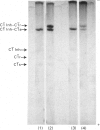
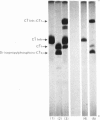
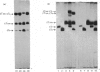
Abstract
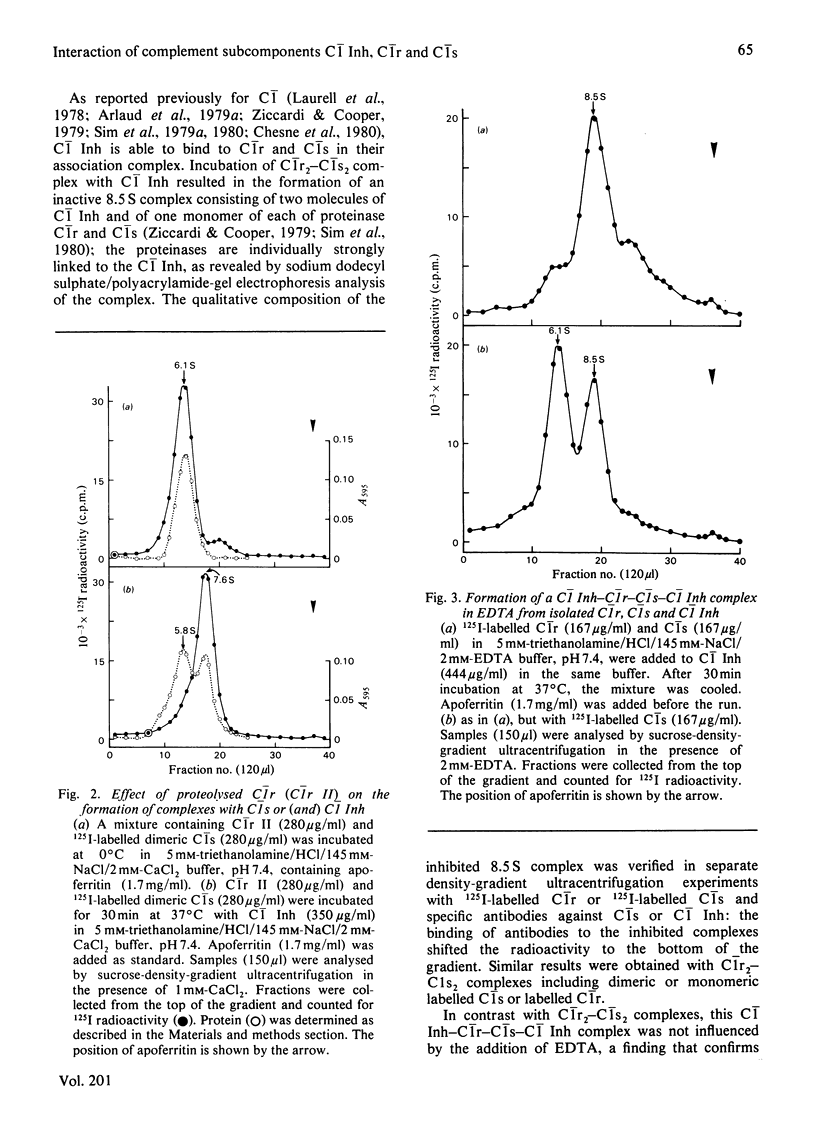
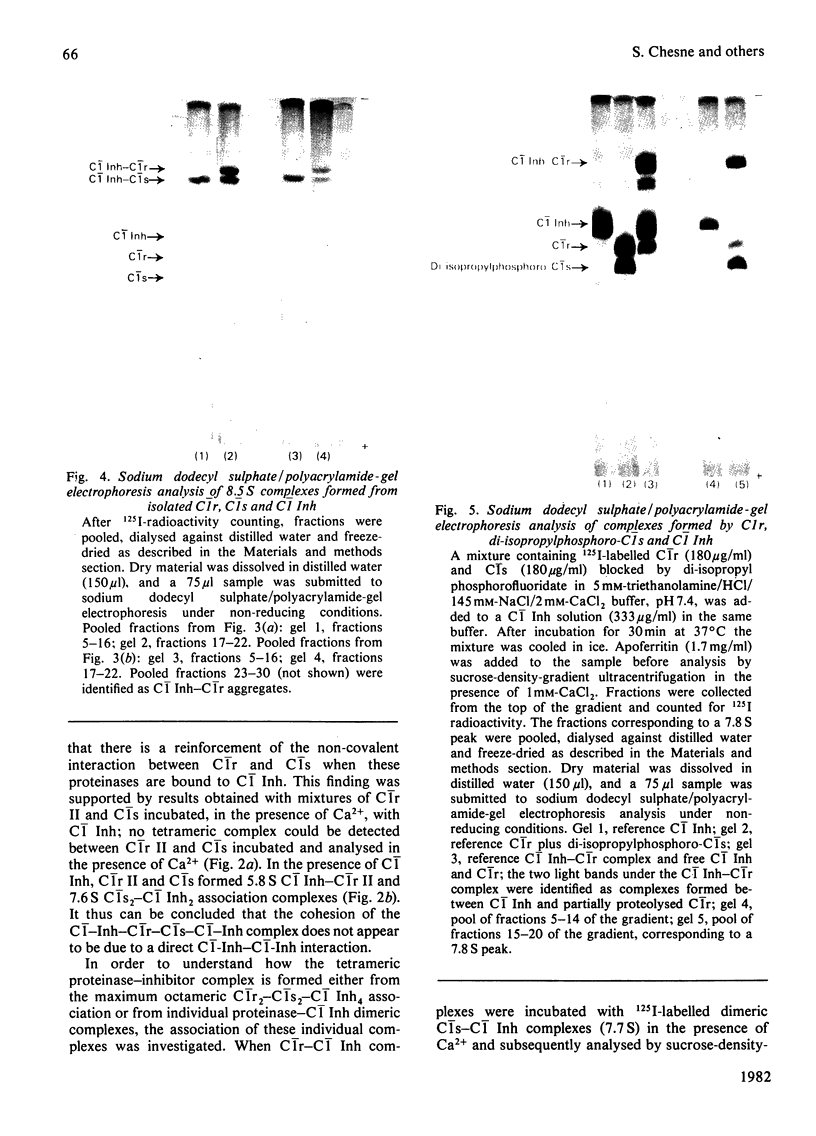
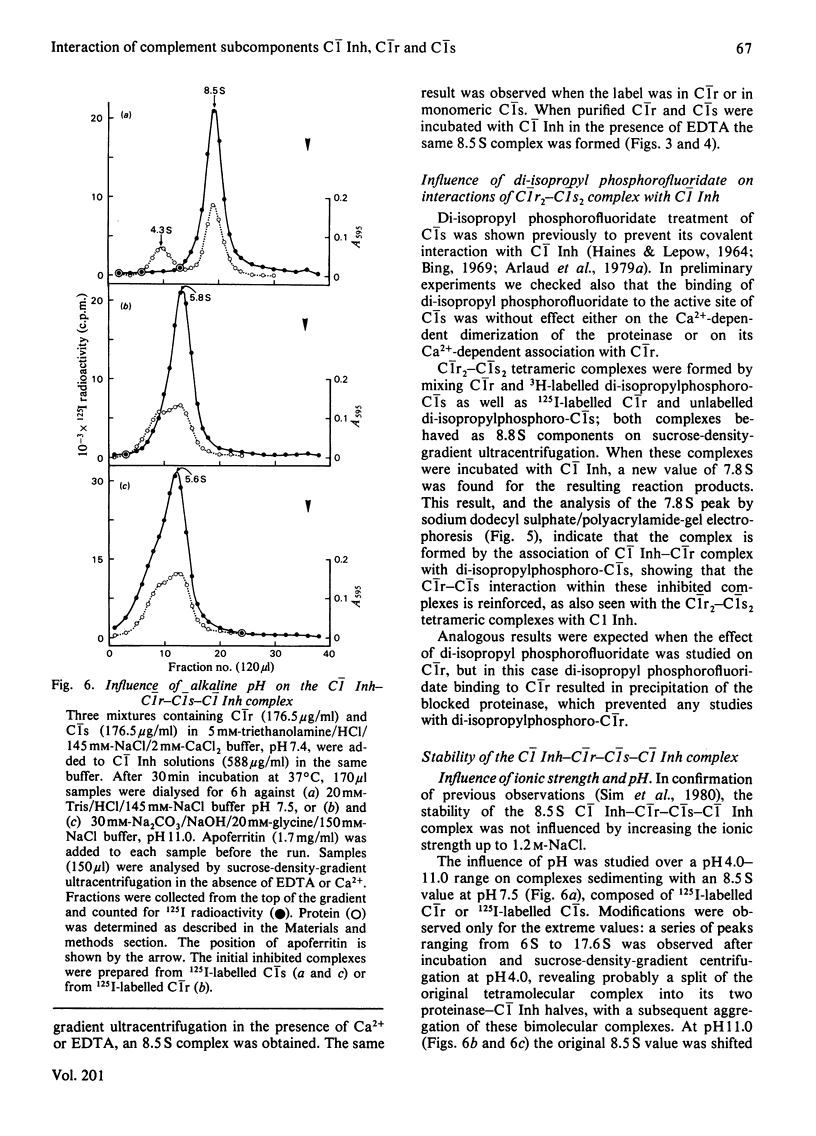
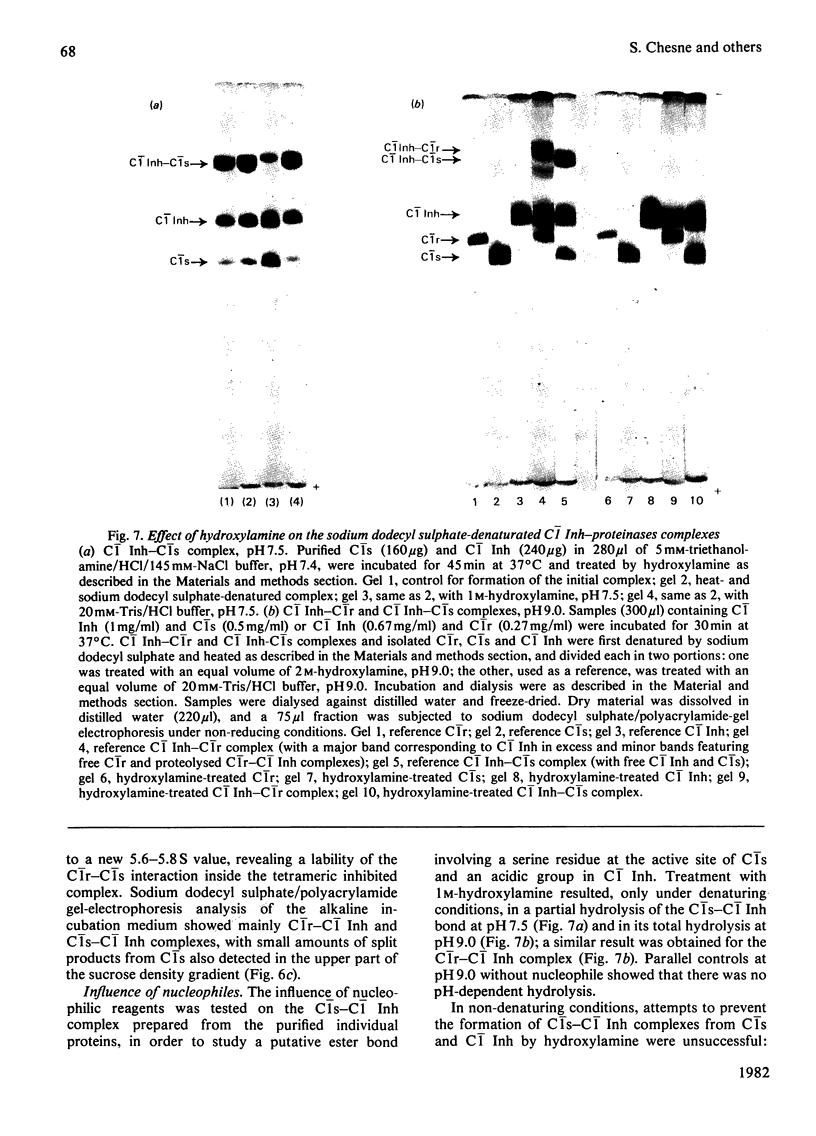
Interactions between proenzymic or activated complement subcomponents of C1 and C1 Inh (C1 inhibitor) were analysed by sucrose-density-gradient ultracentrifugation and sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. The interaction of C1 Inh with dimeric C1r in the presence of EDTA resulted into two bimolecular complexes accounting for a disruption of C1r. The interaction of C1 Inh with the Ca2+-dependent C1r2-C1s2 complex (8.8 S) led to an 8.5 S inhibited C1r-C1s-C1 Inh complex (1:1:2), indicating a disruption of C1r2 and of C1s2 on C1 Inh binding. The 8.5 S inhibited complex was stable in the presence of EDTA; it was also formed from a mixture of C1r, C1s and C1 Inh in the presence of EDTA or from bimolecular complexes of C1r-C1 Inh and C1s-C1 Inh. C1r II, a modified C1r molecule, deprived of a Ca2+-binding site after autoproteolysis, did not lead to an inhibited tetrameric complex on incubation with C1s and C1 