Abstract
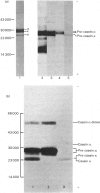
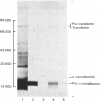
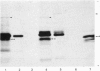
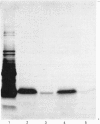
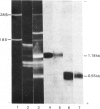
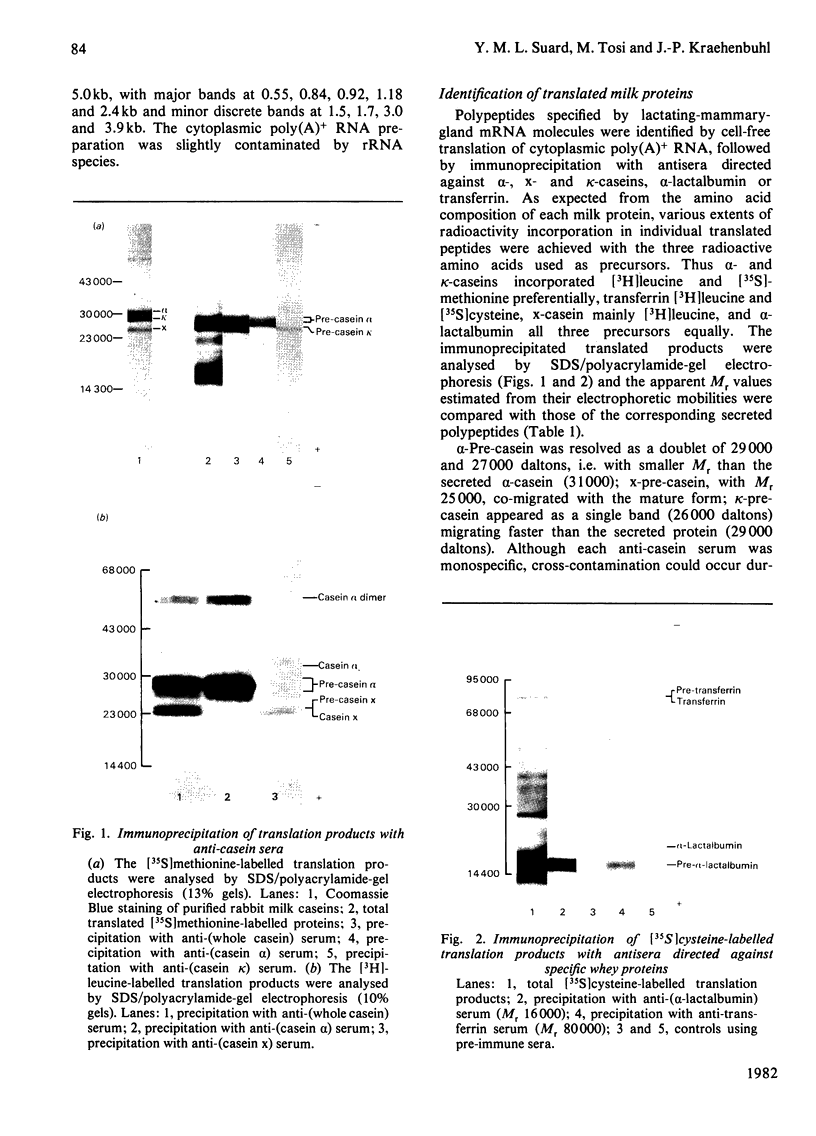
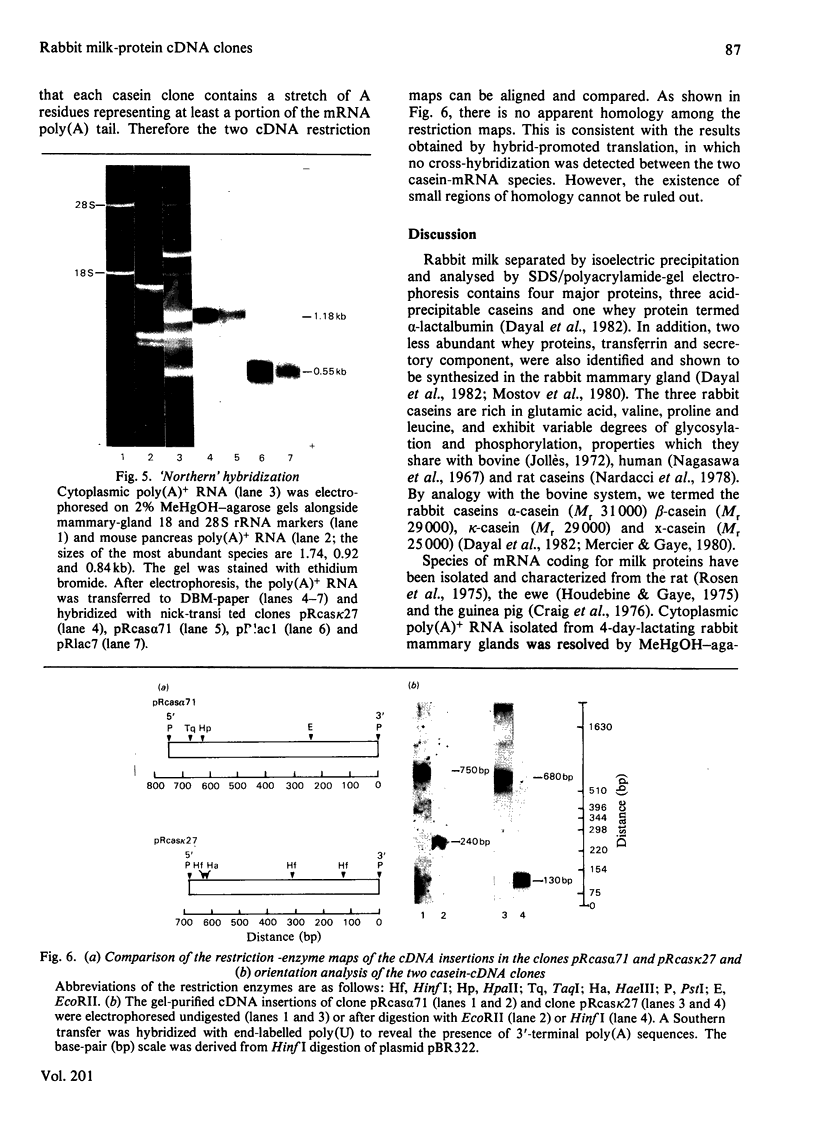
Total cytoplasmic polyadenylated RNA from lactating rabbit mammary glands was analysed on methylmercury hydroxide-agarose gels. The size of the most abundant mRNA species ranged between 0.5 and 5.0 kb (kilobases), with major bands at 0.55, 0.84, 0.92, 1.18 and 2.4 kb and discrete minor bands of 1.5, 1.7, 3.0 and 3.9 kb. Translation in vitro of total mRNA with [3H]leucine or [35S]methionine as precursor yielded four major bands with apparent Mr values of 16 000, 25 000, 26 000 and 29 000. The four protein bands were identified by immunoprecipitation by using specific antisera as alpha-lactalbumin and x-, kappa- and alpha-caseins, respectively. Labelling with (35S]cysteine followed by immunoprecipitation with anti-transferrin or anti-alpha-lactalbumin sera allowed the identification of two whey proteins. Translated transferrin was resolved as an 80 000-dalton band and alpha-lactalbumin appeared as a 16 000-dalton protein. A library of recombinant plasmids containing cDNA (complementary DNA) sequences representing cytoplasmic polyadenylated RNA was used to isolate clones for the major rabbit caseins and alpha-lactalbumin. A preliminary characterization of these cDNA clones was achieved by colony hybridization with enriched RNA fractions as probes. Positive clones were identified by use of hybrid-promoted translation in vitro and immunoprecipitation of the translation products. The corresponding mRNA species were further identified by hybridizing RNA blots with radioactively labelled cDNA clones. We present the restriction map of alpha-casein and kappa-casein cDNA clones.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Banerjee M. R., Terry P. M., Sakai S., Lin F. K., Ganguly R. Hormonal regulation of casein messenger RNA (mRNA). In Vitro. 1978 Jan;14(1):128–139. doi: 10.1007/BF02618179. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Boulton A. P., Harrison O. S., Parker D., Campbell P. N. Studies on the intracellular segregation of polyribosome-associated messenger ribonucleic acid species in the lactating guinea-pig mammary gland. Biochem J. 1979 Sep 1;181(3):737–756. doi: 10.1042/bj1810737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Brown P. A., Harrison O. S., McIlreavy D., Campbell P. N. Guinea-pig milk-protein synthesis. Isolation and characterization of messenger ribonucleic acids from lactating mammary gland and identification of caseins and pre-alpha-lactalbumin as translation products in heterologous cell-free systems. Biochem J. 1976 Oct 15;160(1):57–74. doi: 10.1042/bj1600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Hall L., Parker D., Campbell