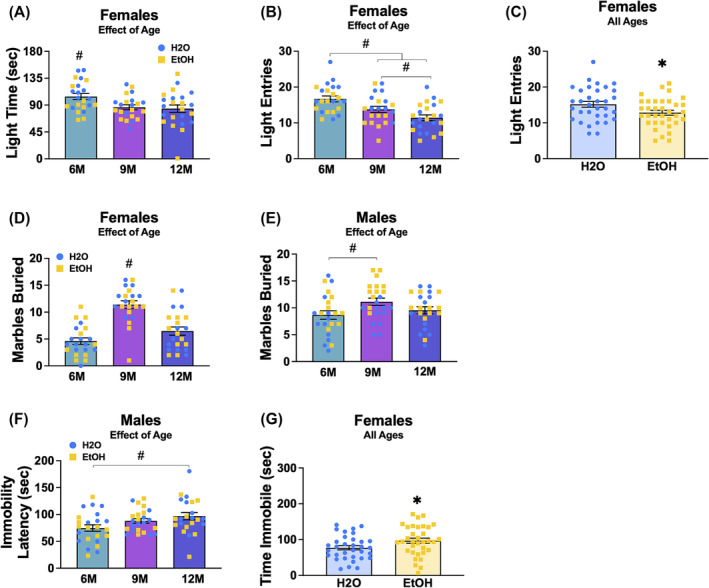
Depiction of the significant age by drinking history ANOVA results for the observed behaviour in the light dark box shuttle, marble‐burying and forced swim tests for female and male mice. (A) Total time spent by female mice on the light side, showing a significant main age effect with 6‐month‐old (6M) females spending more time in the light side compared to other age groups. (B) Total number of light‐side entries by female mice, showing a significant main age effect with fewer entries by 12‐month‐old (12M) females and more entries by 6M females compared to other age groups. (C) Total number of light‐side entries by female mice, showing a significant main drinking history effect. (D) Number of marbles buried by female mice, showing a significant age effect with 9‐month‐old (9M) females burying more marbles than other age groups. (E) Number of marbles buried by male mice, showing a significant age effect with 9M males burying more marbles than 6M males. (F) Latency to first exhibit immobility in the forced swim test for male mice, showing a significant age effect with a shorter latency in 6M males compared to 12M males. (G) Total time spent immobile by female mice in the forced swim test, showing a significant drinking history effect. Figures show means ± SEMs. *p < 0.05, EtOH versus H2O; # p < 0.05, age difference.
3.2.2. Marble burying test
A significant main effect of age was observed for the number of marbles buried by female mice [F(2,62) = 25.62, p < 0.001, ɳ 2 = 0.452; Figure 3D]. Post hoc tests determined that 9M females buried more marbles than both the 6M (p < 0.001) and 12M (p < 0.001) female mice. Similarly, a significant main effect of age was detected in male mice [F(2,66) = 3.52, p = 0.035, ɳ 2 = 0.096; Figure 3E], with 9M males burying significantly more marbles than the 6M males (p = 0.035). Figures depicting the means ± SEMS for all measures from the light dark shuttle box test are presented separately for males and females in Figure S1G,H.
3.2.3. Forced swim test
In male mice, a significant main effect of Age was detected for the latency to first float in the forced swim test [F(2,65) = 3.88, p = 0.026, ɳ 2 = 0.107; Figure 3F]. Tests for multiple comparisons indicated that 6M males exhibited a shorter latency to first float than the 12M males (p = 0.008). Additionally, for female mice, a significant main drinking history effect was found for the total time spent immobile [F(2,61) = 5.25, p = 0.025, ɳ 2 = 0.079; Figure 3G], with binge‐drinking females spending more time immobile than their water‐drinking controls. Figures depicting the means ± SEMs for all measures from the forced swim test are presented separately for males and females in Figure S1I–N.
3.2.4. Acoustic startle and prepulse inhibition of acoustic startle
The only statistically significant results regarding acoustic startle was an amplitude × age interaction [F(3.49, 104.73) = 2.67, p = 0.043, ɳ 2 = 0.082] in male mice that reflected lower startle amplitude in response to the background noise (st0) by 12–month‐old males (p's > 0.016) and a lower response to the 90 dB startle stimulus by 6–month‐old males (p = 0.007). This result and figures depicting all measures from acoustic startle testing are presented in Figure S2.
3.3. Morris water maze
3.3.1. Flag test
In female mice, a significant main age effect was observed for the latency to locate the flagged platform [F(2,62) = 3.75, p = 0.029, ɳ 2 = 0.108; Figure 4A]. Post hoc comparisons revealed that 6M female mice located the visible platform more quickly than 12M female mice (p = 0.009). Figures depicting the means ± SEMs for all measures from the flag test are presented in Figure S3A–E.
FIGURE 4.
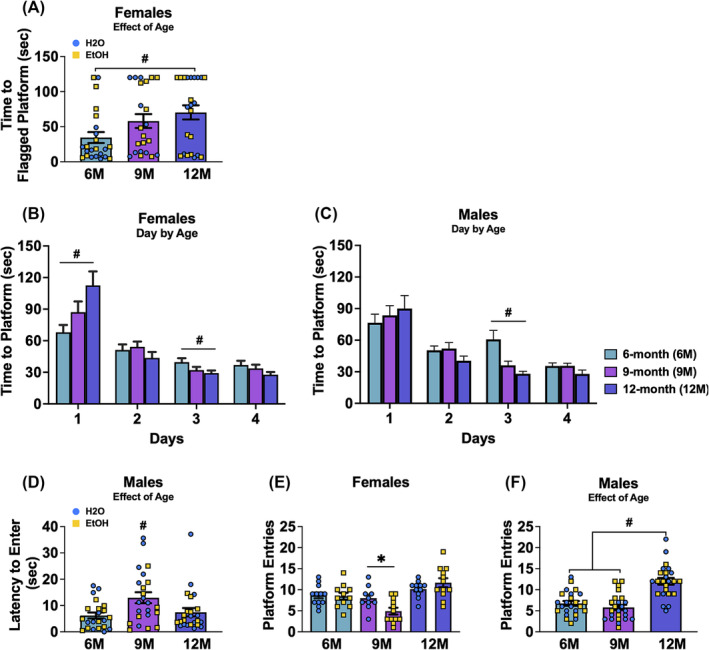
Depiction of the significant results for the Morris water maze for female and male mice. (A) Time to locate the flagged hidden platform in female mice, showing a significant main age effect with 6‐month‐old (6M) females showing a shorter latency compared to 12‐month‐old (12M) females. (B) Latency to locate the hidden platform during the acquisition phase in female mice, showing a significant day × age interaction. (C) Latency to locate the hidden platform during the acquisition phase in male mice, showing a significant day × age interaction. (D) Latency to enter the platform's former location in the NE quadrant during the probe test in male mice, showing a significant main age effect with 9‐month‐old (9M) males showing a longer latency compared to 6M and 12M males. (E) Number of entries to the former platform location in female mice, showing a significant age by drinking history interaction, with 9M binge‐drinking females making fewer entries compared to 9M water‐drinking females. (F) Number of platform entries during the probe test in male mice, showing a significant age effect with 12M males making more entries compared to other age groups. Figures show means ± SEMs. *p < 0.05, EtOH versus H2O; # p < 0.05, age difference.
3.3.2. Maze acquisition
In female mice, a significant day × age interaction was found for the average latency to locate the hidden platform during the Moris maze acquisition [F(3.34, 101.95) = 5.16, p = 0.002, ɳ 2 = 0.145], which was then analysed across the day factor. As illustrated in Figure 4B, 12M females required significant more time to locate the platform than their 6M counterparts on day 1 of training (p = 0.004), while the opposite pattern was observed on day 3, with 12M females locating the platform faster than 6M females (p = 0.022). Similarly, in male mice, a significant day × age interaction was detected [F(3.39, 103.49 = 3.05, p = 0.026, ɳ 2 = 0.091; Figure 4C]. This interaction was also deconstructed along the day factor and found that on day 3 of training, 6M males required more time to reach the platform than the 9M (p ≤ 0.001) and 12M (p < 0.001) male mice. Figures depicting the means ± SEMs for all measures from the acquisition phase of the Morris water maze are presented in Figure S3F–K.
3.3.3. Probe test
In male mice, a significant main effect of age was observed for the latency to re‐enter the former platform location [F(2,63) = 4.45, p = 0.016, ɳ 2 = 0.124]. As shown in Figure 4D, post hoc analyses revealed that 9M males had a longer latency to re‐enter the former location compared with the 6M (p = 0.006) and 12M males (p = 0.020). For female mice, a significant age × drinking history interaction was observed for the number of times female mice entered into the platform's former location in the maze during the 2‐min probe test [F(2,62) = 3.79, p = 0.028, ɳ 2 = 0.109; Figure 4E], prompting an analysis across the age factor to identify differences between alcohol and water‐drinking mice. Pairwise comparisons revealed that 9M binge‐drinking females made fewer entries to the platform's former location than their water‐drinking counterparts (p = 0.013). In male mice, a significant main age effect was observed for the number of entries into the former platform location [F(2, 66) = 24.88, p < 0.001, ɳ 2 = 0.430; Figure 4F]. Post hoc analyses indicated that 12M males made more entries compared to both 6M (p < 0.001) and 9M (p < 0.001) male mice.
3.3.4. Reversal test
We failed to detect any significant group differences in behaviour during the reversal test conducted in the Morris water maze (Figure S3L‐Q).
3.4. Radial arm maze
3.4.1. Time taken to complete the radial arm maze
A significant day × age × drinking history interaction was detected for the time taken by female mice to complete the radial arm maze [F(10,310) = 2.75, p = 0.003, ɳ 2 = 0.081]. Deconstruction of this interaction along the age factor detected a significant day × drinking history interaction for the 9M females [F(3.28, 65.50) = 3.51, p = 0.017, ɳ 2 = 0.149; Figure 5A], which reflected a longer time taken by 9M water‐drinking females to complete the maze on day 4 of training (p = 0.005). A significant day × drinking history interaction was also detected for 12M females [F(3.59, 75.37) = 2.88, p = 0.033, ɳ 2 = 0.121; Figure 5B], which reflected a longer time taken by binge‐drinking versus water controls on day 3 of training (p = 0.013). Akin to females, a significant day × age × drinking history interaction was detected for the male mice [F(10,330) = 2.82, p = 0.002, ɳ 2 = 0.079]. Deconstruction of this interaction along the age factor detected a significant day × drinking history interaction for the 6M males [F(5,110) = 4.53, p = 0.001, ɳ 2 = 0.171; Figure 5C], which reflected less time by binge‐drinking mice to complete the maze compared to their water‐drinking counterparts on days 2, 6 and 7 (p's < 0.046; all other p's > 0.162). Figures depicting the means ± SEMs for all measures from the time taken to complete the radial arm water maze are presented in Figure S4A–C.
FIGURE 5.
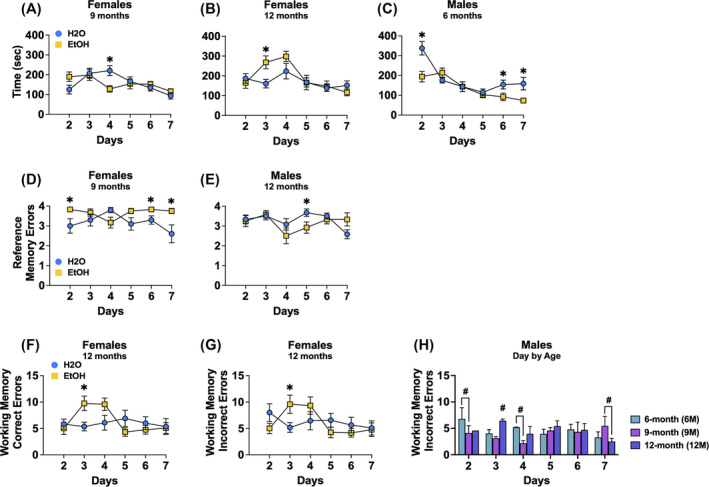
Depiction of the significant results for the radial arm water maze for female and male mice. (A) Depiction of the significant day by drinking history interaction for time taken to complete the maze for female 9M mice, reflecting a longer time taken by water‐drinking versus binge‐drinking females on day 4. (B) Depiction of the same interaction for the 12M females, reflecting a longer time taken by binge‐drinking versus water controls on day 3. (C) Depiction of the significant day by age by drinking history interaction was observed for the time taken by male mice to complete the radial arm maze for which 6M binge‐drinking males completed the maze more quickly on days 2, 6 and 7 versus their water controls. A significant day by age × drinking history interaction for number of reference memory errors was detected in female mice that reflected (D) a significant day by drinking history interaction for the 9M females. A three‐way interaction was also detected for this variable in males that reflected (E) less errors by 12M binge‐drinking males on day 5 compared to water‐drinking controls. A significant day by age by drinking history interaction for the number of working memory correct errors was also detected in females that (F) more errors by 12M binge‐drinking females versus their water controls on day 3. (G) Depiction of the significant day by drinking history interaction was observed for the number of working memory incorrect errors in the 12M females, with binge‐drinking females committing more errors than water controls on day 3. (H) A depiction of the significant day by age interaction for this variable in males, reflected more errors by 6M versus 9M mice on days 2 and 4, while on day 3, 12M mice committed the most errors and on day 7, 9M mice committed more errors than the 12M mice. Figures show means ± SEMs. *p < 0.05, EtOH versus H2O; # p < 0.05, age difference.