Inh. These findings suggest that, when C1 Inh binds to C1r2-C1s2 complex, the intermonomer links inside C1r2 or C1s2 are weakened, whereas the non-covalent Ca2+-independent interaction between C1r2 and C1s2 is strengthened. The nature of the proteinase-C1 Inh link was investigated. Hydroxylamine (1M) was able to dissociate the complexes partially (pH 7.5) or totally (pH 9.0) when the incubation was performed in denaturing conditions. An ester link between a serine residue at the active site of C1r or C1s and C1 Inh is postulated.
Full text
PDF


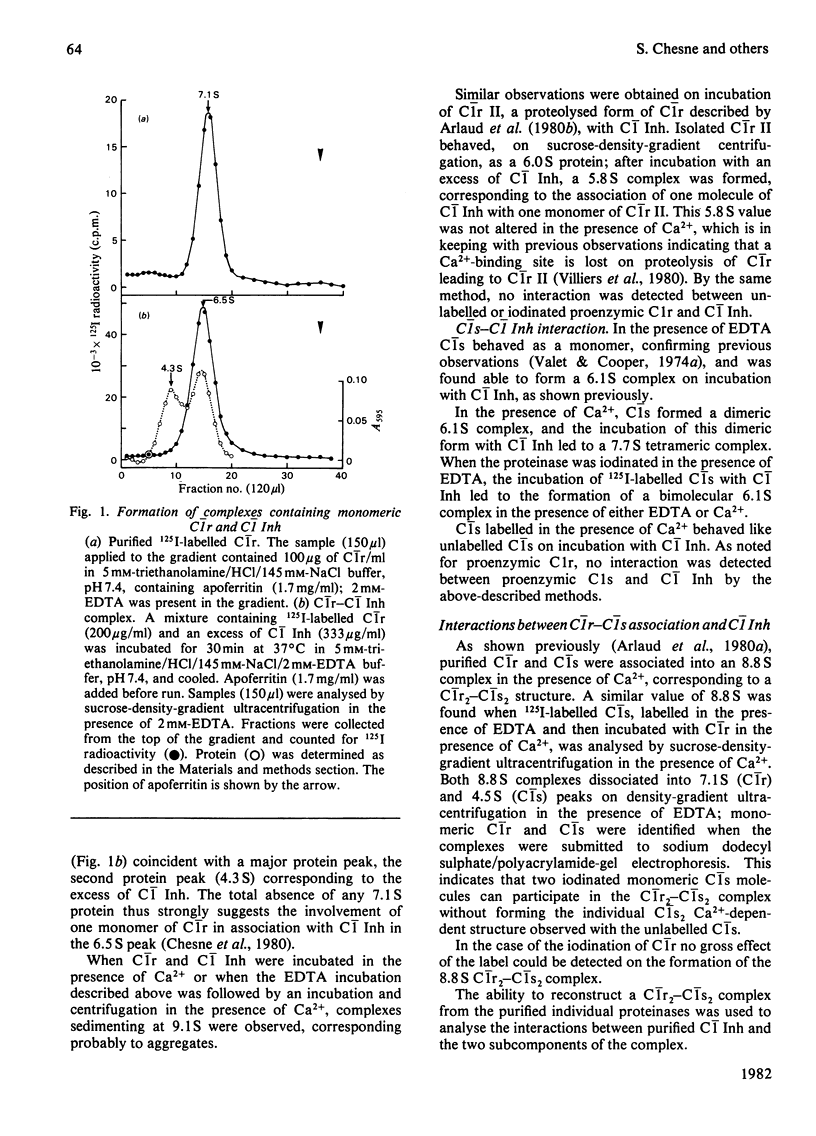


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlaud G. J., Chesne S., Villiers C. L., Colomb M. G. A study on the structure and interactions of the C1 sub-components C1r and C1s in the fluid phase. Biochim Biophys Acta. 1980 Nov 6;616(1):105–115. doi: 10.1016/0005-2744(80)90268-5. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Reboul A., Colomb M. G. Proenzymic C1s associated with catalytic amounts of C1r. Study of the activation process. Biochim Biophys Acta. 1977 Nov 23;485(1):227–235. doi: 10.1016/0005-2744(77)90209-1. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Reboul A., Meyer C. M., Colomb M. G. Purification of proenzymic and activated human C1s free ofC1r. Effect of calcium and ionic strength on activated C1s. Biochim Biophys Acta. 1977 Nov 23;485(1):215–225. doi: 10.1016/0005-2744(77)90208-x. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Sim R. B., Duplaa A. M., Colomb M. G. Differential elution of Clq, Clr and Cls from human Cl bound to immune aggregates. Use in the rapid purification of Cl subcomponents. Mol Immunol. 1979 Jul;16(7):445–450. doi: 10.1016/0161-5890(79)90069-5. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Villiers C. L., Chesne S., Colomb M. G. Purified proenzyme C1r. Some characteristics of its activation and subsequent proteolytic cleavage. Biochim Biophys Acta. 1980 Nov 6;616(1):116–129. doi: 10.1016/0005-2744(80)90269-7. [DOI] [PubMed] [Google Scholar]
- Bing D. H. Nature of the active site of a subunit of the first component of human complement. Biochemistry. 1969 Nov;8(11):4503–4510. doi: 10.1021/bi00839a042. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- HAINES A. L., LEPOW I. H. STUDIES ON HUMAN C'1-ESTERASE. II. FUNCTION OF PURIFIED C'1-ESTERASE IN THE HUMAN COMPLEMENT SYSTEM. J Immunol. 1964 Mar;92:468–478. [PubMed] [Google Scholar]