P. N. The construction, identification and partial characterization of plasmids containing guinea-pig milk protein complementary DNA sequences. Biochem J. 1981 Mar 15;194(3):989–998. doi: 10.1042/bj1940989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., McIlreavy D., Hall R. L. Separation and partial characterization of guinea-pig caseins. Biochem J. 1978 Aug 1;173(2):633–641. doi: 10.1042/bj1730633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Perera P. A., Mellor A., Smith A. E. Initiation and processing in vitro of the primary translation products of guinea-pig caseins. Biochem J. 1979 Nov 15;184(2):261–267. doi: 10.1042/bj1840261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayal R., Hurlimann J., Suard Y. M., Kraehenbuhl J. P. Chemical and immunochemical characterization of caseins and the major whey proteins of rabbit milk. Biochem J. 1982 Jan 1;201(1):71–79. doi: 10.1042/bj2010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinoy E., Houdebine L. M., Delouis C. Role of prolactin and glucocorticoids in the expression of casein genes in rabbit mammary gland organ culture. Quantification of casein mRNA. Biochim Biophys Acta. 1978 Feb 16;517(2):360–366. doi: 10.1016/0005-2787(78)90202-2. [DOI] [PubMed] [Google Scholar]
- Gaye P., Gautron J. P., Mercier J. C., Hazé G. Amino terminal sequences of the precursors of ovine caseins. Biochem Biophys Res Commun. 1977 Dec 7;79(3):903–911. doi: 10.1016/0006-291x(77)91196-2. [DOI] [PubMed] [Google Scholar]
- Gaye P., Hue D., Mercier J. C., Haze G. Enzymatic processing of precursors of ovine lactoproteins by mammary microsomal membranes and a deoxycholate-soluble extract from rough microsomes. FEBS Lett. 1979 May 1;101(1):137–142. doi: 10.1016/0014-5793(79)81312-5. [DOI] [PubMed] [Google Scholar]
- Gordon J. I., Deeley R. G., Burns A. T., Paterson B. M., Christmann J. L., Goldberger R. F. In vitro translation of avian vitellogenin messenger RNA. J Biol Chem. 1977 Nov 25;252(22):8320–8327. [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hall L., Craig R. K., Campbell P. N. mRNA species directing synthesis of milk proteins in normal and tumour tissue from human mammary gland. Nature. 1979 Jan 4;277(5691):54–56. doi: 10.1038/277054a0. [DOI] [PubMed] [Google Scholar]
- Hall L., Davies M. S., Craig R. K. The construction, identification and characterisation of plasmids containing human alpha-lactalbumin cDNA sequences. Nucleic Acids Res. 1981 Jan 10;9(1):65–84. doi: 10.1093/nar/9.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Primary structure of rabbit alpha-lactalbumin. Biochemistry. 1979 Nov 13;18(23):5182–5191. doi: 10.1021/bi00590a024. [DOI] [PubMed] [Google Scholar]
- Houdebine L. M., Devinoy E., Delouis C. Stabilization of casein mRNA by prolactin and glucocorticoids. Biochimie. 1978;60(1):57–63. doi: 10.1016/s0300-9084(78)80198-9. [DOI] [PubMed] [Google Scholar]