3.4.2. Reference memory errors
A very strong statistical trend for a day × age × drinking history interaction was observed for the number of reference memory errors committed by female mice during radial arm maze testing [F(7.76, 240.63) = 1.98, p = 0.051, ɳ 2 = 0.060]. Deconstruction of the 3‐way interaction along the age factor detected a significant day × drinking history interaction for the 9M females [F(5,100) = 3.19, p = 0.010, ɳ 2 = 0.138; Figure 5D] that reflected more reference errors on days 2, 6 and 7 by binge‐drinking females, compared to their water‐drinking counterparts (p's < 0.032; all other p's > 0.057). Similarly, a significant day × age × drinking history interaction was observed for the male mice [F(10,330) = 2.36, p = 0.010, ɳ 2 = 0.067]. For the 12M males, a significant day × drinking history interaction was observed [F(5,110) = 2.34, p = 0.046, ɳ 2 = 0.096; Figure 5E] and reflected fewer reference memory errors by binge‐drinking versus water‐drinking males on day 5 (p = 0.040). Figures depicting the means ± SEMs for all measures from number of reference memory errors committed during the radial arm water maze are presented in Figure S4D–G.
3.4.3. Working memory correct errors
A significant day × age × drinking history interaction was detected for the number of working memory correct errors committed by female mice [F(10,310) = 2.32, p = 0.012, ɳ 2 = 0.070]. Deconstruction of this interaction along the age factor revealed a significant day × drinking history interaction for the 12M female mice [F(5, 105) = 2.85, p = 0.019, ɳ 2 = 0.120; Figure 5F], which reflected more errors by binge‐drinking females than water controls on day 3 (day 3: p = 0.014). Figures depicting the means ± SEMs for all measures from number of working memory correct errors committed during the radial arm water maze are presented in Figure S4H–L.
3.4.4. Working memory incorrect errors
A significant day × age × drinking history interaction was found for the number of working memory incorrect errors committed by the female mice [F(10,310) = 2.00, p = 0.033, ɳ 2 = 0.061]. Deconstruction of the interaction along the age factor identified a significant day × drinking history interaction for the 12M females [F(5,105) = 2.64, p = 0.028, ɳ 2 = 0.112; Figure 5G] that reflected more working memory incorrect errors committed by binge‐drinking versus water controls on day 3 (day 3: p = 0.040; other days: p's > 0.131). For the male mice, a significant day × age interaction was observed for the number working memory incorrect errors [F(10,330) = 2.66, p = 0.004, ɳ 2 = 0.075]. As shown in Figure 5H, test for simple main effects revealed that on days 2 and 4, 6M male mice committed more working memory incorrect errors compared to their 9M counterparts (day 2: p = 0.049; day 4: p = 0.011). On day 3, the 12M males had more working memory errors relative to both the 6M (p = 0.034) and 9M (p = 0.004) male mice. However, on day 7, 9M males exhibited a greater number of working memory incorrect errors than the 12M males (p = 0.025). Figures depicting the means ± SEMs for all measures from number of working memory incorrect errors committed during the radial arm water maze are presented in Figure S4M–Q.
3.5. Immunoblotting
The immunoblotting results below are organized by brain region. For the sake of clarity, only statistically significant outcomes are highlighted in the main text. Full statistical details, including null results, are provided in Tables 1, 2 and 3. Graphical depictions of the negative results for protein expression within the entorhinal cortex, prefrontal cortex and amygdala are presented in Figures S5–S10.
3.6. Entorhinal cortex
3.6.1. Group 1 mGluRs
A significant interaction between sex and drinking history was detected for mGlu1 expression only in 6M mice [F(1,34) = 7.71, p = 0.009, ɳ 2 = 0.185; Figure 6A]. LSD tests for simple main effects showed that 6M binge‐drinking male mice exhibited a significantly higher mGlu1 expression versus both their water‐drinking counterparts (p = 0.006) and the female binge‐drinking mice (p < 0.001). Additionally, a significant main effect of drinking history was detected for the mGlu5 monomer expression in both 9M [F(1,34) = 4.73, p = 0.037, ɳ 2 = 0.122; H2O > EtOH; Figure 6B] and 12M mice [F(1,27) = 12.59, p = 0.001, ɳ 2 = 0.318; EtOH > H2O; Figure 6C].
FIGURE 6.
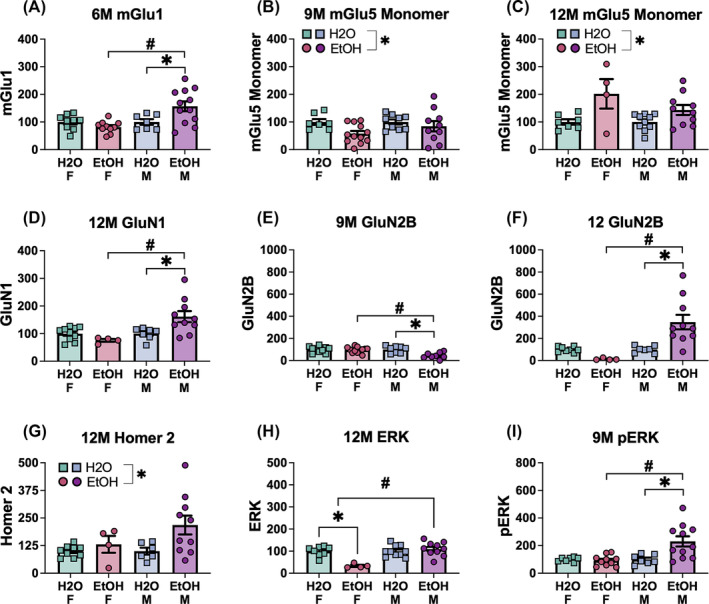
Summary of the significant effects of the sex by drinking history ANOVA on the expression of glutamate‐related proteins in the entorhinal cortex. Group 1 mGlu receptors: (A) 6M mGlu1 and (B,C) 9M and 12M mGlu5 (monomer), the NMDA receptor subunits: (D) 12M GluN1, (E,F) 9M and 12M GluN2B, (G) 12M Homer 2a/b, (H) 12M ERK and (I) 9M pERK. Data represent means ± SEMs, with specific significant interactions and main effects highlighted. *p < 0.05, EtOH versus H2O; # p < 0.05, age difference.
3.6.2. NMDA subunits
A significant sex × drinking history interaction for GluN2B was detected in 9M mice [F(1,32) = 5.47, p = 0.009, ɳ 2 = 0.185; Figure 6E] and LSD tests for simple main effects indicated lower GluN2B expression in binge‐drinking males versus both their water controls (p < 0.001) and binge‐drinking females (p < 0.001). Additionally, significant interactions for both GluN1 and GluN2B subunits were detected in 12M mice [GluN1: F(1,27) = 5.91, p = 0.010, ɳ 2 = 0.220; GluN2B: F(1,25) = 11.24, p = 0.003, ɳ 2 = 0.310]. Analysis of simple main effects revealed that 12M binge‐drinking mice exhibited higher levels of both GluN1 and GluN2B than both male water‐drinking controls (GluN1: p = 0.006; GluN2B: p < 0.001) and female alcohol‐drinking mice (GluN1: p < 0.001; GluN2B: p < 0.001), as shown in Figure 6D,F, respectively.
3.6.3. Homer proteins
A significant main effect of drinking history was detected for Homer 2a/b levels in the 12M mice [F(1,25) = 4.59, p = 0.042, ɳ 2 = 0.155; EtOH > H2O; Figure 6G).
3.6.4. ERK
A significant interaction was observed for p (Tyr204)‐ERK in 9M mice [F(1,31) = 7.30, p = 0.011, ɳ 2 = 0.191; Figure 6I] that reflect higher phospho‐ERK levels in binge‐drinking males, compared to both the male water‐drinking mice (p = 0.001) and female binge‐drinking mice (p < 0.001). Additionally, in 12M mice, a significant interaction was detected for ERK expression [F(1,28) = 15.52, p < 0.001, ɳ 2 = 0.357; Figure 6H].
3.6.5. Tau proteins
A significant sex × drinking history interaction was detected in 9M mice [F(1,33) = 5.96, p = 0.020, ɳ 2 = 0.153; Figure 7A] and simple main effects analysis indicated revealed elevated p (Thr217)‐Tau levels in binge‐drinking males, relative to both their male water‐drinking controls (p = 0.002) and female alcohol‐drinking counterparts (p < 0.001). Additionally, a significant drinking history effect was also detected in 12M mice [F(1,28) = 16.51, p < 0.001, ɳ 2 = 0.371; EtOH > H2O; Figure 7B]. For p (Ser936)‐Tau, a significant main effect of drinking history was found in 9M mice [F(1,33) = 15.17, p < 0.001, ɳ 2 = 0.315; H2O > EtOH; Figure 7C].
FIGURE 7.
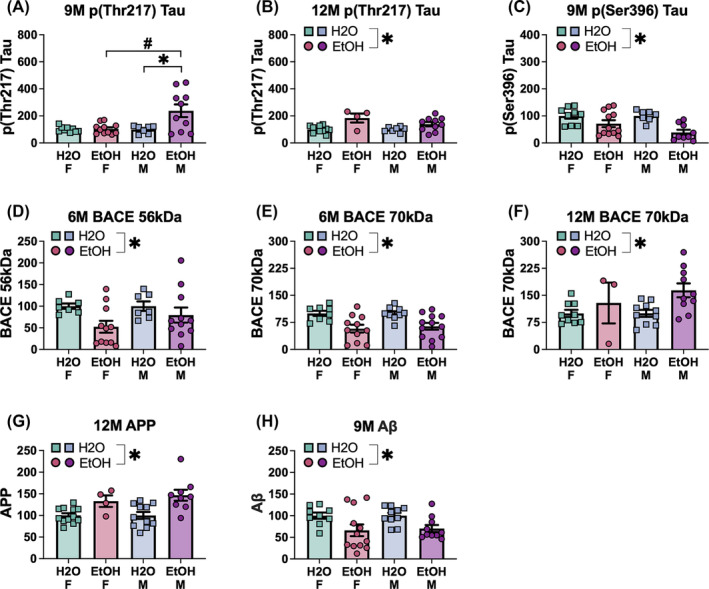
Summary of the significant effects of the sex by drinking history ANOVA on the expression of indices of neuropathology in the entorhinal cortex. Tau proteins: (A,B) 9M and 12M p (Thr217)‐tau and (C) 9M p (Ser396)‐tau, the BACE isoforms: (D) 6M BACE 56 kDa and (E,F) 6M and 12M BACE 70 kDa, (G) 12M APP and (H) 9M Aβ. Data represent means ± SEMs, with specific significant interactions and main effects highlighted. *p < 0.05, EtOH versus H2O; # p < 0.05, age difference.
3.6.6. BACE isoforms
Significant main drinking history effects were observed in 6M mice for both BACE 56 kDa [F(1,32) = 5.27, p = 0.028, ɳ 2 = 0.141; H2O > EtOH; Figure 7D] and BACE 70 kDa [F(1,36) = 17.63, p < 0.001, ɳ 2 = 0.329; H2O > EtOH; Figure 7E]. Moreover, a significant drinking history effect was observed for BACE 70 kDa in 12M animals [F(1,28) = 5.53, p = 0.026, ɳ 2 = 0.157; H2O < EtOH; Figure 7F].
3.6.7. APP and Aβ
A significant main drinking history effect was observed in 12M mice for APP [F(1,34) = 15.82, p < 0.001, eta = 0.331; H2O < EtOH; Figure 7G]. Additionally, for Aβ, a significant main drinking history effect was detected in 9M mice [F(1,37) = 10.09, p = 0.003, ɳ 2 = 0.241; H2O > EtOH; Figure 7H].