- Harpel P. C. C1 inactivator. Methods Enzymol. 1976;45:751–750. doi: 10.1016/s0076-6879(76)45068-1. [DOI] [PubMed] [Google Scholar]
- Laurell A. B., Johnson U., Mårtensson U., Sjöholm A. G. Formation of complexes composed of C1r, C1s, and C1 inactivator in human serum on activation of C1. Acta Pathol Microbiol Scand C. 1978 Dec;86C(6):299–306. doi: 10.1111/j.1699-0463.1978.tb02594.x. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Owen W. G. Evidence for the formation of an ester between thrombin and heparin cofactor. Biochim Biophys Acta. 1975 Oct 20;405(2):380–387. doi: 10.1016/0005-2795(75)90103-8. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The fractionation of rabbit gamma-globulin by partition chromatography. Biochem J. 1955 Mar;59(3):405–410. doi: 10.1042/bj0590405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul A., Arlaud G. J., Sim R. B., Colomb M. G. A simplified procedure for the purification of C1-inactivator from human plasma. Interaction with complement subcomponents C1r and C1s. FEBS Lett. 1977 Jul 1;79(1):45–50. doi: 10.1016/0014-5793(77)80347-5. [DOI] [PubMed] [Google Scholar]
- Reboul A., Thielens N., Villiers M. B., Colomb M. G. Purification of human complement subcomponent C4. C4 cleavage by C1s. FEBS Lett. 1979 Jul 1;103(1):156–161. doi: 10.1016/0014-5793(79)81271-5. [DOI] [PubMed] [Google Scholar]