- Houdebine L. M. Effects of prolactin and progesterone on expression of casein genes. Titration of casein mRNA by hybridization with complementary DNA. Eur J Biochem. 1976 Sep;68(1):219–225. doi: 10.1111/j.1432-1033.1976.tb10781.x. [DOI] [PubMed] [Google Scholar]
- Houdebine L. M., Gaye P. Regulation of casein synthesis in the rabbit mammary gland. Titration of mRNA activity for casein under prolactin and progesterone treatments. Mol Cell Endocrinol. 1975 Jul;3(1):37–55. doi: 10.1016/0303-7207(75)90030-1. [DOI] [PubMed] [Google Scholar]
- Katz L., Williams P. H., Sato S., Leavitt R. W., Helinski D. R. Purification and characterization of covalently closed replicative intermediates of ColEl DNA from Escherichia coli. Biochemistry. 1977 Apr 19;16(8):1677–1683. doi: 10.1021/bi00627a024. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Prasad R., Ebner K. E., Blobel G. Coupled cell-free synthesis, segregation, and core glycosylation of a secretory protein. Proc Natl Acad Sci U S A. 1978 May;75(5):2338–2342. doi: 10.1073/pnas.75.5.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusik R. J., Rosen J. M. Prolactin induction of casein mRNA in organ culture. A model system for studying peptide hormone regulation of gene expression. J Biol Chem. 1978 Apr 10;253(7):2343–2347. [PubMed] [Google Scholar]
- Matusik R. J., Rosen J. M. Prolactin regulation of casein gene expression: possible mediators. Endocrinology. 1980 Jan;106(1):252–259. doi: 10.1210/endo-106-1-252. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Beard P., Engers H. D., Hirt B. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J Virol. 1981 Apr;38(1):317–326. doi: 10.1128/jvi.38.1.317-326.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier J. C., Gaye P. Study of secretory lactoproteins: primary structures of the signals and enzymatic processing. Ann N Y Acad Sci. 1980;343:232–251. doi: 10.1111/j.1749-6632.1980.tb47255.x. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Kraehenbuhl J. P., Blobel G. Receptor-mediated transcellular transport of immunoglobulin: synthesis of secretory component as multiple and larger transmembrane forms. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7257–7261. doi: 10.1073/pnas.77.12.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T., Ryoki T., Kiyosawa I., Kuwahara K. Studies on human casein. I. Fractionation of human casein by diethylaminoethyl cellulose column chromatography. Arch Biochem Biophys. 1967 Aug;121(2):502–507. doi: 10.1016/0003-9861(67)90105-1. [DOI] [PubMed] [Google Scholar]
- Nardacci N. J., Lee J. W., McGuire W. L. Differential regulation of alpha-lactalbumin and casein messenger RNA's in mammary tissue. Cancer Res. 1978 Sep;38(9):2694–2699. [PubMed] [Google Scholar]
- Ono M., Oka T. The differential actions of cortisol on the accumulation of alpha-lactalbumin and casein in midpregnant mouse mammary gland in culture. Cell. 1980 Feb;19(2):473–480. doi: 10.1016/0092-8674(80)90522-x. [DOI] [PubMed] [Google Scholar]