3.7. Prefrontal cortex
3.7.1. Group 1 mGluRs
A significant main effect of drinking history was detected for PFC mGlu1 expression in 6M mice [F(1,37) = 4.59, p = 0.039, η 2 = 0.110; Figure 8A] that reflected elevated mGlu1 expression in binge‐drinking mice of both sexes, compared to their water‐drinking counterparts. Additionally, a significant main drinking history effect was detected for mGlu5 monomer expression in 6M mice [F(1,33) = 6.41, p = 0.016, η 2 = 0.163; EtOH > H2O; Figure 8B]. In 9M mice, a significant sex × drinking history interaction was observed for mGlu5 monomer levels [F(1,39) = 5.40, p = 0.025, η 2 = 0.122; Figure 8C] and LSD tests for simple main effects detected elevated mGlu5 monomer levels in male water‐ versus male binge‐drinking mice (p = 0.010), in addition to higher mGlu5 monomer expression in female versus male binge‐drinking mice (p = 0.001).
FIGURE 8.
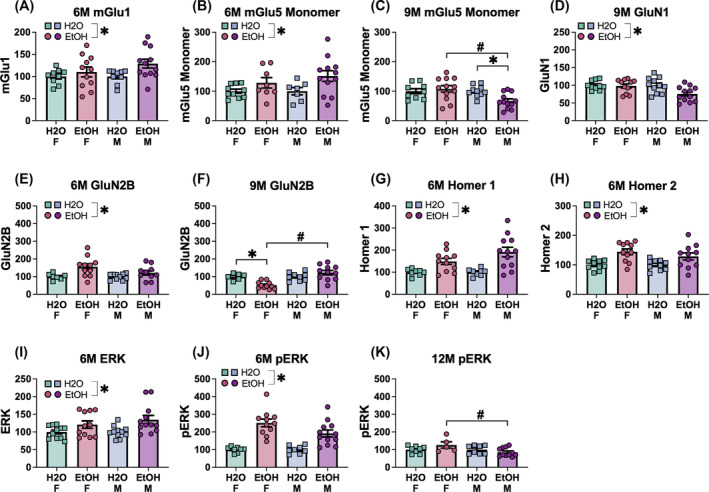
Summary of the significant effects of the sex by drinking history ANOVA on the expression of glutamate‐related proteins in the prefrontal cortex. Group 1 mGlu receptors: (A) 6M mGlu1, (B,C) 6M and 9M mGlu5 (monomer), the NMDA receptor subunits: (D) 9M GluN1 and (E,F) 6M and 9M GluN2B, (G) 6M Homer 1b/c and (H) 6M Homer 2a/b, (I) 6M ERK and (J,K) 6M and 12M pERK. Data represent means ± SEMs, with specific significant interactions and main effects highlighted. *p < 0.05, EtOH versus H2O; # p < 0.05, age difference.
3.7.2. NMDA subunits
A significant main drinking history effect on GluN1 was detected for the 9M mice [F(1,40) = 5.02, p = 0.031, η 2 = 0.112; H2O > EtOH; Figure 8D]. A significant main drinking history effect was identified for GluN2B expression in 6M mice [F(1,34) = 9.36, p = 0.004, η 2 = 0.216; EtOH > H2O; Figure 8E], while a significant interaction was detected in 9M mice [F(1,33) = 13.36, p < 0.001, η 2 = 0.288; Figure 8F], that reflected lower GluN2B expression in female binge‐drinking mice, relative to both their female water‐drinking counterparts (p = 0.002) and the male binge‐drinking mice (p < 0.001).
3.7.3. Homer proteins
A significant main drinking history effect was observed for both Homer 1b/c [F(1,35) = 19.09, p < 0.001, η 2 = 0.353; EtOH > H2O; Figure 8G] and Homer 2a/b [F(1,38) = 16.93, p < 0.001, η 2 = 0.308; EtOH > H2O; Figure 8H] expression within the PFC of 6M mice.
3.7.4. ERK
A significant main drinking history effect was observed for ERK expression in the PFC for 6M mice [F(1,41) = 9.76, p = 0.003, η 2 = 0.192; EtOH > H2O; Figure 8I]. Similarly, a significant main effect of drinking history was observed for PFC p (Tyr204)‐ERK expression in 6M mice [F(1,35) = 44.94, p < 0.001, η 2 = 0.562; EtOH > H2O; Figure 8J]. Moreover, a significant sex × drinking history interaction for p (Tyr204)‐ERK expression was observed in 12M mice [F(1,28) = 4.57, p = 0.042, η 2 = 0.140; Figure 8K], which reflected higher phospho‐ERK expression for the female binge‐drinking mice when compared to the their male binge‐drinking counterparts (p = 0.007).
3.7.5. Tau proteins
A significant main drinking history effect was detected for PFC expression of p (Thr217)‐Tau in 6M mice [F(1,34) = 4.86, p = 0.034, η 2 = 0.125; EtOH > H2O; Figure 9A]. Additionally, for the 12M cohort, a significant main effect of drinking history was found for p (Ser396), with binge‐drinking mice exhibiting lower levels of p (Ser396)‐Tau in comparison to their water‐drinking counterparts [F(1,28) = 10.01, p = 0.004, η 2 = 0.263; Figure 9B].
FIGURE 9.
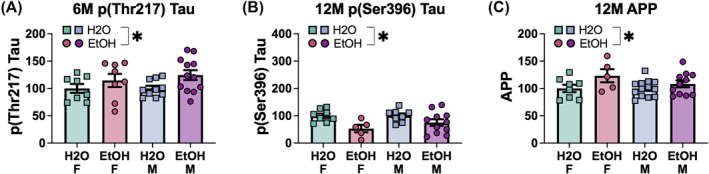
Summary of the significant effects of the sex by drinking history ANOVA on the expression of indices of neuropathology in the prefrontal cortex. Tau proteins: (A) 6M p (Thr217)‐tau and (B) 12M p (Ser396)‐tau and (C) 12M APP. Data represent means ± SEMs, with specific significant main effects highlighted. *p < 0.05, EtOH versus H2O.
3.7.6. APP and Aβ
A significant drinking history effect was observed for APP expression in the PFC of 12M mice [F(1,33) = 5.36, p = 0.027, η 2 = 0.140; EtOH > H2O; Figure 9C].
3.8. Amygdala
3.8.1. Group 1 mGluRs
Sex × drinking history ANOVAs detected a significant interaction for amygdala expression of mGlu1 in 6M mice [F(1,30) = 4.50, p = 0.042, η 2 = 0.130; Figure 10A], which reflected higher mGlu1 levels in female binge‐drinking mice versus their female water‐drinking controls (p = 0.001) and male binge‐drinking counterparts (p = 0.002). Additionally, a significant main drinking history effect was detected in 12M mice [F(1,33) = 7.93, p = 0.008, η 2 = 0.194; EtOH > H2O; Figure 10B]. For mGlu5 dimer expression, a significant sex × drinking history interaction was detected in both the 6M [F(1,35) = 5.94, p = 0.020, η 2 = 0.145; Figure 10C] and 12M animals [F(1,33) = 25.93, p < 0.001, η 2 = 0.440; Figure 10E]. In both cases, female binge‐drinking mice exhibited higher mGlu5 dimer expression relative to their female water‐drinking controls (6M: p = 0.029; 12M: p < 0.001) and their male binge‐drinking counterparts (6M and 12M: p's < 0.001). A main drinking day effect was also observed for mGlu5 dimer expression in 9M mice [F(1,34) = 4.38, p = 0.044, η 2 = 0.114; EtOH < H2O; Figure 10D].
FIGURE 10.
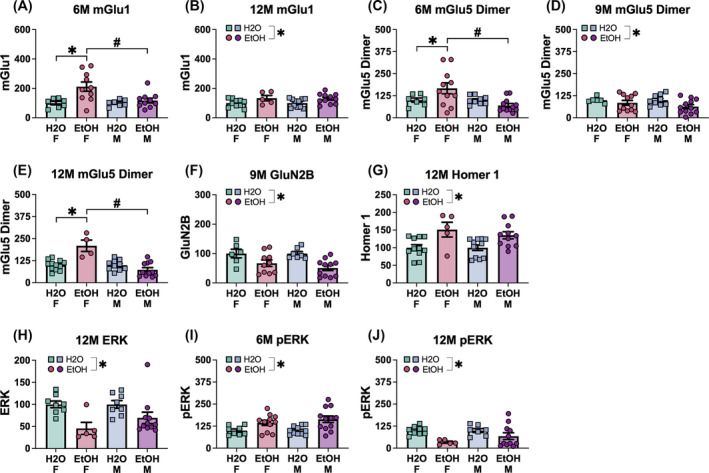
Summary of the significant effects of the sex by drinking history ANOVA on the expression of glutamate‐related proteins in the amygdala. Group 1 mGlu receptors: (A,B) 6M and 12M mGlu1 and (C–E) 6M, 9M and 12M mGlu5 (dimer), (F) 9M GluN2B, (G) 12M Homer 1b/c, (H) 12M ERK and (I,J) 6M and 12M pERK. Data represent means ± SEMs, with specific significant interactions and main effects highlighted. *p < 0.05, EtOH versus H2O; # p < 0.05, age difference.
3.8.2. NMDA subunits
A significant main drinking history effect was observed for GluN2B in 9M mice [F(1,30) = 14.53, p < 0.001, η 2 = 0.0326; EtOH < H2O; Figure 10F].
3.8.3. Homer proteins
A significant drinking history effect was observed for amygdala Homer1b/c in 12M mice [F(1,34) = 15.55, p < 0.001, η 2 = 0.314; EtOH > H2O; Figure 10G].
3.8.4. ERK
A significant drinking history effect on ERK was detected for the 12M mice [F(1,28) = 12.66, p = 0.001, η 2 = 0.311; EtOH < H2O; Figure 10H]. A drinking history effect was also detected for p (Tyr204)‐ERK in 6M [F(1,38) = 14.39, p = 0.001, η 2 = 0.275; EtOH > H2O; Figure 10I]. However, this group difference was reversed in the 12M mice [drinking history effect: F(1,28) = 9.84, p = 0.004, η 2 = 0.260; EtOH < H2O; Figure 10J].
3.8.5. Tau protein
A significant drinking history effect on amygdala Tau expression was observed in 6M mice [F(1,36) = 8.35, p = 0.007, η 2 = 0.188; EtOH < H2O; Figure 11A], with lower Tau levels in binge‐drinking mice compared to water‐drinking mice. In 12M mice, a main drinking history effect was also observed, but in contrast to 6M animals, this effect reflected higher Tau expression in binge‐ versus water‐drinking mice [F(1,27) = 9.30, p = 0.005, η 2 = 0.256; Figure 11B]. For p (Ser396)‐Tau, significant main drinking history effects were observed in both the 9M [F(1,32) = 11.53, p = 0.002, η 2 = 0.265; EtOH < H2O; Figure 11C] and 12M mice [F(1,27) = 16.63, p < 0.001, η 2 = 0.381; EtOH < H2O; Figure 11D].
FIGURE 11.
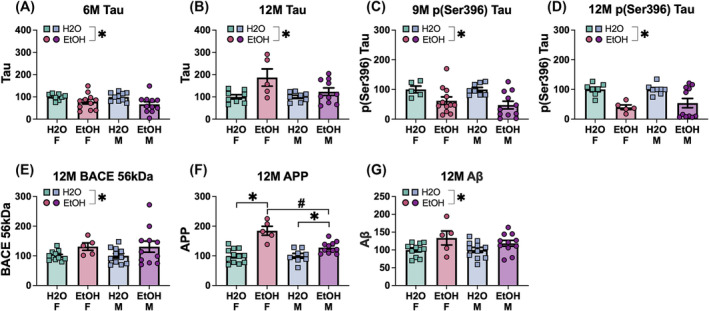
Summary of the significant effects of the sex by drinking history ANOVA on the expression of indices of neuropathology in the amygdala. Tau proteins: (A,B) 6M and 12M tau and (C,D) 9M and 12M p (Ser396)‐tau, (E) 12M BACE 56 kDa, (F) 12M APP and (G) 12M Aβ. Data represent means ± SEMs, with specific significant interactions and main effects highlighted. *p < 0.05, EtOH versus H2O; # p < 0.05, age difference.
3.8.6. BACE isoforms
A significant main effect of drinking history was observed for BACE 56 kDa in 12M mice [F(1,34) = 5.36, p = 0.027, η 2 = 0.136; EtOH > H2O; Figure 11E].
3.8.7. APP and Aβ
A significant sex × drinking history interaction was observed in the 12M mice for APP [F(1,32) = 11.42, p = 0.002, η 2 = 0.263; Figure 11F]. LSD tests for simple main effects indicated a higher APP expression in female binge‐drinking mice, compared to both their female water‐drinking controls (p < 0.001) and male binge‐drinking counterparts (p < 0.001). Moreover, 12M male binge‐drinking mice also displayed higher levels of APP versus their water‐drinking controls (p = 0.016). Additionally, a significant drinking history effect was observed for amygdala Aβ expression in 12M mice [F(1,34) = 7.99, p = 0.008, η 2 = 0.190; EtOH > H2O; Figure 11G].