- Sim R. B., Arlaud G. J., Colomb M. G. C1 inhibitor-dependent dissociation of human complement component C1 bound to immune complexes. Biochem J. 1979 Jun 1;179(3):449–457. doi: 10.1042/bj1790449a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Arlaud G. J., Colomb M. G. Kinetics of reaction of human C1-inhibitor with the human complement system proteases C1r and C1s. Biochim Biophys Acta. 1980 Apr 11;612(2):433–449. doi: 10.1016/0005-2744(80)90126-6. [DOI] [PubMed] [Google Scholar]
- Sim R. B., Porter R. R., Reid K. B., Gigli I. The structure and enzymic activities of the C1r and C1s subcomponents of C1, the first component of human serum complement. Biochem J. 1977 May 1;163(2):219–227. doi: 10.1042/bj1630219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Reboul A., Arlaud G. J., Villiers C. L., Colomb M. G. Interaction of 125I-labelled complement subcomponents C-1r and C-1s with protease inhibitors in plasma. FEBS Lett. 1979 Jan 1;97(1):111–115. doi: 10.1016/0014-5793(79)80063-0. [DOI] [PubMed] [Google Scholar]
- Tenner A. J., Cooper N. R. Analysis of receptor-mediated C1q binding to human peripheral blood mononuclear cells. J Immunol. 1980 Oct;125(4):1658–1664. [PubMed] [Google Scholar]
- Tenner A. J., Cooper N. R. Identification of types of cells in human peripheral blood that bind C1q. J Immunol. 1981 Mar;126(3):1174–1179. [PubMed] [Google Scholar]
- Valet G., Cooper N. R. Isolation and characterization of the proenzyme form of the C1r subunit of the first complement component. J Immunol. 1974 May;112(5):1667–1673. [PubMed] [Google Scholar]
- Valet G., Cooper N. R. Isolation and characterization of the proenzyme form of the C1s subunit of the first complement component. J Immunol. 1974 Jan;112(1):339–350. [PubMed] [Google Scholar]
- Villiers C. L., Arlaud G. J., Painter R. H., Colomb M. G. Calcium binding properties of the C1 subcomponents C1q, C1r and C1s. FEBS Lett. 1980 Aug 11;117(1):289–294. doi: 10.1016/0014-5793(80)80964-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Active disassembly of the first complement component, C-1, by C-1 inactivator. J Immunol. 1979 Aug;123(2):788–792. [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Physicochemical and functional characterization of the C1r subunit of the first complement component. J Immunol. 1976 Feb;116(2):496–503. [PubMed] [Google Scholar]