- Pascall J. C., Boulton A. P., Parker D., Hall L., Craig R. K. Heterogeneity of guinea-pig caseins synthesized and sequestered by cell-free protein-synthesizing systems. Biochem J. 1981 May 15;196(2):567–574. doi: 10.1042/bj1960567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters B., Mous J., van Bellegem H., Rombauts W. The wheat germ cell-free system possesses processing activity for the precursor of human placental lactogen. Biochim Biophys Acta. 1979 Feb 27;561(2):502–516. doi: 10.1016/0005-2787(79)90158-8. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Richards D. A., Blackburn D. E., Rosen J. M. Restriction enzyme mapping and heteroduplex analysis of the rat milk protein cDNA clones. J Biol Chem. 1981 Jan 10;256(1):533–538. [PubMed] [Google Scholar]
- Rosen J. M., Barker S. W. Quantitation of casein messenger ribonucleic acid sequences using a specific complementary DNA hybridization probe. Biochemistry. 1976 Nov 30;15(24):5272–5280. doi: 10.1021/bi00669a012. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Matusik R. J., Richards D. A., Gupta P., Rodgers J. R. Multihormonal regulation of casein gene expression at the transcriptional and posttransciptional levels in the mammary gland. Recent Prog Horm Res. 1980;36:157–193. doi: 10.1016/b978-0-12-571136-4.50011-5. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., O'Neal D. L., McHugh J. E., Comstock J. P. Progesterone-mediated inhibition of casein mRNA and polysomal casein synthesis in the rat mammary gland during pregnancy. Biochemistry. 1978 Jan 24;17(2):290–297. doi: 10.1021/bi00595a016. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Comstock J. P. Regulation of casein messenger RNA during the development of the rat mammary gland. Biochemistry. 1975 Jul;14(13):2895–2903. doi: 10.1021/bi00684a016. [DOI] [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Williams J. G. Quantitative analysis of specific labelled RNA'S using DNA covalently linked to diazobenzyloxymethyl-paper. Nucleic Acids Res. 1979 Jan;6(1):195–203. doi: 10.1093/nar/6.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suard Y. M., Kraehenbuhl J. P., Aubert M. L. Dispersed mammary epithelial cells. Receptors of lactogenic hormones in virgin, pregnant, and lactating rabbits. J Biol Chem. 1979 Oct 25;254(20):10466–10475. [PubMed] [Google Scholar]
- Terry P. M., Banerjee M. R., Lui R. M. Hormone-inducible casein messenger RNA in a serum-free organ culture of whole mammary gland. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2441–2445. doi: 10.1073/pnas.74.6.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]
- Zehavi-Willner T., Lane C. Subcellular compartmentation of albumin and globin made in oocytes under the direction of injected messenger RNA. Cell. 1977 Jul;11(3):683–693. doi: 10.1016/0092-8674(77)90085-x. [DOI] [PubMed] [Google Scholar]