4. DISCUSSION
The present study tracked the progression of the neurodevelopmental effects of a prior history of binge‐drinking during adolescence over the normal aging process through the study of 6‐, 9‐ and 12‐month‐old male and female C57BL6/J mice. This research involved the longitudinal tracking of both cognitive and affective behaviour in relation to aging‐related changes in NMDA and Group 1 mGlu receptor‐related protein expression, as well as protein indices of neuropathology associated with ADRD within the amygdala, the entorhinal cortex (EC) and prefrontal cortex (PFC). To the best of our knowledge, this study is the first to examine the long‐term consequences of adolescent binge‐drinking from mature adulthood into middle age in both female and male mice. Based on a relatively large literature indicating that a history of adolescent alcohol exposure is sufficient to elicit biobehavioural anomalies that can manifest in adulthood, 35 , 59 104 , 105 , 106 , 107 we hypothesized that binge‐drinking during the period of adolescence into young adulthood would accelerate age‐related decline in cognition and emotional regulation, concomitant with perturbations in glutamate‐related signalling and the expression of protein markers of neuropathology. Further, based on the clinical literature demonstrating greater susceptibility to ADRD and alcohol‐induced cognitive impairment in women versus men, 108 , 109 , 110 , 111 in addition to recent results from our laboratory indicating that female mice with a history of binge‐drinking during mature adulthood exhibit more signs of cognitive impairment than their male counterparts, 43 we hypothesized that alcohol‐induced biobehavioural anomalies would have an earlier onset, occur more frequently and/or be more severe, in female versus male mice. While our results are complex, they nevertheless provide evidence supporting enduring consequences of binge‐drinking during the adolescent/young adulthood period of neurodevelopment, particularly with respect to our biochemical measures. Also, consistent with our study of older mice, 43 cognition‐related measures appear to be more consistently impacted by a prior history of binge‐drinking than affective measures. Finally, aligning with data from studies of transgenic murine models of ADRD, 41 , 98 , 112 , 113 the majority of biochemical anomalies and the expression of neuropathology markers tended to precede the manifestation of cognitive anomalies in our aging mice with a prior history of early life binge‐drinking.
4.1. Subject factor interactions in negative affect
Girls and women are purported to exhibit greater vulnerability to, and severity of, anxiety‐related disorders than boys and men. 114 , 115 Further, girls and women are reported to be more sensitive to the affective components of alcohol withdrawal. 15 , 116 However, our prior studies of younger adult mice (aged 1.5–2.5 months) examining for sex differences in basal versus alcohol withdrawal‐induced changes in negative affect yielded relatively few sex differences in the expression of anxiety‐like behaviour, at least in the assays employed herein. 42 , 43 , 48 While the results of our first sex difference study 42 might reflect confounds associated with exposure to pheromones of mice from the opposite sex, 70 our more recent reports, 43 , 48 and the current study, assayed signs of negative affect separately in males and females to eliminate this confounding variable. Yet, despite these efforts, we detected only a few sex differences in our anxiety‐related measures in studies of either 6‐ and 18‐month‐old mice 43 or 2‐month‐old mice. 48 Moreover, in our prior study of older mice, 43 the sex differences in affective behaviour varied by age (being more prevalent in 6‐ versus 18‐month‐old mice), but did not vary as a function of sex as the alcohol effects were observed in both male and female subjects. Consistent with this published work, the present study revealed few sex‐related differences in negative affect in mice ranging from 6 to 12 months of age. Specifically, we detected an age‐related reduction in the both the time spent in, as well as the number of entries into, the light side of the light–dark box test that was selective for female mice (Figure 3A,B). Additionally, 9M females buried more marbles than 6M and 12M females (Figure 3D), indicating peak anxiety‐like behaviour at this age. Interestingly, males also showed an age‐related effect in the number of marbles buried, with 9M males burying more marbles than the 6M males (Figure 3E). Conversely, only males exhibited an age‐related increase in the latency to first float in the forced swim test (Figure 3F), arguing that males switch their coping strategy in this assay from passive to active as they age. All three of these sex by age interactions were independent of the alcohol history of the mice.
In contrast to a relatively recent report on the long‐term effects of a prior history of adolescent binge‐drinking on measures of negative affect in 3xTG‐AD mice, 41 we detected only two alcohol effects with respect to our negative affect measures—a reduction in the number of light‐side entries (Figure 3C) and an increase in the time spent immobile in the forced swim test (Figure 3G)—both of which were age‐independent and observed in females only. While we have reported little to no sex differences in the expression of alcohol‐induced negative affect during early withdrawal, 42 , 48 these results are our first demonstration of a female‐selective, long‐term, effect of prior adolescent binge‐drinking history on negative affect, which likely reflects the fact that mice in the present study binge‐drank throughout adolescence into young adulthood, while binge‐drinking procedures were restricted to the 2‐week period corresponding to adolescence in our earlier work. 42 , 48 As the massive neuroplasticity associated with the adolescent period of development continues into early adulthood in both humans and laboratory animals, 117 , 118 it is perhaps not surprising that repeated bouts of binge‐drinking during adolescence into young adulthood would have a larger or longer‐lasting impact on brain function and behaviour than that during adolescence alone. Indeed, we detected numerous alcohol‐associated changes in protein indices of glutamate transmission and neuropathology in our aging mice with a prior binge‐drinking history during adolescence/young adulthood (see Tables 1, 2, 3 and 4) to indicate that early life binge‐drinking has long‐term consequences for the brain. Further, approximately a third of these changes varied as a function of sex, with alcohol‐experienced females exhibiting the most pronounced biochemical effects.
TABLE 4.
Summary of the differential protein expression patterns organized by age groups across various brain regions.
| A | Glutamate‐related proteins—all brain regions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entorhinal cortex (EC) | Prefrontal cortex (PFC) | Amygdala (AMY) | |||||||
| 6M | 9M | 12M | 6M | 9M | 12M | 6M | 9M | 12M | |
| mGlu1 |
EtOH M > H2O M EtOH M > EtOH F |
EtOH > H2O |
EtOH F > H2O F EtOH F > EtOH M |
EtOH > H2O | |||||
| mGlu5 Dimer | EtOH F > H2O F EtOH F > EtOH M | H2O > EtOH |
EtOH F > H2O F EtOH F > EtOH M |
||||||
| mGlu5 Monomer | H2O > EtOH | EtOH > H2O | EtOH > H2O |
EtOH M < EtOH F EtOH M < H2O M |
|||||
| GluN1 | EtOH M > H2O M EtOH M > EtOH F | EtOH < H2O | |||||||
| GluN2B |
EtOH M < H2O M EtOH M < EtOH F |
EtOH M > H2O M EtOH M > EtOH F | EtOH > H2O |
EtOH F < H2O F EtOH F < EtOH M |
H2O > EtOH | ||||
| Homer 1b/c | EtOH > H2O | EtOH > H2O | |||||||
| Homer 2a/b | EtOH > H2O | EtOH > H2O | |||||||
| ERK |
EtOH F < H2O F EtOH F < EtOH M |
EtOH > H2O | H2O > EtOH | ||||||
| pERK |
EtOH M > H2O M EtOH M > EtOH F |
EtOH > H2O | EtOH F > EtOH M | EtOH > H2O | H2O > EtOH | ||||
| B | Neuropathological protein expression—all brain regions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tau | H2O > EtOH | EtOH > H2O | |||||||
| pThr(217) Tau |
EtOH M > H2O M EtOH M > EtOH F |
EtOH > H2O | EtOH > H2O | ||||||
| pSer(396) Tau | H2O > EtOH | H2O > EtOH | H2O > EtOH | H2O > EtOH | |||||
| BACE 56 kDa | H2O > EtOH | EtOH > H2O | |||||||
| BACE 70 kDa | H2O > EtOH | EtOH > H2O | |||||||
| APP | EtOH > H2O | EtOH > H2O |
EtOH F > H2O F EtOH F > EtOH M EtOH M > H2O M |
||||||
| Aβ | H2O > EtOH | EtOH > H2O | |||||||
Given our biochemical results, particularly those for the amygdala and PFC (see Table 4) that are key neural loci governing emotional reactivity, 119 , 120 , 121 , 122 one might question why so few alcohol‐related changes in affective behaviour were apparent in the present study? Clearly, the lengthy duration of drug withdrawal is not a major factor as robust changes in brain biochemistry and neuropathology were detected at these late withdrawal time‐points. Perhaps our alcohol‐induced biochemical and/or neuropathological changes were simply of an insufficient magnitude and/or require more time to accumulate in brain to drive more overt changes in behaviour? Related to this latter possibility, biochemical/neuropathological changes in the brain often precede behavioural anomalies in many transgenic models of neurodegenerative disease (e.g., Parkinson's and Alzheimer's disease), 123 , 124 , 125 which may also hold true for the long‐term consequences of early life alcohol exposure. Lastly, the possibility exists that the behavioural paradigms employed in this study to assay negative affect are not as sensitive to the long‐term effects of early alcohol exposure as they are for detecting its short‐term effects. Indeed, we based our decision to assay negative affect using the light–dark shuttle‐box, marble‐burying and forced swim tests in the present study on data collected from adolescent and young adult (primarily male) mice demonstrating that these three assays were the most reliable at detecting both the short‐ and longer‐term (i.e. 1 versus 30 days withdrawal) effects of prior binge‐drinking history. 45 , 46 , 49 , 69 However, our more recent study of 6‐ and 18‐month‐old mice detected robust alcohol effects in behavioural paradigms that we abandoned over the years that we have studied adolescent binge‐drinking, including the elevated plus‐maze and novel object reactivity tests. 43 Thus, a recommendation for any future work aimed at characterizing alcohol‐induced changes in the behaviour of older mice is to employ a broad repertoire of assays for negative affect should they prove to be differentially sensitive to the age of the subjects tested.
4.2. Subject factor interactions in cognition
Aligning with our results for negative affect, we also detected relatively few age‐related deficits in cognitive performance in the present study, particularly in the radial arm maze where the only significant age‐related effect related to the number of working memory incorrect errors but was inconsistent over the course of maze acquisition and only observed in male mice (Figure 5H). That being said, some evidence indicated that cognitive performance varied with age and did so in a sex‐selective manner. Specifically, we observed an age‐dependent increase in the latency of female mice to find the flagged platform at the outset of Morris water maze testing (Figure 4A). Females also showed a significant age‐dependent increase in the latency to find the hidden platform on the first day of Morris water maze training that was not as robust in male subjects (Figure 4B vs. 4C). Other age‐related differences in Morris water maze performance were also male‐selective, including a longer latency to first enter the zone that formerly contained the hidden platform behaviour by 9M males (Figure 4D) and an age‐related increase in the number of entries into the former platform location during the memory probe test (Figure 4F). However, in contrast to the data from females, these age‐related effects in males are difficult to interpret as the former and latter result is indicative of poorer and better spatial memory, respectively. While only a few age‐related effects were detected, such findings are notable as they align with the bulk of the extant human and laboratory animal literature arguing that females are more sensitive to normal age‐related cognitive decline than males. 126 , 127 , 128 , 129 , 130
A prior history of early life binge‐drinking also exerted few effects on cognitive performance in the Morris water maze in B6 mice. This contrasts with the results of an earlier study of 3XTg‐AD mice where prior adolescent intermittent alcohol exposure (5 g/kg/day) was found to markedly enhance the cognitive impairment exhibited by these transgenic mice months later in adulthood. 41 However, it is noteworthy that the only effect observed (a reduction in the number of platform entries) was apparent in 9M females (Figure 4E), particularly considering that the alcohol‐related effects on cognitive performance in the radial arm maze were most consistently expressed by female subjects (Figure 5). At the present time, it is not clear why alcohol effects apparent in 9M females (e.g. fewer entries into the former platform location (Figure 4E) and more reference memory errors toward the end of radial arm maze training (Figure 5D)) were not expressed also by the older females in this study. However, it is worth noting that 12M females with a prior history of early life binge‐drinking exhibited the most consistent pattern of cognitive deficits during radial arm maze testing (Figure 5). This latter result aligns well with the extant literature indicating that females are more sensitive to alcohol‐induced acceleration of cognitive decline (e.g. 43 , 131 , 132 ) and demonstrate for the first time that inbred B6 females are more vulnerable to the very long‐term cognitive consequences of early life binge‐drinking. An important goal of future work is to extend these findings for alcohol to even older mice (e.g. 18–24 months of age) that exhibit more signs of age‐related cognitive decline upon which to assess the effects of early life binge‐drinking.
4.3. Subject factor interactions in protein markers of glutamate transmission and neuropathology
As mentioned above, we detected a large number of alcohol‐related protein changes in all three brain regions examined, despite relatively few overt changes in affective or cognitive behaviour. As apparent from Table 4, the specific protein changes varied by brain region and by the age of the mice at assay, with the number of protein changes observed within the EC and amygdala increasing in an age‐dependent manner, while the number of protein changes in the PFC declined with aging. Further, we failed to detect a single change in protein expression that was consistent across all age groups, even within a given brain region. It remains to be determined if that observation reflects age‐related dynamics in protein expression or an artefact related to the procedural design of our study given that the tissue from 6M, 9M and 12M mice were collected at different times. Nevertheless, it is clear from Table 4 that the majority of alcohol‐related protein changes were apparent in both sexes and that a prior history of binge‐drinking during adolescence/young adulthood is not only capable of altering cellular activity (indexed by Egr‐1 expression 69 ) and dysregulating glutamate neurotransmission in brain during early withdrawal (e.g. 45 , 46 , 57 ) but is sufficient to induce very long‐term perturbations in cellular activity (indexed by p (Tyr204)‐ERK expression) and the expression of glutamate receptor‐related proteins (the vast majority of which were up‐regulated during alcohol withdrawal) that are linked to neurodegenerative mechanisms, such as amyloid‐beta deposition and tau hyperphosphorylation—hallmarks of both normal age‐related cognitive decline (e.g. 127 , 133 , 134 ) and ADRD‐related behavioural pathologies. 88 , 89 , 90 , 91 , 92 , 93
Aligning with this, we detected a host of alcohol‐related changes in the expression of such markers of dementia‐ and ADRD‐associated neuropathology, including an increase in the following: the highly specific biomarker of AD p (Thr217)‐tau, the purported early marker of AD neuropathology p (Ser396)‐tau (Janelidze et al. 2020), the key component of amyloid plaques Aβ β‐amyloid peptide, 135 , 136 , 137 both the 56 and 70 kD isoforms of the β‐site amyloid precursor protein‐cleaving enzyme BACE and its target APP (c.f. 135 , 137 ). These results are consistent with those from our earlier study of older B6 mice, 44 as well as studies of 3xTg‐AD transgenic mice, 98 in which both male and female mice with a history of alcohol‐drinking exhibited comparable changes in the expression of many ADRD‐associated biomarkers in brain. APP is demonstrated to directly interact with GluN2B subunits of NMDA receptors, to affect synaptic function and promote amyloidogenic fragments that exacerbate abnormal NMDA receptor activity with age. 138 While a causal relationship between our alcohol‐induced changes in glutamate receptor expression and in the levels of neuropathology markers remain to be determined, the present results extend our earlier findings from more aged mice 44 by demonstrating that a history of binge‐drinking during early life is sufficient to perturb glutamate receptor expression within the EC, PFC and amygdala and to augment the expression of neuropathology markers during the normal aging process. Noteworthy is the fact that the majority of our alcohol‐related protein changes were apparent in both male and female mice, despite the fact that the behavioural effects of early life drinking were primarily sex‐selective. Whether or not this brain‐behaviour discrepancy reflects sex differences in the behavioural manifestation of brain anomalies (i.e. the notion that males and females employ different neural mechanisms to generate behaviour) and/or differential behavioural sensitivity to brain anomalies (i.e. females may be more sensitive to smaller changes in biochemistry than males) cannot be discerned from the results of the present study. Further, it is possible that a greater congruency between behavioural and biochemical outcomes might be observed in older mice that naturally exhibit higher expression of ADRD‐related biomarkers (e.g. 139 , 140 , 141 ). Nevertheless, it does appear from this collection of behavioural and immunoblotting data that, at least with respect to the proteins examined herein, the long‐term biochemical effects of early life binge‐drinking within the EC, PFC and amygdala do not map perfectly onto the behaviour of mature adult and middle‐aged mice and may in fact precede abnormal behaviour.
While the majority of alcohol‐related protein changes observed were not sex‐selective, some sex‐selectivity was noted for alcohol‐related changes in protein expression that appeared to vary with the brain region examined (Table 4). For example, all but one alcohol effect on protein expression within the EC was male‐selective; binge‐drinking males exhibiting higher mGlu1 expression at 6M, lower GluN2B at 9M, with higher GluN1, GluN2B, p (Thr217)‐tau and p (Tyr204)‐ERK at 12M. The only female‐selective alcohol‐related protein change observed in the EC was reduced ERK expression in 12M mice. The male‐selectivity of the alcohol effect on protein expression within the EC aligns with the results of our recent study of more aged mice in which males exhibited a number of age and/or alcohol‐related changes in glutamate receptor expression within the hippocampus that were not apparent in female mice. 44 Unfortunately, we were not able to assay protein expression within the hippocampus herein. However, given that the EC is a major afferent to the hippocampus (e.g. 142 ), we would predict similar patterns of alcohol‐related changes in hippocampal protein expression and the hippocampus will be an important target of future work related to the long‐term biochemical consequences of early life binge‐drinking.
Also consistent with our earlier report in which both male and female older mice exhibited robust alcohol‐related changes in glutamate receptor expression within the PFC, 44 we detected many alcohol‐related changes in protein expression within PFC, only a few of which were sex‐selective. These included: lower mGlu5 monomer expression in 12M males, higher GluN2B in 9M females and higher p (Tyr204)‐ERK in 12M. While we did not assay for amygdala protein expression in our earlier study of binge‐drinking in older adult mice, a history of binge‐drinking during adolescence is reported to increase AD‐associated inflammation biomarkers within the amygdala of adult female, but not male, 3XTg‐AD mice. 41 Consistent with a greater sensitivity of female transgenic mice to alcohol‐induced neuropathology in the amygdala, we detected no male‐selective alcohol‐related protein changes in the amygdala while female binge‐drinking mice exhibited higher expression of mGlu1 and the mGlu5 dimer at 6M, as well as higher mGlu5 dimer and APP levels at 12M. Whether and how any of our changes in protein expression drive the few sex differences in alcohol‐related changes in affective and cognitive function observed herein is an important research question we seek to address in future work.
5. CONCLUSIONS
A prior month‐long history of binge‐drinking during adolescence into young adulthood is sufficient to elicit changes in affect and cognition that manifest into middle age, particularly in female mice. Moreover, a prior history of binge‐drinking over the period of adolescence and early young adulthood produces many biochemical changes in the EC, amygdala and PFC that are also apparent into middle age, to include increased expression of ADRD‐associated biomarkers. Most of the alcohol‐related changes in protein expression are not sex‐selective, although male‐selective protein changes were prevalent within the EC, while female‐selective changes were prevalent in the amygdala. While correlational in nature, the present results add to the growing body of preclinical experimental evidence that a prior history of excessive alcohol‐drinking during early life can impact brain and behaviour in the very long‐term in a manner that can be sex‐dependent.
ETHICS APPROVAL STATEMENT
This work.
Supporting information
Table S1: Summary of the full factorial ANOVAs results in (A) tests for negative affect and (B) tests for cognitive functioning.
Figure S1. Depiction of nonsignificant Age by Drinking History ANOVA results for the observed behaviour in the light–dark box shuttle, marble‐burying, and forced swim tests for female and male mice. (A,B) Latency to enter the light side for female and (B) male mice. (C) Total time spent in the light side for female and (C) male mice. (E) Total number of entries to the light side for female and (F) male mice. (G) Total number of marbles buried for female and (H) male mice. (I) Latency to first immobile episodes for female and (J) male mice. (K) Total time spent immobile for female and (L) male mice. (M) Total number of immobile episodes for female and (N) male mice. Data represent means ± SEMs.
Figure S2. Depiction of the observed behaviour in the acoustic startle test for female and male mice. Analyses of the acoustic stimulus‐startle response curve for the female (A‐C) and male (G‐J) mice indicated no significant Age by Drinking History by Stimulus interactions at any of the stimulus intensities. No significant Age by Drinking History by Prepulse Stimulus interaction was noted for the (D‐F) female or (K‐M) male mice. Data represent means ± SEMs.
Figure S3. Depiction of the nonsignificant results for the Morris water maze for female and male mice. (A) No significant Age by Drinking History ANOVA interaction was observed for the latency to locate the flagged platform by female or (B) male mice. (C) No differences were observed in the latency to enter the platform's former location during the probe test for the female and (D) male mice. (E) Male mice showed no differences in entries to the platform's former location during the probe test. Mixed‐model Day by Age by Drinking History ANOVAs for the latency to locate the hidden platform during acquisition of the Morris maze indicated no group differences in either (F‐H) female or (I‐K) male mice. In the reversal test, neither the (L‐N) female or (O‐Q) male mice exhibited significant interactions in the time taken to locate the repositioned platform. Data represent means ± SEMs.
Figure S4. Depicition of the nonsignificant results for the Radial Arm Water Mater Maze for female and male mice. No significant interactions were observed for the time taken to complete maze for (A) 6‐month females, (B) 9‐month males, and (C) 12‐month males. (D) No differences were observed in the number of reference memory errors committed by 6‐month females, (E) 12‐month females, (F) 6‐month males, and (G) 9‐month males. Similarily, no significant interactions or differences were noted regarding the total number of working memory correct errors for (H) 6‐month females, (I) 9‐month females, and (J‐L) males of all three age groups. For the total number of working memory incorrect errors, no significant interactions were detected for (M) 6‐month females, (N) 9‐month females, and (O‐Q) males of all three age groups. Data represent means ± SEMs; (ns) p > 0.05, not statistically significant
Figure S5. Summary of the nonsignificant effects of the Sex by Drinking History ANOVA on the expression of glutamate‐related proteins in the entorhinal cortex. (A) mGlu1, (B) mGlu5 (monomer) and (C) mGlu5 (dimer), (D) ERK, (E) GluN1, (F) GluN2B, (G) pERK, (H) Homer 1b/c and (I) Homer 2a/b. Data represent means ± SEMs.
Figure S6. Summary of the nonsignificant effects of the Sex by Drinking History ANOVA on the expression of indices of neuropathology in the entorhinal cortex. (A) Tau, (B) p(Tau217)‐Tau, (C) p(Ser396)‐Tau, (D) BACE 56 kDa, (E) BACE 70 kDa, (F) APP and (G) Aβ. Data represent means ± SEMs.
Figure S7. Summary of the nonsignificant effects of the Sex by Drinking History ANOVA on the expression of glutamate‐related proteins in the prefrontal cortex. (A) mGlu1, (B) mGlu5 (dimer) and (C) mGlu5 (monomer), (D) GluN1, (E) GluN2B, (F) Homer 1b/c, (G) Homer 2a/b, (H) ERK and (I) pERK. Data represent means ± SEMs.
Figure S8. Summary of the nonsignificant effects of the Sex by Drinking History ANOVA on the expression of indices of neuropathology in the prefrontal cortex. (A) Tau, (B) BACE 56 kDa, (C) p(Tau217)‐Tau, (D) BACE 70 kDa, (E) p(Ser396)‐Tau, (F) APP and (G) Aβ. Data represent means ± SEMs.
Figure S9. Summary of the nonsignificant effects of the Sex by Drinking History ANOVA on the expression of glutamate‐related proteins in the amygdala. (A) mGlu1, (B) mGlu5 (monomer), (C) GluN1, (D) GluN2B, (E) Homer 1b/c, (F) Homer 2a/b, (G) ERK and (H) pERK. Data represent means ± SEMs.
Figure S10. Summary of the nonsignificant effects of the Sex by Drinking History ANOVA on the expression of indices of neuropathology in the amygdala. (A) Tau, (B) p(Tau217)‐Tau, (C) p(Ser396)‐Tau, (D) BACE 56 kDa, (E) BACE 70 kDa, (F) APP and (G) Aβ. Data represent means ± SEMs.
Chavez CLJ, Scheldrup GP, Madory LE, et al. Biochemical changes precede affective and cognitive anomalies in aging adult C57BL/6J mice with a prior history of adolescent alcohol binge‐drinking. Addiction Biology. 2024;29(12):e70006. doi: 10.1111/adb.70006
Funding information This work was supported by NIH grant AA024044, UCSB Faculty Research Assistant Program awards to KKS and NSF DMR 1720256 awarded to the Materials Research Science and Engineering Center at UC Santa Barbara. CLJC was supported by an NSF Graduate Research Program Fellowship 2139319. CJED and LEM were supported by NIH grant DA053328 (KKS).
DATA AVAILABILITY STATEMENT
The data are available upon request.
REFERENCES
- 1. Dorn LD. Measuring puberty. J Adolesc Health. 2006;39(5):625‐626. doi: 10.1016/j.jadohealth.2006.05.014 [DOI] [PubMed] [Google Scholar]
- 2. Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9(2):60‐68. doi: 10.1016/j.tics.2004.12.008 [DOI] [PubMed] [Google Scholar]
- 3. Spear L. The behavioral neuroscience of adolescence. Norton & Company; 2010. [Google Scholar]
- 4. Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2009;35(1):217‐238. doi: 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Novier A, Diaz‐Granados JL, Matthews DB. Alcohol use across the lifespan: an analysis of adolescent and aged rodents and humans. Pharmacol Biochem Behav. 2015;133:65‐82. doi: 10.1016/j.pbb.2015.03.015 [DOI] [PubMed] [Google Scholar]
- 6. Spear LP, Varlinskaya EI. Adolescence: alcohol sensitivity, tolerance, and alcohol. Recent Dev Alcohol. 2005;17:143‐159. doi: 10.1007/0-306-48626-1_7 [DOI] [PubMed] [Google Scholar]
- 7. New Mexico Department of Health (NM‐IBIS) . Binge drinking (past 30 days) by year, grades 9–12, New Mexico and U.S., 2001 to 2017. Indicator‐Based Information System for Public Health. 2022. Retrieved from https://ibis.doh.nm.gov/ [Google Scholar]
- 8. National Institute on Alcohol Abuse and Alcoholism (NIAAA) . (2023). Drinking levels defined. Retrieved from https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking [Google Scholar]
- 9. Chung T, Creswell KG, Bachrach R, Clark DB, Martin CS. Adolescent binge drinking: developmental context and opportunities for prevention. Alcohol Res. 2018;39(1):5‐15. [PMC free article] [PubMed] [Google Scholar]
- 10. Substance Abuse Mental Health Services Administration (SAMHSA) . Key substance use and mental health indicators in the United States: results from the 2020 National Survey on drug use and health. 2021. Retrived from https://rb.gy/yaw0fc
- 11. Duka T, Gentry J, Malcolm R, et al. Consequences of multiple withdrawals from alcohol. Alcohol Clin Exp Res. 2004;28(2):233‐246. doi: 10.1097/01.alc.0000113780.41701.81 [DOI] [PubMed] [Google Scholar]
- 12. Addolorato G, Vassallo GA, Antonelli G, et al. Binge drinking among adolescents is related to the development of alcohol use disorders: results from a cross‐sectional study. Sci Rep. 2018;8(1):12624. doi: 10.1038/s41598-018-29311-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cruz B, Borgonetti V, Bajo M, Roberto M. Sex‐dependent factors of alcohol and neuroimmune mechanisms. Neurobiol Stress. 2023;26:100562. doi: 10.1016/j.ynstr.2023.100562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flores‐Bonilla A, Richardson H. Sex differences in the neurobiology of alcohol use disorder. Alcohol Res. 2020;40(2):04. doi: 10.35946/arcr.v40.2.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peltier MR, Verplaetse TL, Mineur YS, et al. Sex differences in stress‐related alcohol use. Neurobiol Stress. 2019;10:100149. doi: 10.1016/j.ynstr.2019.100149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verplaetse TL, Moore KE, Pittman BP, et al. Intersection of stress and gender in association with transitions in past year DSM‐5 substance use disorder diagnoses in the United States. Chronic Stress. 2018;2:247054701775263. doi: 10.1177/2470547017752637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Albert PR. Why is depression more prevalent in women? J Psychiatry Neurosci. 2015;40(4):219‐221. doi: 10.1503/jpn.150205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guinle MIB, Sinha R. The role of stress, trauma, and negative affect in the development of alcohol misuse and alcohol use disorders in women. Alcohol Res. 2020;40(2):05. doi: 10.35946/arcr.v40.2.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mumenthaler MS, Taylor JL, O'Hara R, Yesavage JA. Gender differences in moderate drinking effects. Alcohol Res Health, 1999;23(1):55–64. Retrived from https://pubmed.ncbi.nlm.nih.gov/10890798/ [PMC free article] [PubMed] [Google Scholar]
- 20. Agabio R, Pisanu C, Gessa GL, Franconi F. Sex differences in alcohol use disorder. Curr Med Chem. 2017;24(24):2661‐2670. doi: 10.2174/0929867323666161202092908 [DOI] [PubMed] [Google Scholar]
- 21. Connor JP, Haber PS, Hall WD. Alcohol use disorders. The Lancet. 2016;387(10022):988‐998. doi: 10.1016/s0140-6736(15)00122-1 [DOI] [PubMed] [Google Scholar]
- 22. Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease. Circulation. 1999;99(9):1165‐1172. doi: 10.1161/01.cir.99.9.1165 [DOI] [PubMed] [Google Scholar]
- 23. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12‐month alcohol use, high‐risk drinking, and DSM‐IV alcohol use disorder in the United States, 2001‐2002 to 2012‐2013. JAMA Psychiatry. 2017;74(9):911‐923. doi: 10.1001/jamapsychiatry.2017.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwarzinger M, Pollock BG, Hasan OSM, et al. Contribution of alcohol use disorders to the burden of dementia in France 2008–13: a nationwide retrospective cohort study. Lancet Public Health. 2018;3(3):e124‐e132. doi: 10.1016/s2468-2667(18)30022-7 [DOI] [PubMed] [Google Scholar]
- 25. Podcasy JL, Epperson CN. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci. 2016;18(4):437‐446. doi: 10.31887/dcns.2016.18.4/cepperson [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bahorik A, Bobrow K, Hoang T, Yaffe K. Increased risk of dementia in older female US veterans with alcohol use disorder. Addiction. 2021;116(8):2049‐2055. doi: 10.1111/add.15416 [DOI] [PubMed] [Google Scholar]
- 27. Carbia C, Cadaveira F, Caamaño‐Isorna F, Rodríguez‐Holguín S, Corral M. Binge drinking during adolescence and young adulthood is associated with deficits in verbal episodic memory. PLoS ONE. 2017;12(2):e0171393. doi: 10.1371/journal.pone.0171393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crego A, Holguín SR, Parada M, Mota N, Corral M, Cadaveira F. Binge drinking affects attentional and visual working memory processing in young university students. Alcohol Clin Exp Res. 2009;33(11):1870‐1879. doi: 10.1111/j.1530-0277.2009.01025.x [DOI] [PubMed] [Google Scholar]
- 29. Lees B, Meredith LR, Kirkland AE, Bryant BE, Squeglia LM. Effect of alcohol use on the adolescent brain and behavior. Pharmacol Biochem Behav. 2020;192:172906. doi: 10.1016/j.pbb.2020.172906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32(3):386‐394. doi: 10.1111/j.1530-0277.2007.00602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Bellis MD, Clark DB, Beers SR, et al. Hippocampal volume in adolescent‐onset alcohol use disorders. Am J Psychiatry. 2000;157(5):737‐744. doi: 10.1176/appi.ajp.157.5.737 [DOI] [PubMed] [Google Scholar]
- 32. Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res Neuroimaging. 2005;139(3):181‐190. doi: 10.1016/j.pscychresns.2005.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ibáñez J, Herrero MT, Insausti R, et al. Chronic alcoholism decreases neuronal nuclear size in the human entorhinal cortex. Neurosci Lett. 1995;183(1–2):71‐74. doi: 10.1016/0304-3940(94)11117-2 [DOI] [PubMed] [Google Scholar]
- 34. Hyman BT, Van Hoesen GW, Kromer LJ, Damasio AR. Perforant pathway changes and the memory impairment of Alzheimer's disease. Ann Neurol. 1986;20(4):472‐481. doi: 10.1002/ana.410200406 [DOI] [PubMed] [Google Scholar]
- 35. Crews FT, Braun CJ, Hoplight B, Switzer RC III, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24(11):1712‐1723. doi: 10.1097/00000374-200011000-00014 [DOI] [PubMed] [Google Scholar]
- 36. van Hoesen GW, Hyman BT, Damasio AR. Entorhinal cortex pathology in Alzheimer's disease. Hippocampus. 1991;1(1):1‐8. doi: 10.1002/hipo.450010102 [DOI] [PubMed] [Google Scholar]
- 37. Sipos E, Kurunczi A, Kasza Á, et al. β‐Amyloid pathology in the entorhinal cortex of rats induces memory deficits: implications for Alzheimer's disease. Neuroscience. 2007;147(1):28‐36. doi: 10.1016/j.neuroscience.2007.04.011 [DOI] [PubMed] [Google Scholar]
- 38. McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27(1):1‐28. doi: 10.1146/annurev.neuro.27.070203.144157 [DOI] [PubMed] [Google Scholar]
- 39. Gonzalez‐Rodriguez M, Villar‐Conde S, Astillero‐Lopez V, et al. Human amygdala involvement in Alzheimer's disease revealed by stereological and dia‐PASEF analysis. Brain Pathol. 2023;33(5):e13180. doi: 10.1111/bpa.13180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal aβ causes the onset of early alzheimer's disease‐related cognitive deficits in transgenic mice. Neuron. 2005;45(5):675‐688. doi: 10.1016/j.neuron.2005.01.040 [DOI] [PubMed] [Google Scholar]
- 41. Barnett A, David E, Rohlman A, et al. Adolescent binge alcohol enhances early Alzheimer's disease pathology in adulthood through proinflammatory neuroimmune activation. Front Pharmacol. 2022;13:884170. doi: 10.3389/fphar.2022.884170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jimenez Chavez CL, Coelho MA, Brewin LW, et al. Incubation of negative affect during protracted alcohol withdrawal is age‐, but not sex‐selective. Brain Sci. 2020;10(6):405. doi: 10.3390/brainsci10060405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jimenez Chavez CL, Van Doren E, Matalon J, et al. Alcohol‐drinking under limited‐access procedures during mature adulthood accelerates the onset of cognitive impairment in mice. Front Behav Neurosci. 2022;16:732375. doi: 10.3389/fnbeh.2022.732375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Szumlinski KK, Herbert JN, Espinoza BM, Madory LE, Scudder SL. Alcohol‐drinking during later life by C57BL/6J mice induces sex‐ and age‐dependent changes in hippocampal and prefrontal cortex expression of glutamate receptors and neuropathology markers. Addict Neurosci. 2023;7:100099. doi: 10.1016/j.addicn.2023.100099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee KM, Coehlo MA, Solton NR, Szumlinski KK. Negative affect and excessive alcohol intake incubate during protracted withdrawal from binge‐drinking in adolescent, but not adult, mice. Front Psychol. 2017a;8:1128. doi: 10.3389/fpsyg.2017.01128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee KM, Coelho MA, McGregor HA, Solton NR, Cohen M, Szumlinski KK. Adolescent mice are resilient to alcohol withdrawal‐induced anxiety and changes in indices of glutamate function within the nucleus accumbens. Front Cell Neurosci. 2016;10:265. doi: 10.3389/fncel.2016.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee KM, Coelho MA, Sern KR, Class MA, Bocz MD, Szumlinski KK. Anxiolytic effects of buspirone and MTEP in the Porsolt forced swim test. Chronic Stress. 2017b;1:247054701771298. doi: 10.1177/2470547017712985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jimenez Chavez CL, Van Doren E, Scheldrup G, et al. A subchronic history of binge‐drinking elicits mild, age‐ and sex‐selective, affective, and cognitive anomalies in C57BL/6J mice. Front Behav Neurosci. 2023;17:1192076. doi: 10.3389/fnbeh.2023.1192076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Szumlinski KK, Coelho MA, Lee KM, et al. DID it or DIDn't it? Exploration of a failure to replicate binge‐like alcohol‐drinking in C57BL/6J mice. Pharmacol Biochem Behav. 2019;178:3‐18. doi: 10.1016/j.pbb.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Burnett EJ, Chandler LJ, Trantham‐Davidson H. Glutamatergic plasticity and alcohol dependence‐induced alterations in reward, affect and cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:309‐320. doi: 10.1016/j.pnpbp.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rao PSS, Bell RL, Engleman EA, Sari Y. Targeting glutamate uptake to treat alcohol use disorders. Front Neurosci. 2015;9:144. doi: 10.3389/fnins.2015.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roberto M, Varodayan FP. Synaptic targets: chronic alcohol actions. Neuropharmacology. 2017;122:85‐99. doi: 10.1016/j.neuropharm.2017.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brust JC. Ethanol and cognition: indirect effects, neurotoxicity and neuroprotection: a review. Int J Environ Res Public Health. 2010;7(4):1540‐1557. doi: 10.3390/ijerph7041540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peng B, Yang Q, Joshi B, et al. Role of alcohol drinking in Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis. Int J Mol Sci. 2020;21(7):2316. doi: 10.3390/ijms21072316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Campbell RR, Domingo RD, Williams AR, et al. Increased alcohol‐drinking induced by manipulations of mGlu5 phosphorylation within the bed nucleus of the stria terminalis. J Neurosci. 2019;39(14):2745‐2761. doi: 10.1523/jneurosci.1909-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee KM, Coelho MA, Class MA, Szumlinski KK. mGlu5‐dependent modulation of anxiety during early withdrawal from binge‐drinking in adult and adolescent male mice. Drug Alcohol Depend. 2018a;184:1‐11. doi: 10.1016/j.drugalcdep.2017.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee KM, Coelho MA, Class MA, Sern KR, Bocz MD, Szumlinski KK. mGlu5 receptor blockade within the nucleus accumbens shell reduces behavioral indices of alcohol withdrawal‐induced anxiety in mice. Front Pharmacol. 2018b;9:1306. doi: 10.3389/fphar.2018.01306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alaux‐Cantin S, Warnault V, Legastelois R, et al. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521‐531. doi: 10.1016/j.neuropharm.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 59. Crews FT, Vetreno RP, Broadwater MA, Robinson DL. Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharmacol Rev. 2016;68(4):1074‐1109. doi: 10.1124/pr.115.012138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Quoilin C, Didone V, Tirelli E, Quertemont E. Chronic ethanol exposure during adolescence alters the behavioral responsiveness to ethanol in adult mice. Behav Brain Res. 2012;229(1):1‐9. doi: 10.1016/j.bbr.2011.12.039 [DOI] [PubMed] [Google Scholar]
- 61. Flurkey K, McUrrer J, Harrison D. Mouse models in aging research. In: The mouse in biomedical research. Elsevier; 2007:637‐672. doi: 10.1016/b978-012369454-6/50074-1 [DOI] [Google Scholar]
- 62. Fukumoto H, Rosene DL, Moss MB, Raju S, Hyman BT, Irizarry MC. Beta‐secretase activity increases with aging in human, monkey, and mouse brain. Am J Pathol. 2004;164(2):719‐725. doi: 10.1016/s0002-9440(10)63159-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta‐secretase in Alzheimer's disease. Ann Neurol. 2002;51(6):783‐786. doi: 10.1002/ana.10208 [DOI] [PubMed] [Google Scholar]
- 64. Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta‐secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59(9):1381‐1389. doi: 10.1001/archneur.59.9.1381 [DOI] [PubMed] [Google Scholar]
- 65. Janelidze S, Stomrud E, Smith R, et al. Cerebrospinal fluid p‐tau217 performs better than p‐tau181 as a biomarker of Alzheimer's disease. Nat Commun. 2020a;11(1):1683. doi: 10.1038/s41467-020-15436-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004;117(24):5721‐5729. doi: 10.1242/jcs.01558 [DOI] [PubMed] [Google Scholar]
- 67. Cozzoli DK, Courson J, Wroten MG, et al. Binge alcohol drinking by mice requires intact group1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology. 2014;39(2):435‐444. doi: 10.1038/npp.2013.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee KM, Coelho MA, Sern KR, Szumlinski KK. Homer2 within the central nucleus of the amygdala gates withdrawal‐induced anxiety in a mouse model of binge‐drinking. Neuropharmacology. 2018c;128:448‐459. doi: 10.1016/j.neuropharm.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee KM, Coelho M, McGregor HA, Waltermire RS, Szumlinski KK. Binge alcohol drinking elicits persistent negative affect in mice. Behav Brain Res. 2015;291:385‐398. doi: 10.1016/j.bbr.2015.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jimenez Chavez CL, Szumlinski KK. Modulation of marble‐burying behavior in adult versus adolescent C57BL/6J mice of both sexes by ethologically relevant chemosensory stimuli. Oxford Open Neurosci. 2024;3:kvae009. doi: 10.1093/oons/kvae009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9(1):37‐44. doi: 10.1016/0149-7634(85)90030-2 [DOI] [PubMed] [Google Scholar]
- 72. Gallo I, Rattazzi L, Piras G, et al. Formyl peptide receptor as a novel therapeutic target for anxiety‐related disorders. PLoS ONE. 2014;9(12):e114626. doi: 10.1371/journal.pone.0114626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Datko MC, Hu JH, Williams M, et al. Behavioral and neurochemical phenotyping of mice incapable of homer1a induction. Front Behav Neurosci. 2017;11:208. doi: 10.3389/fnbeh.2017.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lominac KD, Oleson EB, Pava M, et al. Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J Neurosci. 2005;25(50):11586‐11594. doi: 10.1523/JNEUROSCI.3764-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Szumlinski KK, Lominac KD, Kleschen MJ, et al. Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes Brain Behav. 2005a;4(5):273‐288. doi: 10.1111/j.1601-183X.2005.00120.x [DOI] [PubMed] [Google Scholar]
- 76. Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2001;Chapter8:Unit 8.10A. 10.1002/0471142301.ns0810as14 [DOI] [PubMed] [Google Scholar]
- 77. Denning CJE, Madory LE, Herbert JN, Cabrera RA, Szumlinski KK. Neuropharmacological evidence implicating drug‐induced glutamate receptor dysfunction in affective and cognitive sequelae of subchronic methamphetamine self‐administration in mice. Int J Mol Sci. 2024;25(3):1928. doi: 10.3390/ijms25031928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jarrard LE, Okaichi H, Steward O, Goldschmidt RB. On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behav Neurosci. 1984;98(6):946‐954. doi: 10.1037//0735-7044.98.6.946 [DOI] [PubMed] [Google Scholar]
- 79. Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial‐arm maze. Physiol Behav. 2000;70(3–4):311‐317. doi: 10.1016/s0031-9384(00)00259-6 [DOI] [PubMed] [Google Scholar]
- 80. Wills TA, Baucum AJ 2nd, Holleran KM, et al. Chronic intermittent alcohol disrupts the GluN2B‐associated proteome and specifically regulates group I mGlu receptor‐dependent long‐term depression. Addict Biol. 2017;22(2):275‐290. doi: 10.1111/adb.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cozzoli DK, Courson J, Caruana AL, et al. Nucleus accumbens mGluR5‐associated signaling regulates binge alcohol drinking under drinking‐in‐the‐dark procedures. Alcohol Clin Exp Res. 2012;36(9):1623‐1633. doi: 10.1111/j.1530-0277.2012.01776.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cozzoli DK, Goulding SP, Zhang PW, et al. Binge drinking upregulates accumbens mGluR5‐Homer2‐PI3K signaling: functional implications for alcoholism. J Neurosci. 2009;29(27):8655‐8668. doi: 10.1523/JNEUROSCI.5900-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Swartzwelder HS, Risher ML, Miller KM, Colbran RJ, Winder DG, Wills TA. Changes in the adult GluN2B associated proteome following adolescent intermittent ethanol exposure. PLoS ONE. 2016;11(5):e0155951. doi: 10.1371/journal.pone.0155951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Szumlinski KK, Lominac KD, Oleson EB, et al. Homer2 is necessary for EtOH‐induced neuroplasticity. J Neurosci. 2005b;25(30):7054‐7061. doi: 10.1523/JNEUROSCI.1529-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xiao B, Tu JC, Petralia RS, et al. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer‐related, synaptic proteins. Neuron. 1998;21(4):707‐716. doi: 10.1016/s0896-6273(00)80588-7 [DOI] [PubMed] [Google Scholar]
- 86. Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE. Accumbens Homer2 overexpression facilitates alcohol‐induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology. 2008;33(6):1365‐1378. doi: 10.1038/sj.npp.1301473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cozzoli DK, Courson J, Rostock C, et al. Protein kinase c epsilon activity in the nucleus accumbens and central nucleus of the amygdala mediates binge alcohol consumption. Biol Psychiatry. 2016;79(6):443‐451. doi: 10.1016/j.biopsych.2015.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Banning LCP, Ramakers IHGB, Rosenberg PB, Lyketsos CG, Leoutsakos JS. Alzheimer's disease biomarkers as predictors of trajectories of depression and apathy in cognitively normal individuals, mild cognitive impairment, and Alzheimer's disease dementia. Int J Geriatr Psychiatry. 2021;36(1):224‐234. doi: 10.1002/gps.5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cheignon C, Tomas M, Bonnefont‐Rousselot D, Faller P, Hureau C, Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol. 2018;14:450‐464. doi: 10.1016/j.redox.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dodart JC, Mathis C, Bales KR, Paul SM. Does my mouse have Alzheimer's disease? Genes Brain Behav. 2002;1(3):142‐155. doi: 10.1034/j.1601-183x.2002.10302.x [DOI] [PubMed] [Google Scholar]
- 91. Hamley IW. The amyloid beta peptide: a chemist's perspective. Role in Alzheimer's and fibrillization. Chem Rev. 2012;112(10):5147‐5192. doi: 10.1021/cr3000994 [DOI] [PubMed] [Google Scholar]
- 92. Hersi M, Irvine B, Gupta P, Gomes J, Birkett N, Krewski D. Risk factors associated with the onset and progression of Alzheimer's disease: a systematic review of the evidence. Neurotoxicology. 2017;61:143‐187. doi: 10.1016/j.neuro.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 93. Perl DP. Neuropathology of Alzheimer's disease. Mt Sinai J Med. 2010;77(1):32‐42. doi: 10.1002/msj.20157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer‐type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology. 1992;42(9):1681‐1688. doi: 10.1212/wnl.42.9.1681 [DOI] [PubMed] [Google Scholar]
- 95. Haroutunian V, Perl DP, Purohit DP, et al. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Arch Neurol. 1998;55(9):1185‐1191. doi: 10.1001/archneur.55.9.1185 [DOI] [PubMed] [Google Scholar]
- 96. O'Brien RJ, Resnick SM, Zonderman AB, et al. Neuropathologic studies of the Baltimore longitudinal study of aging (BLSA). J Alzheimers Dis. 2009;18(3):665‐675. doi: 10.3233/JAD-2009-1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Troncoso JC, Cataldo AM, Nixon RA, et al. Neuropathology of preclinical and clinical late‐onset Alzheimer's disease. Ann Neurol. 1998;43(5):673‐676. doi: 10.1002/ana.410430519 [DOI] [PubMed] [Google Scholar]
- 98. Hoffman JL, Faccidomo S, Kim M, et al. Alcohol drinking exacerbates neural and behavioral pathology in the 3xTg‐AD mouse model of Alzheimer's disease. Int Rev Neurobiol. 2019;148:169‐230. doi: 10.1016/bs.irn.2019.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu M, Guo S, Huang D, et al. Chronic alcohol exposure alters gene expression and neurodegeneration pathways in the brain of adult mice. J Alzheimers Dis. 2022;86(1):315‐331. doi: 10.3233/JAD-215508 [DOI] [PubMed] [Google Scholar]
- 100. Salling MC, Faccidomo SP, Li C, et al. Moderate alcohol drinking and the amygdala proteome: identification and validation of calcium/calmodulin dependent kinase ii and AMPA receptor activity as novel molecular mechanisms of the positive reinforcing effects of alcohol. Biol Psychiatry. 2016;79(6):430‐442. doi: 10.1016/j.biopsych.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chiu AS, Kang MC, Huerta Sanchez LL, et al. Preclinical evidence to support repurposing everolimus for craving reduction during protracted drug withdrawal. Neuropsychopharmacology. 2021;46(12):2090‐2100. doi: 10.1038/s41386-021-01064-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Huerta Sanchez LL, Sankaran M, Li TL, et al. Profiling prefrontal cortex protein expression in rats exhibiting an incubation of cocaine craving following short‐access self‐administration procedures. Front Psych. 2023;13:1031585. doi: 10.3389/fpsyt.2022.1031585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Blanca MJ, Alarcón R, Arnau J. Non‐normal data: is ANOVA still a valid option? Psicothema. 2017;29(4):552‐557. doi: 10.7334/psicothema2016.383 [DOI] [PubMed] [Google Scholar]
- 104. Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS ONE. 2012;7(2):e31466. doi: 10.1371/journal.pone.0031466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hicks BM, Iacono WG, McGue M. Consequences of an adolescent onset and persistent course of alcohol dependence in men: adolescent risk factors and adult outcomes. Alcohol Clin Exp Res. 2010;34(5):819‐833. doi: 10.1111/j.1530-0277.2010.01154.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pascale A, Stephenson M, Barr P, et al. Exploring the relationships between adolescent alcohol misuse and later life health outcomes. Alcohol Clin Exp Res. 2022;46(9):1753‐1765. doi: 10.1111/acer.14917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wooden JI, Thompson KR, Guerin SP, Nawarawong NN, Nixon K. Consequences of adolescent alcohol use on adult hippocampal neurogenesis and hippocampal integrity. Int Rev Neurobiol. 2021;160:281‐304. doi: 10.1016/bs.irn.2021.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Squeglia LM, Jacobus J, Tapert SF. The effect of alcohol use on human adolescent brain structures and systems. Handb Clin Neurol. 2014;125:501‐510. doi: 10.1016/b978-0-444-62619-6.00028-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol Clin Exp Res. 2011;35(10):1831‐1841. doi: 10.1111/j.1530-0277.2011.01527.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology (Berl). 2012;220(3):529‐539. doi: 10.1007/s00213-011-2500-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav. 2009;23(4):715‐722. doi: 10.1037/a0016516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kosel F, Pelley JMS, Franklin TB. Behavioural and psychological symptoms of dementia in mouse models of Alzheimer's disease‐related pathology. Neurosci Biobehav Rev. 2020;112:634‐647. doi: 10.1016/j.neubiorev.2020.02.012 [DOI] [PubMed] [Google Scholar]
- 113. Samaey C, Schreurs A, Stroobants S, Balschun D. Early cognitive and behavioral deficits in mouse models for tauopathy and Alzheimer's disease. Front Aging Neurosci. 2019;11:335. doi: 10.3389/fnagi.2019.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Li SH, Graham BM. Why are women so vulnerable to anxiety, trauma‐related and stress‐related disorders? The potential role of sex hormones. Lancet Psychiatry. 2017;4(1):73‐82. doi: 10.1016/S2215-0366(16)30358-3 [DOI] [PubMed] [Google Scholar]
- 115. Smith AR, Jones EL, Subar AR, et al. The role of anxiety and gender in anticipation and avoidance of naturalistic anxiety‐provoking experiences during adolescence: an ecological momentary assessment study. JCPP Advances. 2022;2(3):e12084. doi: 10.1002/jcv2.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Varlinskaya EI, Spear LP. Social consequences of ethanol: impact of age, stress, and prior history of ethanol exposure. Physiol Behav. 2015;148:145‐150. doi: 10.1016/j.physbeh.2014.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Larsen B, Luna B. Adolescence as a neurobiological critical period for the development of higher‐order cognition. Neurosci Biobehav Rev. 2018;94:179‐195. doi: 10.1016/j.neubiorev.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lillard AS, Erisir A. Old dogs learning new tricks: neuroplasticity beyond the juvenile period. Dev Rev. 2011;31(4):207‐239. doi: 10.1016/j.dr.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61‐75. doi: 10.1016/j.brainres.2009.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Salzman CD, Fusi S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annu Rev Neurosci. 2010;33(1):173‐202. doi: 10.1146/annurev.neuro.051508.135256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Warden MR, Selimbeyoglu A, Mirzabekov JJ, et al. A prefrontal cortex‐brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492(7429):428‐432. doi: 10.1038/nature11617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wrase J, Makris N, Braus DF, et al. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165(9):1179‐1184. doi: 10.1176/appi.ajp.2008.07121877 [DOI] [PubMed] [Google Scholar]
- 123. Laforet GA, Sapp E, Chase K, et al. Changes in cortical and striatal neurons predict behavioral and electrophysiological abnormalities in a transgenic murine model of Huntington's disease. J Neurosci. 2001;21(23):9112‐9123. doi: 10.1523/jneurosci.21-23-09112.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Paumier KL, Sukoff Rizzo SJ, Berger Z, et al. Behavioral characterization of A53T mice reveals early and late stage deficits related to Parkinson's disease. PLoS ONE. 2013;8(8):e70274. doi: 10.1371/journal.pone.0070274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tag SH, Kim B, Bae J, Chang KA, Im HI. Neuropathological and behavioral features of an APP/PS1/MAPT (6xTg) transgenic model of Alzheimer's disease. Mol Brain. 2022;15(1):51. doi: 10.1186/s13041-022-00933-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Benice TS, Raber J. Testosterone and dihydrotestosterone differentially improve cognition in aged female mice. Learn Mem. 2009;16(8):479‐485. doi: 10.1101/lm.1428209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex‐differences in age‐related cognitive decline in C57BL/6J mice associated with increased brain microtubule‐associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137(2):413‐423. doi: 10.1016/j.neuroscience.2005.08.029 [DOI] [PubMed] [Google Scholar]
- 128. Jack CR Jr, Wiste HJ, Weigand SD, et al. Age, sex, and apoe ε4 effects on memory, brain structure, and β‐amyloid across the adult life span. JAMA Neurol. 2015;72(5):511‐519. doi: 10.1001/jamaneurol.2014.4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37‐48. doi: 10.2147/CLEP.S37929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Shokouhi S, Taylor WD, Albert K, Kang H, Newhouse PA, Alzheimer's Disease Neuroimaging Initiative . In vivo network models identify sex differences in the spread of tau pathology across the brain. Alzheimers Dement. 2020;12(1):e12016. doi: 10.1002/dad2.12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Fama R, Le Berre AP, Sullivan EV. Alcohol's unique effects on cognition in women: a 2020 (re)view to envision future research and treatment. Alcohol Res. 2020;40(2):03. doi: 10.35946/arcr.v40.2.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Maynard ME, Barton EA, Robinson CR, Wooden JI, Leasure JL. Sex differences in hippocampal damage, cognitive impairment, and trophic factor expression in an animal model of an alcohol use disorder. Brain Struct Funct. 2018;223(1):195‐210. doi: 10.1007/s00429-017-1482-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Fukumoto H, Asami‐Odaka A, Suzuki N, Shimada H, Ihara Y, Iwatsubo T. Amyloid beta protein deposition in normal aging has the same characteristics as that in Alzheimer's disease. Predominance of A beta 42(43) and association of A beta 40 with cored plaques. Am J Pathol. 1996;148(1):259‐265. [PMC free article] [PubMed] [Google Scholar]
- 134. Wiśniewski HM, Ghetti B, Terry RD. Neuritic (senile) plaques and filamentous changes in aged rhesus monkeys. J Neuropathol Exp Neurol. 1973;32(4):566‐584. doi: 10.1097/00005072-197310000-00007 [DOI] [PubMed] [Google Scholar]
- 135. Chen GF, Xu TH, Yan Y, et al. Amyloid beta: structure, biology and structure‐based therapeutic development. Acta Pharmacol Sin. 2017;38(9):1205‐1235. doi: 10.1038/aps.2017.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120(3):885‐890. doi: 10.1016/S0006-291X(84)80190-4 [DOI] [PubMed] [Google Scholar]
- 137. Li J, Wu X, Tan X, et al. The efficacy and safety of anti‐Aβ agents for delaying cognitive decline in Alzheimer's disease: a meta‐analysis. Front Aging Neurosci. 2023;15:1257973. doi: 10.3389/fnagi.2023.1257973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Rajão‐Saraiva J, Dunot J, Ribera A, et al. Age‐dependent NMDA receptor function is regulated by the amyloid precursor protein. Aging Cell. 2023;22(3):e13778. doi: 10.1111/acel.13778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bettio LEB, Rajendran L, Gil‐Mohapel J. The effects of aging in the hippocampus and cognitive decline. Neurosci Biobehav Rev. 2017;79:66‐86. doi: 10.1016/j.neubiorev.2017.04.030 [DOI] [PubMed] [Google Scholar]
- 140. Britton R, Liu AT, Rege SV, Adams JM, et al. Molecular and histological correlates of cognitive decline across age in male C57BL/6J mice. Brain and Behavior. 2022;12(9):e2736. doi: 10.1002/brb3.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hendrickx JO, De Moudt S, Calus E, De Deyn PP, Van Dam D, De Meyer GRY. Age‐related cognitive decline in spatial learning and memory of C57BL/6J mice. Behav Brain Res. 2022;418:113649. doi: 10.1016/j.bbr.2021.113649 [DOI] [PubMed] [Google Scholar]
- 142. Canto CB, Wouterlood FG, Witter MP. What does the anatomical organization of the entorhinal cortex tell us? Neural Plast. 2008;2008:381243. doi: 10.1155/2008/381243 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Summary of the full factorial ANOVAs results in (A) tests for negative affect and (B) tests for cognitive functioning.
Figure S1. Depiction of nonsignificant Age by Drinking History ANOVA results for the observed behaviour in the light–dark box shuttle, marble‐burying, and forced swim tests for female and male mice. (A,B) Latency to enter the light side for female and (B) male mice. (C) Total time spent in the light side for female and (C) male mice. (E) Total number of entries to the light side for female and (F) male mice. (G) Total number of marbles buried for female and (H) male mice. (I) Latency to first immobile episodes for female and (J) male mice. (K) Total time spent immobile for female and (L) male mice. (M) Total number of immobile episodes for female and (N) male mice. Data represent means ± SEMs.
Figure S2. Depiction of the observed behaviour in the acoustic startle test for female and male mice. Analyses of the acoustic stimulus‐startle response curve for the female (A‐C) and male (G‐J) mice indicated no significant Age by Drinking History by Stimulus interactions at any of the stimulus intensities. No significant Age by Drinking History by Prepulse Stimulus interaction was noted for the (D‐F) female or (K‐M) male mice. Data represent means ± SEMs.
Figure S3. Depiction of the nonsignificant results for the Morris water maze for female and male mice. (A) No significant Age by Drinking History ANOVA interaction was observed for the latency to locate the flagged platform by female or (B) male mice. (C) No differences were observed in the latency to enter the platform's former location during the probe test for the female and (D) male mice. (E) Male mice showed no differences in entries to the platform's former location during the probe test. Mixed‐model Day by Age by Drinking History ANOVAs for the latency to locate the hidden platform during acquisition of the Morris maze indicated no group differences in either (F‐H) female or (I‐K) male mice. In the reversal test, neither the (L‐N) female or (O‐Q) male mice exhibited significant interactions in the time taken to locate the repositioned platform. Data represent means ± SEMs.
Figure S4. Depicition of the nonsignificant results for the Radial Arm Water Mater Maze for female and male mice. No significant interactions were observed for the time taken to complete maze for (A) 6‐month females, (B) 9‐month males, and (C) 12‐month males. (D) No differences were observed in the number of reference memory errors committed by 6‐month females, (E) 12‐month females, (F) 6‐month males, and (G) 9‐month males. Similarily, no significant interactions or differences were noted regarding the total number of working memory correct errors for (H) 6‐month females, (I) 9‐month females, and (J‐L) males of all three age groups. For the total number of working memory incorrect errors, no significant interactions were detected for (M) 6‐month females, (N) 9‐month females, and (O‐Q) males of all three age groups. Data represent means ± SEMs; (ns) p > 0.05, not statistically significant
Figure S5. Summary of the nonsignificant effects of the Sex by Drinking History ANOVA on the expression of glutamate‐related proteins in the entorhinal cortex. (A) mGlu1, (B) mGlu5 (monomer) and (C) mGlu5 (dimer), (D) ERK, (E) GluN1, (F) GluN2B, (G) pERK, (H) Homer 1b/c and (I) Homer 2a/b. Data represent means ± SEMs.
Figure S6. Summary of the nonsignificant effects of the Sex by Drinking History ANOVA on the expression of indices of neuropathology in the entorhinal cortex. (A) Tau, (B) p(Tau217)‐Tau, (C) p(Ser396)‐Tau, (D) BACE 56 kDa, (E) BACE 70 kDa, (F) APP and (G) Aβ. Data represent means ± SEMs.
Figure S7. Summary of the nonsignificant effects of the Sex by Drinking History ANOVA on the expression of glutamate‐related proteins in the prefrontal cortex. (A) mGlu1, (B) mGlu5 (dimer) and (C) mGlu5 (monomer), (D) GluN1, (E) GluN2B, (F) Homer 1b/c, (G) Homer 2a/b, (H) ERK and (I) pERK. Data represent means ± SEMs.
Figure S8. Summary of the nonsignificant effects of the Sex by Drinking History ANOVA on the expression of indices of neuropathology in the prefrontal cortex. (A) Tau, (B) BACE 56 kDa, (C) p(Tau217)‐Tau, (D) BACE 70 kDa, (E) p(Ser396)‐Tau, (F) APP and (G) Aβ. Data represent means ± SEMs.
Figure S9. Summary of the nonsignificant effects of the Sex by Drinking History ANOVA on the expression of glutamate‐related proteins in the amygdala. (A) mGlu1, (B) mGlu5 (monomer), (C) GluN1, (D) GluN2B, (E) Homer 1b/c, (F) Homer 2a/b, (G) ERK and (H) pERK. Data represent means ± SEMs.
Figure S10. Summary of the nonsignificant effects of the Sex by Drinking History ANOVA on the expression of indices of neuropathology in the amygdala. (A) Tau, (B) p(Tau217)‐Tau, (C) p(Ser396)‐Tau, (D) BACE 56 kDa, (E) BACE 70 kDa, (F) APP and (G) Aβ. Data represent means ± SEMs.
Data Availability Statement
The data are available upon request.


